Abstract
For several years, our laboratory has investigated the role for the HPA axis in cocaine reinforcement. Two classes of drugs that we have studied include corticosterone synthesis inhibitors (e.g., metyrapone) and benzodiazepine receptor agonists (e.g., oxazepam). In the experiments described in this manuscript, we tested the effects of various doses of metyrapone and oxazepam against several doses of self-administered cocaine. Behavioral, endocrine and pharmacokinetic measures of the effects of the combination of metyrapone and oxazepam on cocaine reward are presented. Combinations of metyrapone and oxazepam at doses that produced no observable effects when administered separately significantly reduced cocaine self-administration without affecting food-maintained responding during the same sessions. Changes in pharmacokinetics or endocrine function do not appear to mediate these effects, suggesting a central mechanism of action. Therefore, although these drugs produce their effects through distinct mechanisms, an additive effect on cocaine self-administration is obtained when these drugs are administered together, suggesting that combinations of low doses of metyrapone and oxazepam may be useful in reducing cocaine seeking with a reduced incidence of unwanted side effects and a decreased potential for abuse.
Keywords: cocaine, corticosterone, benzodiazepine, reinforcement, metyrapone, oxazepam
Introduction
Over the last several years, our laboratory, as well as a number of others, has explored the complex relationship between stress and the subsequent activation of the hypothalamic-pituitary-adrenal (HPA) axis in psychomotor stimulant reinforcement (Goeders, 2002, 2007; Majewska, 2002; Winhusen and Somoza, 2001; Sarnyai et al, 2001). In this context, we have investigated the effects of drugs that attenuate HPA axis activity on cocaine self-administration and the drugand cue-induced reinstatement of extinguished cocaine seeking (Goeders, 2004, 2007).
In our earliest work in this area, we investigated the effects of benzodiazepine receptor agonists on intravenous cocaine self-administration in rats. We studied this class of drugs not only because they are among the most widely prescribed drugs for the treatment of anxiety (Uhlenhuth et al., 1995; Baldessarini, 1996), but also since these drugs can decrease plasma corticosterone (Keim and Sigg, 1977), cortisol and ACTH (Meador-Woodruff and Greden, 1988; Torpy et al., 1993) and attenuate cocaine-induced increases in plasma corticosterone (Yang et al., 1992). We initially reported that pretreatment with chlordiazepoxide significantly decreased intravenous cocaine self-administration (Goeders et al., 1989). This effect was attenuated when the unit dose of cocaine was increased, suggesting that chlordiazepoxide decreased the efficacy of cocaine as a reinforcer. However, since these decreases in drug-intake may have resulted from a non-specific disruption of the ability of the rats to respond, an additional study was conducted whereby another benzodiazepine receptor agonist, alprazolam, was tested in rats responding under a multiple schedule of intravenous cocaine presentation and food reinforcement (Goeders et al., 1993). Initially, alprazolam reduced responding maintained by both food and cocaine. However, while tolerance quickly developed to the sedative effects of alprazolam on food-maintained responding during subsequent testing, no tolerance was observed in the ability of alprazolam to reduce cocaine self-administration, suggesting that the effects of benzodiazepines may result from specific actions on cocaine reinforcement rather than non-specific effects on responding.
We have also investigated the effects of corticosterone synthesis inhibitors on cocaine self-administration. Metyrapone blocks the 11β-hydroxylation reaction in the production of corticosterone to decrease plasma concentrations of the hormone (Haleem et al., 1988; Haynes, 1990). Pretreatment with metyrapone resulted in significant dose-related decreases in cocaine self-administration and plasma corticosterone in rats (Goeders et al., 1996). However, since it was once again not clear whether these effects were specific for cocaine reinforcement or were the result of nonspecific effects on the ability of the rats to respond, an additional experiment was designed to address this problem through the use of a multiple, alternating schedule of food presentation and cocaine self-administration, this time following pretreatment with ketoconazole. Ketoconazole is an oral antimycotic agent with a broad spectrum of activity and low toxicity (Sonino, 1987; Thienpont et al., 1979) that also inhibits the 11β-hydroxylation and 18-hydroxylation steps in the synthesis of adrenocorticosteroids (Engelhardt et al., 1985). In these experiments, rats were allowed alternating 15-min periods of access to food reinforcement and cocaine self-administration during daily 2-hour sessions. Pretreatment with ketoconazole reduced cocaine self-administration without affecting food-reinforced responding, suggesting that corticosterone synthesis inhibitors decrease cocaine reinforcement at doses that do not produce nonspecific motor effects.
Thus, we have demonstrated that benzodiazepine receptor agonists and corticosterone synthesis inhibitors reduce cocaine self-administration. However, both classes of drugs have potential side effects that could limit their usefulness in the treatment of cocaine addiction. For example, benzodiazepines are not usually recommended as the treatment of choice for cocaine dependence since these drugs have the potential for abuse (Chouinard, 2004; Lilja et al., 2001; O'Brien, 2005), worrying some that the use of these drugs might result in a secondary dependence (Wesson and Smith 1985). Corticosterone synthesis inhibitors have the potential to produce adrenal insufficiency, among other things, which could also limit the utility of this class of drugs. However, the incidence of side effects produced by these two classes of drugs may be mitigated by reducing the dose, which is the basis for the design of the experiments described in this manuscript. Our hypothesis was that by combining drugs that affect HPA axis activity through divergent mechanisms and delivering these drugs at concentrations that have no effect when administered alone, we would minimize their potential toxic and unwanted side effects while still reducing cocaine intake. We confirmed our hypothesis, as described in the following experiments, using a combination of metyrapone and oxazepam.
Methods
Subjects
Adult male Wistar rats (Harlan Sprague Dawley, Indianapolis, IN) 80 to 100 days old at the start of the experiments were used. All rats were housed singly in cages equipped with a laminar flow unit and air filter in a temperature- and humidity-controlled, AAALAC-accredited animal care facility on a reversed 12 hr light/dark cycle (lights on at 18:00 hr) with free access to water. Rats were allowed free access to food until their free-feeding body weights increased to approximately 380-400g. These rats were subsequently maintained at 85 to 90% of their freefeeding body weights by food pellets (45 mg, Test Diets, Richmond, IN) during the experimental sessions and supplemental post-session feeding (Harlan Teklad, Madison, WI) throughout the course of the experiments. All procedures were approved by the LSUHSC Institutional Animal Care and Use Committee and were carried out in accordance with the NIH “Principles of laboratory animal care” (NIH publication No. 85-23).
Surgery
Once the targeted weight was reached, the rats were implanted with chronic indwelling jugular catheters under pentobarbital anesthesia (50 mg/kg, ip) with methylatropine nitrate pretreatment (10 mg/kg, ip) using previously reported procedures (Koob and Goeders, 1989; Goeders et al., 1998; Goeders and Clampitt, 2002). The catheter (0.012 in i.d. × 0.025 in o.d., silicone tubing) was inserted into the right posterior facial vein and pushed down into the jugular vein until it terminated outside the right atrium. The catheter was anchored to tissue in the area and continued subcutaneously to the back where it exited just posterior to the scapulae through a Marlex mesh™/dental acrylic/22-gauge guide cannula (Plastics One, Roanoke, VA) assembly that was implanted under the skin for attachment of a leash. The stainless-steel spring leash (Plastics One) was attached to the guide cannula assembly and to a leak-proof fluid swivel suspended above the operant chamber. Tubing connected the swivel to a 20-ml syringe in a motor-driven pump (Razel Scientific Instruments, Stamford, CT) located outside the sound-attenuating enclosure. The swivel and leash assembly was counter-balanced to permit relatively unrestrained movement of the animal. The animals were injected with sterile penicillin G procaine suspension (75,000 units, im) immediately before surgery, and they were allowed a minimum of six days to recover following surgery. The swivel and leash assembly was always connected during all experimental sessions. At the end of each session, the leash was disconnected, the catheter was filled with streptokinase (8,333 IU) to inhibit the formation of blood clots and a dummy cannula was inserted into the guide before the rats were returned to their home cages. The patency of the catheters was tested immediately after the end of the experimental sessions on Wednesdays throughout the course of the experiment. If blood could be obtained via the catheter, then it was judged to be patent. If not, then the rat was injected via the catheter with methohexital sodium (1.5 mg, iv). An immediate light anesthesia indicated that the catheter was functional.
Apparatus
Standard plastic and stainless steel operant conditioning chambers contained within sound-attenuating enclosures (Med-Associates, Inc., St. Albans, VT) were used to run the behavioral experiments. Each experimental chamber was equipped with two response levers mounted on one wall of the chamber with a stimulus light located above each lever. A food pellet dispenser was located between the response levers. One of the levers was designated the “food” lever, while the other was the “cocaine” lever. The enclosures contained an exhaust fan that supplied ventilation and white noise to mask extraneous sounds. An IBM-compatible personal computer and interface system was used to program the procedures and collect the experimental data.
Cocaine Self-Administration
Rats were trained to respond under a multiple, alternating schedule of food reinforcement and cocaine self-administration as described previously (Goeders et al. 1998; Goeders and Guerin, 2000). During the food component of the schedule, a stimulus light located directly above the food response lever was illuminated to indicate the availability of food reinforcement. Initially, each depression of the food response lever resulted in a brief darkening of the food stimulus light (0.6 s) and the delivery of a food pellet (45 mg, Test Diets). A 35-s timeout followed the delivery of each food pellet. During this timeout, the stimulus light was darkened and responses on the food lever were counted but had no scheduled consequences. Responding on the other (i.e., cocaine) lever during the food component also had no scheduled consequences. The response requirement for the food lever was gradually increased over several sessions from continuous reinforcement to a fixed-ratio 4 (FR4) schedule whereby 4 responses were required for food presentation. Following 15 min of access to food, all stimulus lights in the chamber were darkened for a 1-min timeout. Following the timeout, the stimulus light above the cocaine response lever was illuminated to indicate the availability of cocaine. Initially, each depression of the cocaine response lever resulted in a brief darkening of the stimulus light and an infusion of cocaine (0.25 mg/kg/infusion in 200 μl 0.9% NaCl delivered over 5.6 s). A 25-s timeout period followed each infusion. The response requirement for cocaine was gradually increased to a FR4 schedule of reinforcement. After 15 min of access to cocaine and another 1-min timeout, the rats were again allowed 15 min of access to the food component of the schedule. Access to food and cocaine alternated in this manner every 15 min during the 2-h behavioral sessions so that each rat was exposed to food and cocaine for four 15-min periods each. Each behavioral session began with 15 min of access to either food or cocaine, and this alternated daily. Sessions were conducted at the same time each day, typically between 09:00 and 10:00, Monday through Friday. Stable baselines of responding occurred when the total number of cocaine and food presentations, as well as the number of presentations during each of the four exposures each session, varied less than 10% for three consecutive sessions.
Extinction Training
Prior to testing, the rats were repeatedly exposed to extinction (Goeders et al. 1998; Goeders and Guerin, 2000). On extinction test days, a saline vehicle syringe was substituted for the cocaine syringe normally present, and responses on the “cocaine” lever during the cocaine component of the schedule only resulted in infusions of saline. Likewise, responding on the “food” lever during the food component of the session activated the feeder mechanism, but no pellets were delivered. The rats were presented with these “extinction probes” on drug test days (i.e., Tuesdays and Fridays) until stable, reproducible, “extinction-like” behavior was observed. Extinction-like behavior was deemed to occur when the rates and patterns of responding were less than 50% of baseline and did not vary more than 10% during at least two consecutive extinction probes. Extinction probes continued to be run approximately every 2 weeks until the conclusion of all of the experiments to ensure that consistent extinction-like behavior was reliably produced.
Dose determination and testing
Once consistent and reproducible behavior during self-administration and extinction was obtained, the animals received vehicle (5% emulphor in heparinized 0.9% saline) 30 minutes before the start of the behavioral session to establish baseline behaviors. Then the “effective” and “ineffective” doses of metyrapone and oxazepam were individually determined for each rat. The “effective” dose of each drug was the dose that reduced cocaine-maintained responding by at least 50% without affecting responding on the “food” lever. The “ineffective” dose was that dose that reduced cocaine-maintained responding by less than 10%. These doses were individually determined to ensure that each drug combination consisted of an ineffective dose of each drug for each rat.
The effective and ineffective doses were determined for one drug (either metyrapone or oxazepam) before the doses for the second drug were identified, and the drug that was tested first was randomly divided among the rats. The rats only received pretreatments with oxazepam or metyrapone when the baselines for cocaine and food self-administration were stable according to the criteria described above, and at least two sessions elapsed between each pretreatment. The rats were initially pretreated (30 min, IP) with either vehicle or a dose of metyrapone or oxazepam that had been shown in previous and pilot studies to be an effective dose in most rats (i.e., 10 mg/kg oxazepam or 50 mg/kg metyrapone). If cocaine self-administration was not reduced by at least 50% without affecting food-maintained responding, the dose was increased (e.g., to 20 mg/kg oxazepam or 100 mg/kg metyrapone) for the next pretreatment. The dose continued to be increased in this way until the effective dose of each drug was identified for each rat. The dose of each drug was then incrementally reduced in the same manner until cocaine intake was altered by less than 10%.
Metyrapone/Oxazepam combination pharmacotherapy testing
When the “effective” and “ineffective” doses for each drug were identified, combination pharmacotherapy testing began. During this phase of the experiment, rats were pretreated (30 minutes, IP) with a combination of the individually-determined “ineffective” doses of metyrapone and oxazepam. Stable baselines of responding were required between tests (typically conducted on Tuesdays and Fridays) according to the criteria described above. The “ineffective” dose combinations determined for use in this study included: oxazepam/metyrapone; 5 mg/kg/50 mg/kg (n=1), 10 mg/kg/50 mg/kg (n=2), 20 mg/kg/50 mg/kg (n=1), 30 mg/kg/50 mg/kg (n=2); 40 mg/kg/25 mg/kg (n=1), and 45mg/kg/20 mg/kg (n=1), IP). Once combination testing during cocaine self-administration at the 0.25 mg/kg/infusion dose was completed, the dose of cocaine was increased to 0.5 mg/kg/infusion or decreased to 0.125 mg/kg/infusion and self-administration was allowed to stabilize at the new cocaine dose. Vehicle and extinction probes were conducted at each dose. Once self-administration and extinction met the criteria described above, the rats were tested with the same individually-determined dose combinations of metyrapone and oxazepam that were tested when 0.25 mg/kg/infusion was the cocaine dose self-administered. Following these tests, the third dose of cocaine was made available for self-administration and the same combinations of metyrapone and oxazepam were tested as described above. The decision to initially increase or decrease the cocaine dose was randomly determined for each rat.
All of the dose determinations (i.e., the “effective” and “ineffective” doses of metyrapone and oxazepam as well as the dose combinations) were replicated a minimum of two times for inter-injection reliability and also to check for the appearance of tolerance or sensitization. The effects of vehicle pretreatment were also redetermined once the effective and ineffective doses of each drug were identified to check for any shift in baseline responding. As above, rats were only pretreated with oxazepam, metyrapone, the metyrapone/oxazepam combinations and vehicle when the baselines for cocaine and food self-administration were stable, and at least two sessions elapsed between each pretreatment.
Corticosterone Measurements
Blood was collected via the implanted catheters at the end of the behavioral sessions several times during the course of this experiment for the measurement of plasma corticosterone. Blood was collected following pretreatment with vehicle, following extinction, following pretreatment with the “effective” and “ineffective” doses of metyrapone and oxazepam, and following pretreatment with the combination of the “ineffective” doses of metyrapone and oxazepam. Blood was also collected following extinction and following pre-treatment with vehicle and the combination of the “ineffective” doses of metyrapone and oxazepam when the other two doses of cocaine were tested. The catheter was removed from the bottom of the swivel and blood (100 μl) was rapidly obtained from the catheter while the rats were still in the experimental chambers at the end of the behavioral sessions. Plasma corticosterone (ng/ml) was subsequently determined by specific radioimmunoassay using the ImmuChem™ double antibody [125I]corticosterone kit (ICN Biomedicals, Costa Mesa, CA).
Pharmacokinetic Studies
Ten adult male Wistar rats (90 to 120 days old) were implanted with chronic, indwelling jugular catheters as described above and were allowed to recover from surgery. On the test day, the rats were pretreated with intraperitoneal injections of various combinations of oxazepam and metyrapone (oxazepam/metyrapone; 5 mg/kg/25 mg/kg, 10 mg/kg/25 mg/kg, 10 mg/kg/50 mg/kg, 30 mg/kg/50 mg/kg, 40 mg/kg/25 mg/kg, IP) or vehicle (5% emulphor in saline) 30 minutes before the cocaine injections were administered. These oxazepam/metyrapone combinations were selected from our behavioral studies to encompass the range of doses that reduced cocaine self-administration without affecting food-maintained responding. Beginning 30 minutes following the drug combination or vehicle injection, the rats received intravenous injections of cocaine (0.25 mg/kg/infusion) every 2 minutes for 1 hour. After the final injection of cocaine, blood was collected from the catheter for the analysis of cocaine and its metabolites, ecgonine methyl ester and benzoylecgonine. Concentrations of metyrapone and its active metabolite, metyrapol, as well as oxazepam were also determined. All drug concentrations were determined using gas chromatography-mass spectrometry (GCMS) procedures as described below.
Blood samples were collected via the intravenous jugular catheter into 1.5 ml microcentrifuge tubes. 500 ul of methanol were added to 300 ul of blood, which was shaken and then centrifuged (10,000 × g for 5 min). The supernatant was removed and the pellet was extracted with an additional 500 ul of methanol. The combined supernatants were stored at −10°C until analysis.
The samples were brought to room temperature and were acidified by adding 2 ml of 0.2M KH2PO4 (pH 4.75). Prior to analysis, the samples were passed through a Waters Oasis MCX 3cc (60 mg) solid phase extraction column. Before the samples were added to the column, they were pre-equilibrated with 2 ml of methanol followed by 2 ml of deionized water. The samples were added to the column, washed with 2 ml of 0.1 N HCl, 2 ml of deionized water, and finally 2 ml of methanol. The analytes were eluted with 2.5 ml dichloromethane:2-propanol:ammonium hydroxide (39:10:1). The samples were evaporated to dryness under a stream of dry nitrogen in a 70°C water bath. The samples were derivatized by adding 50 ul of pentafluoropropanol and 50 ul of pentafluoropropionic acid anhydride and incubating for 20 minutes in a 70°C water bath. The residual derivatization reagent was removed by evaporating to dryness at 70°C under a stream of dry nitrogen. Finally, the residue was reconstituted with 75 ul of ethyl acetate.
The GC/MS system consisted of a Hewlett Packard 5890 Series II GC equipped with a Hewlett Packard 7673 autosampler. Detection was accomplished using a Hewlett Packard 5972 Mass Selective Detector. GC separation was carried out using a Restek Rtx-5, 30 m × 0.25 mm column, with a 0.25 um film thickness using helium for the mobile phase. The injection port temperature was 250°C, and the detector temperature was 305°C. The injection volume was 1.5 ul, and the mode was splitless at a constant flow pressure of 20 psi at 265°C, with the inlet purge valve initially off and turned on at 1 minute. The GC initial temperature was held at 100°C for 1 minute. After the initial 1 minute, the oven was programmed to 160°C at 60°C/min, then to 175°C at 10°C/min, and finally to 300°C at 25°C/min, which was held for 1.5 min. Detection was achieved in the selected ion monitoring mode as follows:
8.00 – 8.89 min, m/z 182.10, 303.20, 272.10 (cocaine RT = 8.17)
3.68 – 5.68 min, m/z 182.10, 345.10, 314.00 (ecgonine methyl ester RT = 4.59)
7.40 – 8.00 min, m/z 300.10, 421.10, 316.00 (benzoylecgonine RT = 7.64)
6.75 – 7.40 min, m/z 120.10, 106.00, 226.10 (metyrapone RT = 6.90)
5.68 – 6.75 min, m/z 120.10, 211.10, 374.10 (metyrapol RT = 6.50)
8.89 – 10.00 min, m/z 257.00, 241.10, 418.10 (oxazepam RT = 9.53)
Quantification was achieved by comparison to external standard calibration curves for metyrapone and metyrapol and internal standard curves using deuterated analogues for ecgonine methyl ester, benzoylecgonine, cocaine, and oxazepam.
Drugs
Cocaine HCl was obtained from the National Institute on Drug Abuse (Research Triangle Park, NC) and was dissolved in bacteriostatic, heparinized 0.9% saline. Cocaine was self-administered at doses of 0.125, 0.25 and 0.5 mg/kg/infusion and was delivered in a 200-μl volume over 5.6 sec. Metyrapone was purchased from Biomol International L.P. (Plymouth Meeting, PA) and Sigma-Aldrich (St. Louis, MO) and oxazepam was purchased from Sigma-Aldrich. These drugs were administered intraperitoneally as a suspension in 5% emulphor (Alkamuls EL-620, Rhodia, Cranberry, NJ) in heparinized 0.9% saline at a volume of 1 ml/kg.
Data Analysis
Data collected included the total number of cocaine infusions and food pellets delivered per session during baseline responding and following pretreatment with vehicle and the various doses of metyrapone, oxazepam and the metyrapone/oxazepam combinations. For the extinction probes, the number of saline injections and feeder mechanism activations were counted, and the data analyses only included those data obtained once the criteria for successful extinction-like behavior were met. Plasma corticosterone was measured as described above and expressed as ng/ml plasma. In the pharmacokinetic studies, cocaine and its metabolites benzoylecgonine and ecgonine methyl ester, metyrapone and its metabolite metyrapol, and oxazepam were measured as described above and expressed as ng/ml plasma. The significance of the differences among the various conditions was determined with a one-way or two-way analysis of variance as appropriate. Tukey's all pair-wise multiple comparison procedures were then used to isolate differences between the experimental groups. For all analyses, significance was set at p<0.05.
Results
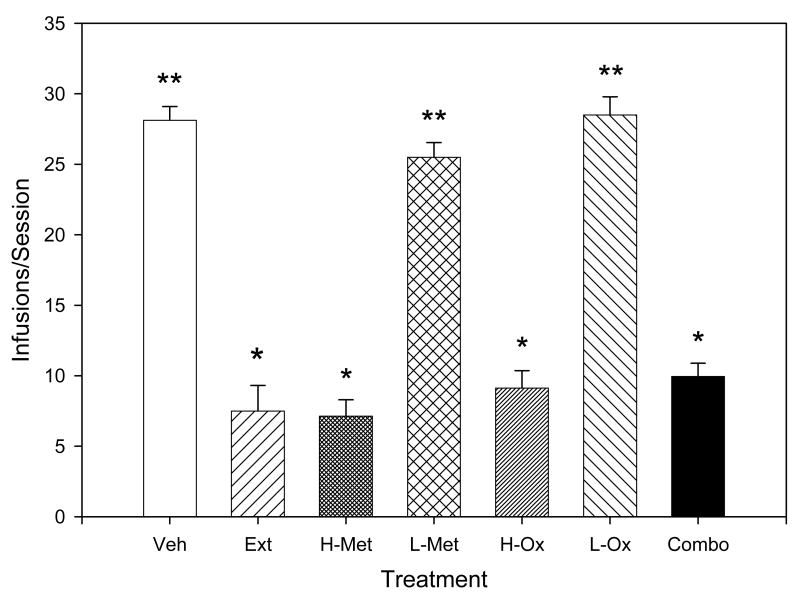
Stable baselines of cocaine- and food-reinforced responding were obtained following approximately 15 to 20 experimental sessions with 0.25 mg/kg/infusion cocaine. The effects of the “effective” and “ineffective” doses of metyrapone and oxazepam delivered individually and the effects of the “ineffective” doses of metyrapone and oxazepam delivered in combination are depicted in Figure 1. A two-way analysis of variance revealed a significant effect of the treatment conditions on cocaine self-administration [F(6,55)=75.578, p<0.001], with responding on the cocaine lever significantly reduced during cocaine extinction (q=17.551, p<0.05) and following pretreatment the high doses of metyrapone (q=17.870, p<0.05) and oxazepam (q=16.169, p<0.05) compared to pretreatment with vehicle. In contrast, the effects of pretreatment with the ineffective doses of metyrapone (q=2.234) and oxazepam (q=0.375) were no different from pretreatment with vehicle. However, pretreatment with a combination of the ineffective doses of metyrapone and oxazepam significantly reduced cocaine self-administration (q=15.459, p<0.05) compared to pretreatment with vehicle and was not significantly different from responding observed during extinction (q=2.092). The number of infusions delivered during each of the four 15-min self-administration bins per session following pretreatment with the combination of oxazepam and metyrapone was also significantly decreased compared to vehicle pretreatment [F(7,63)=17.661, p<0.001]. In addition, there were no differences in the number of infusions among the four bins for each treatment condition, indicating that the combination reduced cocaine seeking throughout the behavioral sessions. Although a two-way analysis of variance indicated a significant effect of treatment on food-maintained responding [F(6,55)=93.649, p<0.001], the only significant effects on responding were between extinction and all other treatment conditions (Table 1). The combination of oxazepam and metyrapone had no significant effect on food-maintained responding.
Figure 1.
The effects of the various treatment conditions on intravenous cocaine (0.25 mg/kg/infusion) self-administration in rats. Values represent the means (± S.E.M.) for N=8. Veh refers to pretreatment with the vehicle (0.5% emulphor in saline); Ext to responding during extinction conditions; H-Met to the high dose of metyrapone; L-Met to the low, ineffective dose of metyrapone; H-Ox to the high dose of oxazepam; L-Ox to the low, ineffective dose of oxazepam; and Combo to the co-administration of the low, ineffective doses of metyrapone and oxazepam. Pretreatment with the high doses of metyrapone and oxazepam reduced cocaine intake to levels seen during extinction. Although the low doses of each drug did not affect drug intake when administered alone, the co-administration of ineffective doses of metyrapone and oxazepam reduced cocaine self-administration to levels seen during extinction. The ineffective dose combinations (Combo) determined for use in this study included: oxazepam/metyrapone; 5 mg/kg/50 mg/kg (n=1), 10 mg/kg/50 mg/kg (n=2), 20 mg/kg/50 mg/kg (n=1), 30 mg/kg/50 mg/kg (n=2); 40 mg/kg/25 mg/kg (n=1), and 45mg/kg/20 mg/kg (n=1), IP).
* p<0.05 compared to vehicle pretreatment
** p<0.05 compared to extinction conditions
Table 1. Effects of the various treatment conditions on food-reinforced responding.
Rats were trained to respond under a multiple, alternating schedule of cocaine (0.25 mg/kg/infusion) and food reinforcement. Values represent the means (± S.E.M.) for N=8. None of the treatments (except for extinction conditions) significantly altered food-maintained responding from values seen when the vehicle was administered.
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
|
Foods
± SEM |
Vehicle | Extinction | Metyrapone
High |
Metyrapone
Low |
Oxazepam
High |
Oxazepam
Low |
Combo |
| 99.3
± 0.4 |
29.5*
± 6.9 |
95.4
± 1.7 |
97.0
± 1.7 |
97.3
± 0.8 |
98.4
± 0.6 |
97.2
± 0.4 |
|
p<0.05 compared to all other treatment conditions.
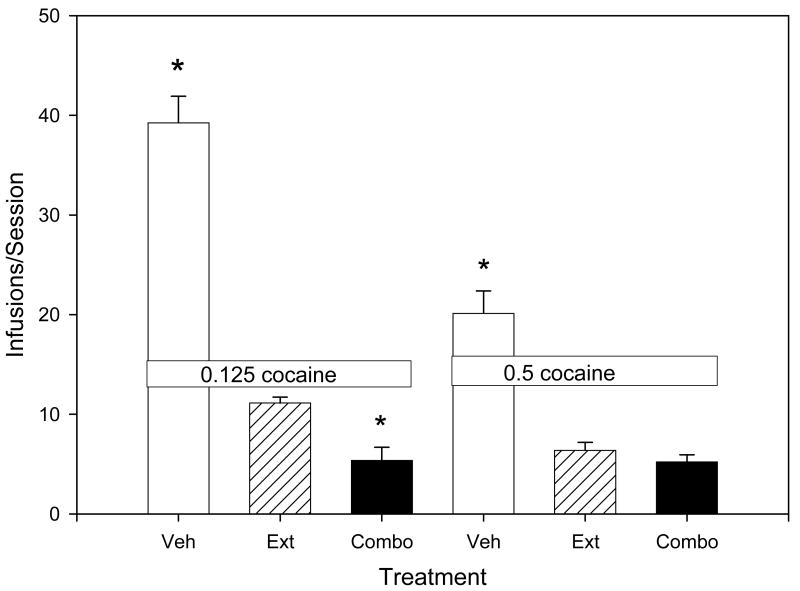
Similar effects on self-administration were observed when the cocaine unit dose was either increased or decreased (Figure 2). At the 0.125 mg/kg/infusion dose of cocaine [F(2,23)=142.786, p<0.001), pretreatment with a combination of the ineffective doses of metyrapone and oxazepam significantly reduced self-administration compared to pretreatment with vehicle (q=22.330, p<0.05) and to responding during extinction (q=3.790, p<0.05). Similarly, at the 0.5 mg/kg/infusion dose of cocaine [F(2,23)=46.503, p<0.001), pretreatment with a combination of the ineffective doses of metyrapone and oxazepam significantly reduced self-administration compared to pretreatment with vehicle (q=12.258, p<0.05) and was no different from responding during extinction (q=0.951). Finally, while a two-way analysis of variance indicated a significant effect of treatment on food-maintained responding at the 0.125 mg/kg/infusion [F(2,23)=173.969, p<0.001] and 0.5 mg/kg/infusion [F(2,23)=280.203, p<0.001] doses of cocaine, the only significant effects on responding were between extinction and all other treatment conditions (Table 2). The combination of oxazepam and metyrapone had no significant effect on food-maintained responding.
Figure 2.
The effects of the treatment conditions on cocaine self-administration at the 0.125 mg/kg/infusion (left side) and 0.5 mg/kg/infusion (right side) unit doses. Values represent the means (± S.E.M.) for N=8. Veh refers to pretreatment with the vehicle; Ext to responding during extinction conditions; and Combo to the co-administration of ineffective doses of metyrapone and oxazepam. The co-administration of low, ineffective doses of metyrapone and oxazepam reduced drug intake to levels seen during extinction with both unit doses of cocaine. The ineffective dose combinations (Combo) determined for use in this study included: oxazepam/metyrapone; 5 mg/kg/50 mg/kg (n=1), 10 mg/kg/50 mg/kg (n=2), 20 mg/kg/50 mg/kg (n=1), 30 mg/kg/50 mg/kg (n=2); 40 mg/kg/25 mg/kg (n=1), and 45mg/kg/20 mg/kg (n=1), IP).
* p<0.05 compared to extinction conditions
Table 2. Effects of the various treatment conditions on food-reinforced responding.
Rats were trained to respond under a multiple, alternating schedule of food and either 0.125 mg/kg/infusion (left side) or 0.5 mg/kg/infusion (right side) cocaine reinforcement. Values represent the means (± S.E.M.) for N=8. None of the treatments (except for extinction conditions) significantly altered food-maintained responding from values seen when the vehicle was administered.
| Treatment | ||||||
|---|---|---|---|---|---|---|
|
Foods
± SEM |
0.125 mg/kg/infusion cocaine | 0.5 mg/kg/infusion cocaine | ||||
| Vehicle | Extinction | Combo | Vehicle | Extinction | Combo | |
| 99.4
± 0.4 |
34.6*
± 4.9 |
97.5
± 0.6 |
98.9
± 0.6 |
24.4*
± 4.6 |
97.4
± 0.5 |
|
p<0.05 compared to all other treatment conditions.
The effects of extinction, pretreatment with vehicle, pretreatment with the effective and ineffective doses of metyrapone and oxazepam, and pretreatment with the combination of the ineffective doses of metyrapone and oxazepam on plasma corticosterone are reported in Table 3. Surprisingly, a two-way analysis of variance revealed that there were no significant differences among the treatment groups [F(6,55)=1.999, p=0.087]. We conducted a separate two-way analysis of variance of just the metyrapone data (i.e., vehicle, extinction, effective and ineffective doses), which revealed a significant effect of treatment [F(3,31)=12.020, p<0.001], with plasma corticosterone significantly reduced following the effective dose of metyrapone compared to extinction (q=5.913, p=0.002) or pretreatment with vehicle (q=8.171, p<0.001) or the ineffective dose of metyrapone (q=4.254, p=0.031). A similar analysis of the oxazepam data (i.e., vehicle, extinction, effective and ineffective doses) did not reveal any significant differences among the treatments [F(3,31)=0.358, p=0.784). We also conducted a separate two-way analysis of variance of only the data from pretreatment with the ineffective doses of metyrapone and oxazepam (i.e., vehicle, extinction and the combination), which revealed that the combination did not significantly alter plasma corticosterone [F(2,23)=0.796, p=0.471]. The effects of extinction and pretreatment with vehicle and the combination of the ineffective doses of metyrapone and oxazepam on plasma corticosterone when other doses of cocaine were available for self-administration are shown in Table 4. Once again, two-way analyses of variance revealed that there were no significant differences among the treatments when either 0.125 mg/kg/infusion [F(2,23)=1.476, p=0.262] or 0.5 mg/kg/infusion cocaine [F(2,23)=0.0896, p=0.915] was available for self-administration.
Table 3. Effects of the various treatment conditions on plasma corticosterone (ng/ml).
Rats were trained to respond under a multiple, alternating schedule of cocaine (0.25 mg/kg/infusion) and food reinforcement. Values represent the means (± S.E.M.) for N=8.
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
|
Corticosterone
(ng/ml) ± SEM |
Vehicle | Extinction | Metyrapone
High |
Metyrapone
Low |
Oxazepam
High |
Oxazepam
Low |
Combo |
| 221.1
± 33.6 |
184.4
± 26.6 |
88.1
± 12.4 |
151.9
± 24.6 |
173.9
± 56.7 |
216.8
± 61.8 |
230.4
± 29.5 |
|
Table 4. Effects of the various treatment conditions on plasma corticosterone (ng/ml).
Rats were trained to respond under a multiple, alternating schedule of food and either 0.125 mg/kg/infusion (left side) or 0.5 mg/kg/infusion (right side) cocaine reinforcement. Values represent the means (± S.E.M.) for N=8. None of the treatments significantly altered plasma corticosterone from values seen when the vehicle was administered.
| Treatment | ||||||
|---|---|---|---|---|---|---|
|
Corticosterone
ng/ml ± SEM |
0.125 mg/kg/infusion cocaine | 0.5 mg/kg/infusion cocaine | ||||
| Vehicle | Extinction | Combo | Vehicle | Extinction | Combo | |
| 192.9
± 18.8 |
265.8
± 34.0 |
221.1
± 33.4 |
174.4
± 18.4 |
186.4
± 27.5 |
177.8
± 21.4 |
|
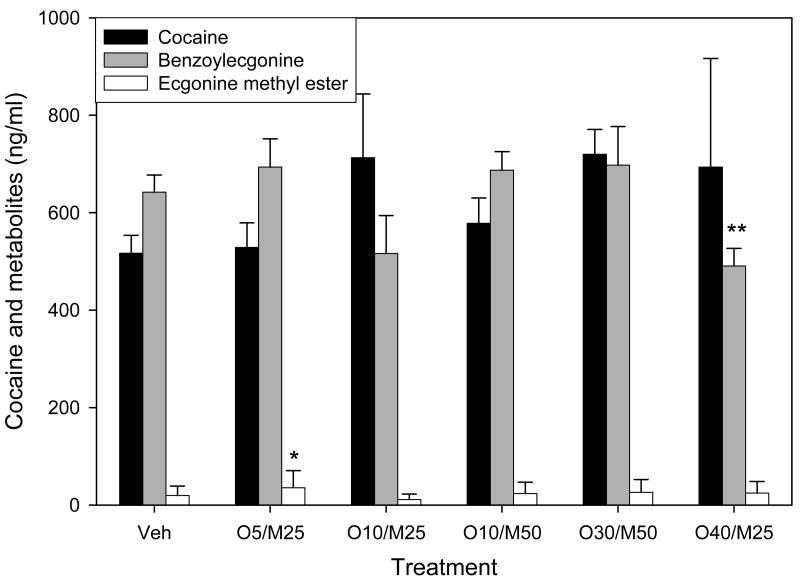
The effects of several dose combinations of metyrapone and oxazepam on the pharmacokinetics of intravenously administered cocaine are depicted in Figure 3. A one-way analysis of variance revealed that neither vehicle nor any of the 5 treatments tested significantly attenuated the plasma concentrations of cocaine [F(5,36)=0.868, p=0.514). We also measured the concentrations of the cocaine metabolites ecgonine methyl ester and benzoylecgonine (Figure 3). A one-way analysis of variance revealed a significant effect of the treatment conditions on ecgonine methyl ester levels [F(5,36)=4.086, p=0.006], but the only significant comparison was between the 5 mg/kg oxazepam/25 mg/kg metyrapone and the 10 mg/kg oxazepam/25 mg/kg metyrapone combinations (q=5.884, p=0.003). A one-way analysis of variance also revealed a significant effect of the treatment conditions on plasma concentrations of benzoylecgonine [F(5,36)=15.214, p<0.001), with benzoylecgonine levels following the highest oxazepam dose combination, 40 mg/kg oxazepam/25 mg/kg metyrapone, significantly decreased compared to all other oxazepam/metyrapone combinations and vehicle (p<0.001).
Figure 3.
Effects of the various treatments on plasma concentrations of cocaine and its metabolites, benzoylecgonine and ecgonine methyl ester. Values represent the means (± S.E.M.) for N=10. Veh refers to pretreatment with vehicle (IP). Five combinations of oxazepam and metyrapone were tested: O5/M25 = 5 mg/kg oxazepam and 25 mg/kg metyrapone; O10/M25 = 10 mg/kg oxazepam and 25 mg/kg metyrapone; O10/M50 = 10 mg/kg oxazepam and 50 mg/kg metyrapone; O30/M50 = 30 mg/kg oxazepam and 50 mg/kg metyrapone; and O40/M25 = 40 mg/kg oxazepam and 25 mg/kg metyrapone. None of the treatment conditions affected plasma concentrations of cocaine, and only minor effects on the cocaine metabolites were observed.
* p<0.05 compared to the 10 mg/kg oxazepam and 50 mg/kg metyrapone combination
** p<0.05 compared to all other treatment conditions
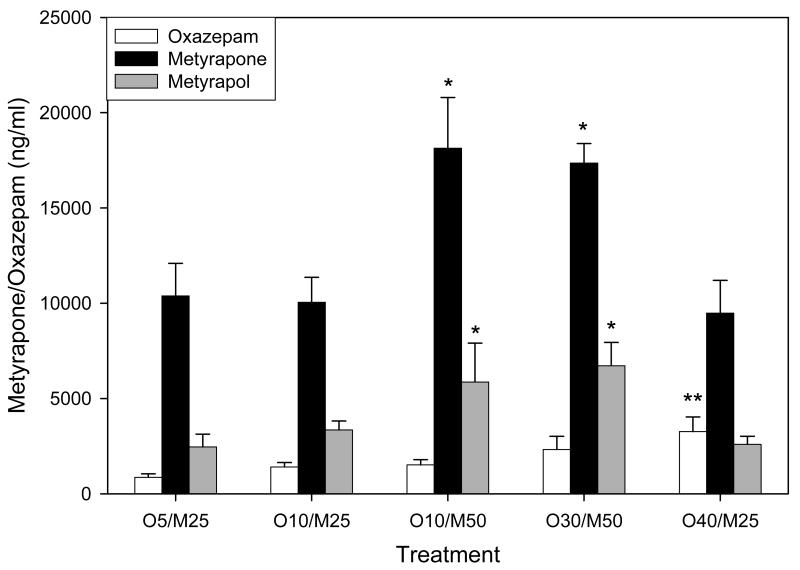
The effects of several dose combinations of metyrapone and oxazepam on the pharmacokinetics of oxazepam and metyrapone following intravenously administered cocaine are shown in Figure 4. A one-way analysis of variance revealed a significant effect of the treatment condition on oxazepam levels [F(4,25)=2.972, p=0.043], but the only significant difference was between the lowest (5 mg/kg oxazepam/25 mg/kg metyrapone) and highest (40 mg/kg oxazepam/25 mg/kg metyrapone) oxazepam dose combinations (q=4.224, p=0.049). A one-way analysis of variance also revealed a significant effect of the treatment conditions on plasma metyrapone concentrations [F(4,25)=6.917, p=0.001], but the only significant differences were between the low (25 mg/kg) and high (50 mg/kg) metyrapone dose combinations (p<0.04). We also measured the concentrations of the active metyrapone metabolite, metyrapol (Figure 4). A one-way analysis of variance revealed a significant effect of the treatment conditions on metyrapol levels [F(4,25)=4.473, p=0.009], but once again the only significant differences were between the low (25 mg/kg) and high (50 mg/kg) metyrapone dose combinations (p<0.05).
Figure 4.
Effects of the various treatments on plasma concentrations of oxazepam, metyrapone and the metyrapone metabolite, metyrapol. Values represent the means (± S.E.M.) for N=10. Five combinations of oxazepam and metyrapone were tested: O5/M25 = 5 mg/kg oxazepam and 25 mg/kg metyrapone; O10/M25 = 10 mg/kg oxazepam and 25 mg/kg metyrapone; O10/M50 = 10 mg/kg oxazepam and 50 mg/kg metyrapone; O30/M50 = 30 mg/kg oxazepam and 50 mg/kg metyrapone; and O40/M25 = 40 mg/kg oxazepam and 25 mg/kg metyrapone (IP). The only significant differences in the concentrations of these compounds were between the lower and higher dose combinations tested.
* p<0.05 compared to dose combinations containing 25 mg/kg metyrapone
** p<0.05 compared to dose combinations containing 5 mg/kg oxazepam
Discussion
Pretreatment with a combination of metyrapone and oxazepam at doses that produced no observable behavioral effects when administered separately significantly attenuated cocaine self-administration in rats, suggesting that the two drugs produced an additive effect to reduce cocaine-seeking and cocaine-taking behavior. The effects of the combination of metyrapone and oxazepam were maintained across several doses of cocaine, with the dose-response curve for cocaine self-administration effectively shifted downward and flattened, suggesting a decrease in the motivation to seek out and take cocaine. These data also suggest that this reduction in cocaine seeking would not easily be overcome by altering the cocaine dose. Food-maintained responding during the same sessions was not affected, indicating that the reduction in cocaine self-administration was not due to nonspecific effects on the ability of the rats to respond. Since ineffective doses of the two drugs were administered and since these two drugs act through distinct mechanisms, the incidence of potential side effects associated with these drugs may also be reduced. These data suggest that similar combinations may be useful in the treatment of cocaine addiction.
Changes in pharmacokinetics do not appear to be involved in the results observed in these experiments. Combinations of oxazepam and metyrapone encompassing a wide range of doses did not affect plasma concentrations of cocaine and did not alter concentrations of oxazepam, metyrapone or the metyrapone metabolite metyrapol either. We did see a small decrease in concentrations of the cocaine metabolite benzoylecgonine with the highest dose of oxazepam tested (40 mg/kg) and a decrease in the concentrations of ecgonine methyl ester when comparing rats injected with 5 mg/kg versus 10 mg/kg oxazepam, but these effects were not orderly and not associated with increases or decreases in plasma cocaine. Therefore, it is unlikely that changes in pharmacokinetics were involved in the effects of combinations of oxazepam and metyrapone on cocaine self-administration.
The mechanisms mediating the additive effects of the combination of oxazepam and metyrapone on cocaine self-administration are not known but are likely, at least in part, to involve the effects of these drugs on the HPA axis. The corticosterone synthesis inhibitor metyrapone blocks 11β-hydroxylation to decrease plasma concentrations of the hormone (Haleem et al., 1988; Haynes, 1990). Benzodiazepines can reduce the elevated cortisol secretion often seen in some psychiatric disorders (Keim and Sigg, 1977; Meador-Woodruff and Greden, 1988; Torpy et al., 1993), attenuate cocaine-induced increases in plasma corticosterone (Yang et al., 1992) and inhibit the cortisol response to adrenocorticotropic hormone (ACTH; Grottoli et al., 2002). Surprisingly, however, the combination of the ineffective doses of metyrapone and oxazepam did not alter plasma corticosterone in the current study, suggesting that the behavioral effects of this combination are mediated through mechanisms not necessarily reflected through this hormone.
Accumulating evidence suggests that these results may actually have been mediated through effects on brain corticotrophin-releasing factor (CRF). We previously reported that pretreatment with the CRF receptor antagonist CP-154,526 also reduces cocaine self-administration (Goeders and Guerin, 2000) and reverses the stress (Shaham et al., 1998) and cue-induced reinstatement of extinguished cocaine seeking (Goeders and Clampitt, 2002), but we failed to observe consistent effects on plasma corticosterone in these experiments as well. These data suggest that these effects may not have occurred through actions on adrenocorticosteroids as initially hypothesized. In another study, we reported that acute and chronic treatment with the corticosterone synthesis inhibitor, ketoconazole, increased the content of CRF in the medial prefrontal cortex (MPC) at doses that did not affect plasma corticosterone (Smagin and Goeders, 2004). Metyrapone has also been reported to increase CRF mRNA in the paraventricular nucleus of the hypothalamus in rhesus monkeys (Van Vugt et al., 1997). We initially identified the involvement of the MPC in cocaine reward in the early 1980s (Goeders and Smith, 1983), and recent reports have further suggested the importance of this structure. For example, the tetrodotoxin-induced inactivation of the MPC has been reported to attenuate the cocaine- (Capriles et al., 2003), stress- (Capriles et al., 2003) and cue-induced (McLaughlin and See, 2003) reinstatement of extinguished cocaine seeking. Furthermore, the direct intracranial injection of cocaine into the MPC reinstates extinguished cocaine-seeking behavior (Park et al., 2002).
Recent studies have demonstrated that there are direct connections between the MPC and hypothalamus (Floyd et al., 2001). We reported that the intracranial delivery of the D1 dopamine agonist SKF 38393 and the D2 agonist quinpirole into the MPC increased plasma corticosterone, suggesting an interaction between MPC dopamine and HPA axis activation (Ikemoto and Goeders, 1998). Interestingly, ketoconazole enhances cocaine-induced increases in dopamine release in the MPC measured using in vivo microdialysis in freelymoving rats (Smagin and Goeders, 2000), which indicates a role for MPC dopamine in the effects of ketoconazole. Cocaine is known to affect CRF activity within the mesocorticolimbic dopaminergic neuronal system, a system widely thought to mediate the reinforcing effects of cocaine and other drugs of abuse (Koob, 1992). Acute cocaine delivery decreases CRF-like immunoreactivity (Sarnyai et al., 1993; Gardi et al., 1997) and increases CRF mRNA (Zhou et al., 1996) in the MPC. Chronic cocaine administration also reduces CRF receptor binding in the MPC and nucleus accumbens (Goeders et al., 1990a; Ambrosio et al., 1997). The localization of CRF and its receptors within this system suggests that the peptide may have modulatory effects on dopaminergic neurotransmission. Accordingly, the administration of CRF directly into the VTA results in a time-dependent decrease in prefrontal cortical dopamine metabolism with an increase in dopamine turnover in the nucleus accumbens (Kalivas et al., 1987). This general pattern of dopaminergic neurotransmission has previously been associated with an enhanced vulnerability to engage in psychomotor stimulant self-administration (Piazza et al., 1991; Goeders and Smith, 1993).
The exact mechanisms underlying the additive effects of the combination of metyrapone and oxazepam are not known, but our hypothesis is that benzodiazepines produce effects in the same brain regions (i.e., the MPC) through processes mediated through the same neurotransmitters (i.e., dopamine, CRF) as corticosterone synthesis inhibitors to produce an additive effect. Benzodiazepine receptor agonists exert their effects by facilitating GABAergic neurotransmission by increasing the affinity of the GABA receptor to GABA (Kostowski, 1995; Lüddens and Korpi, 1995; Oreland, 1988). Stimulation of GABAA receptors opens chloride ion channels, which inhibits neuronal activity. In our hands, chronic injections of cocaine resulted in differential effects on central benzodiazepine receptor binding in various regions of the rat brain (Goeders et al., 1990b; Goeders, 1991). In general, cocaine decreased binding in terminal fields for the mesocorticolimbic dopaminergic system, while increasing labeling in terminal fields for the nigrostriatal system. Decreases in binding in the MPC and increases in the ventral tegmental area were observed for up to two weeks following the final injection of cocaine, suggesting that benzodiazepine receptors in these brain regions may be especially sensitive to the effects of the drug. These cocaine-induced changes in binding appeared to be mediated, at least in part, through the effects of the drug on dopaminergic neuronal activity since intraventricular injections of 6-hydroxydopamine attenuated or reversed these effects (Goeders, 1991). Finally, chronic treatment with benzodiazepines (i.e., alprazolam, diazepam or adinazolam) has also been reported to alter CRF receptors in the frontal cortex and hippocampus (Grigoriadis et al., 1989).
Recently, it was shown that alprazolam can reduce cocaine self-administration in baboons without affecting food-maintained responding (Weerts et al., 2005). Benzodiazepines also reduce the subjective effects of d-amphetamine in humans (Rush et al., 2004). Recognition and the enjoyment of amphetamine and other aspects associated with the psychomotorstimulant effects of amphetamine, including increased heart rate, were significantly decreased in subjects pretreated with alprazolam. GABAA receptor agonists (including benzodiazepines) were also recently reported to decrease the discriminative stimulus effects of cocaine in rats (Barrett et al., 2005) and rhesus monkeys (Negus et al., 2000) and to reduce cocaine self-administration in rats without affecting food-maintained responding (Barrett et al., 2005). These data indicate that benzodiazepines and other agonists at GABAA receptors may be useful in the treatment of psychomotor stimulant addiction (Sofuoglu and Kosten, 2005). However, as mentioned above benzodiazepines are not usually recommended for the treatment of cocaine dependence since these drugs have the potential for abuse (Chouinard, 2004; Lilja et al., 2001; O'Brien, 2005), worrying some that the use of these drugs might result in a secondary dependence (Wesson and Smith 1985). This concern may be mitigated, however, by using low, ineffective doses as described in these experiments.
Metyrapone was recently studied for its safety in cocaine addicts in a placebo-controlled trial (Winhusen et al, 2005). Twelve subjects who were frequent cocaine users were treated with metyrapone or placebo and either cocaine or saline. While metyrapone did not exacerbate cocaine-related physiological changes such as elevated blood pressure, heart rate or other vital signs, cortisol secretion was reduced under both baseline and cocaine-treated conditions. During metyrapone treatment, ACTH was still slightly elevated over baseline during the cocaine infusions, but was lower than levels observed with cocaine alone. Metyrapone had little effect on the subjective feelings produced by cocaine and the “enjoyment” of the drug. Only mild adverse events were reported. Metyrapone was concluded to be safe to use during cocaine use.
Another investigation reported that the cortisol synthesis inhibitor, ketoconazole, was ineffective in reducing cocaine cravings and use in a randomized, double-blind clinical trial (Kosten et al., 2002). However, the investigators also administered cortisol to the subjects receiving ketoconazole to prevent adrenal insufficiency. This was likely unnecessary and actually counterproductive for several reasons. Since ketoconazole was theoretically tested because of its ability to reduce the synthesis of cortisol, the administration of subsequent exogenous injections of cortisol would defeat the purpose of testing a drug like ketoconazole and would be akin to giving dopamine to a schizophrenic patient treated with an antipsychotic drug to forestall the occurrence of extrapyramidal side effects. The antipsychotic would likely be rendered ineffective, similar to the way ketoconazole was rendered ineffective in the aforementioned study. Secondly, as mentioned above, metyrapone has been shown to be safe to administer to cocaine users (Winhusen et al, 2005). Finally, in a small, chronic 6-week, non placebo-controlled, clinical pilot study, we demonstrated that ketoconazole was also safe and did not produce adrenal insufficiency in cocaine-using subjects (unpublished results). Therefore, cortisol supplementation was likely unnecessary in the study by Kosten et al. Another study (Ward et al., 1998) reported that ketoconazole did not reduce the subjective effects of cocaine in a controlled, clinical laboratory study. However, we have also reported (Mantsch and Goeders, 1999) that ketoconazole did not block cocaine discrimination in a two-lever, food-reinforced study in rats. Thus, we do not propose that cortisol synthesis inhibitors or the combination of metyrapone and oxazepam will necessarily block the subjective effects of cocaine. In this case, an addict would likely just increase the amount of cocaine used to overcome the blockade. Rather, we propose that the combination of metyrapone and oxazepam will reduce the motivation to seek cocaine by blocking the ability of cocaineassociated cues to promote craving and subsequent relapse.
Acknowledgments
The authors would like to thank Nandakumar Dorairaj, Angela Burrows, Dempsey Alford and Courtney Keller for their expert technical assistance.
This work was supported in part by USPHS Grant DA06013 from the National Institutes of Health.
Dr. Goeders is Founder and President of Embera NeuroTherapeutics, Inc., a company that funds clinical trials for the treatment of cocaine and other addictions. Dr. Goeders owns common stock in this company and receives compensation as a consultant. Embera NeuroTherapeutics Inc., has filed a patent on the use of combination pharmacotherapy targeting the HPA axis for the treatment of cocaine and other addictions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrosio E, Sharpe LG, Pilotte NS. Regional binding to corticotropin releasing factor receptors in brain of rats exposed to chronic cocaine and cocaine withdrawal. Synapse. 1997;26:272–6. doi: 10.1002/(SICI)1098-2396(199703)25:3<272::AID-SYN6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ. Drugs and the treatment of psychiatric disorders: Psychosis and anxiety. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 1996. pp. 399–430. [Google Scholar]
- Barrett AC, Negus SS, Mello NK, Caine SB. Effect of GABA agonists and GABA-A receptor modulators on cocaine- and food-maintained responding and cocaine discrimination in rats. J Pharmacol Exp Ther. 2005;315:858–71. doi: 10.1124/jpet.105.086033. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berlin) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Chouinard G. Issues in the clinical use of benzodiazepines: potency, withdrawal, and rebound. J Clin Psychiatry. 2004;65 Suppl 5:7–12. [PubMed] [Google Scholar]
- Cunningham SK, Loughlin T, Bertagna X, Girard F, McKenna TJ. Plasma proopiomelanocortin fragments and adrenal steroids following administration of metyrapone to normal and hirsute women. J Endocrinol Invest. 1988;11:247–53. doi: 10.1007/BF03350147. [DOI] [PubMed] [Google Scholar]
- Engelhardt D, Dörr G, Jaspers C, Knorr D. Ketoconazole blocks cortisol secretion in man by inhibition of adrenal 11β-hydroxylase. Klin Wochenschr. 1985;63:607–12. doi: 10.1007/BF01733014. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432:307–28. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- Friesa E, Hellhammer DH, Hellhammer J. Attenuation of the hypothalamic–pituitary–adrenal axis responsivity to the Trier Social Stress Test by the benzodiazepine alprazolam. Psychoneuroendocrinology. 2006;31:1278–88. doi: 10.1016/j.psyneuen.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Gaillard RC, Al-Damluji S. Stress and the pituitary-adrenal axis. Balliere Clin Endocrinol Metab. 1987;2:319–54. doi: 10.1016/s0950-351x(87)80066-6. [DOI] [PubMed] [Google Scholar]
- Gardi J, Bíró É, Sarnyai Z, Vecsernyés M, Julesz J, Telegdy G. Time-dependent alterations in corticotropin-releasing factor-like immunoreactivity in different brain regions after acute cocaine administration to rats. Neuropeptides. 1997;31:15–8. doi: 10.1016/s0143-4179(97)90013-5. [DOI] [PubMed] [Google Scholar]
- Givens JR, Andersen RN, Ragland JB, Wiser WL, Umstot ES. Adrenal function in hirsutism I. Diurnal change and response of plasma androstenedione, testosterone, 17-hydroxyprogesterone, cortisol, LH and FSH to dexamethasone and 1/2 unit of ACTH. J Clin Endocrinol Metab. 1975;40:988–1000. doi: 10.1210/jcem-40-6-988. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–5. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Goeders NE, McNulty MA, Mirkis S, McAllister KH. Chlordiazepoxide alters intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1988;33:859–66. doi: 10.1016/0091-3057(89)90483-8. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Bienvenu OJ, De Souza EB. Chronic cocaine administration alters corticotropin-releasing factor receptors in the rat brain. Brain Res. 1990a;531:322–8. doi: 10.1016/0006-8993(90)90794-c. [DOI] [PubMed] [Google Scholar]
- Goeders N, Bell V, Guidroz A, McNulty M. Dopaminergic involvement in the cocaine-induced up-regulation of benzodiazepine receptors in the rat striatum. Brain Research. 1990b;515:1–8. doi: 10.1016/0006-8993(90)90569-w. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Cocaine differentially affects benzodiazepine receptors in discrete regions of the rat brain: Persistence and potential mechanisms mediating these effects. J Pharmacol Exp Ther. 1991;259:574–81. [PubMed] [Google Scholar]
- Goeders NE, McNulty MA, Guerin GF. Effects of alprazolam on intravenous cocaine self-administration in rats. Pharmacol Biochem Behav. 1993;44:471–4. doi: 10.1016/0091-3057(93)90493-d. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Intracranial cocaine self-administration into the medial prefrontal cortex increases dopamine turnover in the nucleus accumbens. J Pharmacol Exp Ther. 1993;265:592–600. [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Effects of surgical and pharmacological adrenalectomy on the initiation and maintenance of intravenous cocaine self-administration in rats. Brain Research. 1996;722:145–52. doi: 10.1016/0006-8993(96)00206-5. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Peltier RL, Guerin GF. Ketoconazole reduces low dose cocaine self-administration in rats. Drug Alcohol Depend. 1998;53:67–77. doi: 10.1016/s0376-8716(98)00108-2. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Effects of the CRH receptor antagonist CP-154,526 on intravenous cocaine self-administration in rats. Neuropsychopharmacol. 2000;23:577–86. doi: 10.1016/S0893-133X(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–9. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Clampitt DM. Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacol. 2002;161:222–32. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress, Motivation and Drug Addiction. Current Directions in Psychological Science. 2004;13:33–5. [Google Scholar]
- Goeders NE. Hypothalamic-pituitary-adrenocortical axis and addiction. In: al'Absi M, editor. Stress and Addiction. London: Elsevier Neuroscience; 2007. pp. 21–40. [Google Scholar]
- Grigoriadis DE, Pearsall D, De Souza EB. Effects of chronic antidepressant and benzodiazepine treatment on corticotropin-releasing-factor receptors in rat brain and pituitary. Neuropsychopharmacol. 1989;2:53–60. doi: 10.1016/0893-133x(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Grottoli S, Maccagno B, Ramunni J, Di Vito L, Giordano R, Gianotti L, DeStefanis S, Camanni F, Ghigo E, Arvat E. Alprazolam, a benzodiazepine, does not modify the ACTH and cortisol response to hCRH and AVP, but blunts the cortisol response to ACTH in humans. J Endocrinol Invest. 2002;25:420–5. doi: 10.1007/BF03344031. [DOI] [PubMed] [Google Scholar]
- Guthrie GP, Jr, Wilson EA, Quillen DL, Jawad MJ. Adrenal androgen excess and defective 11 beta-hydroxylation in women with idiopathic hirsutism. Arch Intern Med. 1982;142:729–35. [PubMed] [Google Scholar]
- Haleem DJ, Kennet G, Curzon G. Adaptation of female rats to stress: shift to male pattern by inhibition of corticosterone synthesis. Brain Research. 1988;458:339–47. doi: 10.1016/0006-8993(88)90476-3. [DOI] [PubMed] [Google Scholar]
- Haynes RC., Jr . Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. New York: Pergamon Press; 1990. pp. 1431–62. [Google Scholar]
- Ikemoto S, Goeders NE. Microinjections of dopamine agonists and cocaine elevate plasma corticosterone: dissociation effects among the ventral and dorsal striatum and medial prefrontal cortex. Brain Res. 1998;814:171–8. doi: 10.1016/s0006-8993(98)01070-1. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Salposky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–34. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jenkins JS, Meakin JW, Nelson DH, Thorn GW. Inhibition of adrenal steroid 11-oxygenation in the dog. Science. 1995;128:478–89. doi: 10.1126/science.128.3322.478-a. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Latimer G. Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J Pharmacol Exp Ther. 1987;242:757–63. [PubMed] [Google Scholar]
- Keim KL, Sigg EB. Plasma corticosterone and brain catecholamines in stress: Effect of psychotropic drugs. Pharmacol Biochem Behav. 1977;6:79–85. doi: 10.1016/0091-3057(77)90162-9. [DOI] [PubMed] [Google Scholar]
- Koob GF, Goeders NE. Neuroanatomical substrates of drug self-administration. In: Liebman JM, Cooper SJ, editors. Oxford Reviews in Psychopharmacology, Vol 1, Neuropharmacological Basis of Reward. London: Oxford University Press; 1989. pp. 214–63. [Google Scholar]
- Koob G. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–84. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Oliveto A, Sevarino KA, Gonsai K, Feingold A. Ketoconazole increases cocaine and opioid use in methadone maintained patients. Drug Alcohol Depend. 2002;66:173–80. doi: 10.1016/s0376-8716(01)00198-3. [DOI] [PubMed] [Google Scholar]
- Kostowski W. Recent advances in the GABA-A-benzodiazepine receptor pharmacology. Pol J Pharmacol. 1995;47:237–46. [PubMed] [Google Scholar]
- Lilja J, Larsson S, Skinhoj KT, Hamilton D. Evaluation of programs for the treatment of benzodiazepine dependency. Subst Use Misuse. 2001;36:1213–31. doi: 10.1081/ja-100106224. [DOI] [PubMed] [Google Scholar]
- Lüddens H, Korpi ER. Biological function of GABAA/benzodiazepine receptor heterogeneity. J Psychiatr Res. 1995;29:77–94. doi: 10.1016/0022-3956(94)00040-x. [DOI] [PubMed] [Google Scholar]
- Majewska MD. HPA axis and stimulant dependence: an enigmatic relationship. Psychoneuroendocrinology. 2002;27:5–12. doi: 10.1016/s0306-4530(01)00033-6. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Ketoconazole does not block cocaine discrimination or the cocaine-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:65–73. doi: 10.1016/s0091-3057(99)00090-8. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaineseeking behavior in rats. Psychopharmacology (Berlin) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Greden JF. Effects of psychotropic medications on hypothalamic-pituitary-adrenal regulation. Endocrinol Metab Clin North America. 1988;17:225–34. [PubMed] [Google Scholar]
- Meikle AW, Jubiz W, Hutchings MP, West CD, Tyler FH. A simplified metyrapone test with determination of plasma 11-desoxycortisol (metyrapone test with plasma S) J Clin Endocrinol Metab. 1969;29:985–7. doi: 10.1210/jcem-29-7-985. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Fivel PA. Effects of GABA agonists and GABA-A receptor modulators on cocaine discrimination in rhesus monkeys. Psychopharmacol. 2000;152:398–407. doi: 10.1007/s002130000543. [DOI] [PubMed] [Google Scholar]
- O'Brien CP. Benzodiazepine use, abuse, and dependence. J Clin Psychiatry. 2005;66 Suppl 2:28–33. [PubMed] [Google Scholar]
- Oreland L. The benzodiazepines: a pharmacological overview. Acta Anaesthesiol Scand Suppl. 1988;88:13–6. doi: 10.1111/j.1399-6576.1988.tb02826.x. [DOI] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–25. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rougé-Pont F, Deminiére JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991;567:169–74. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Wagner FP, Hays LR, Glaser PEA. Alprazolam attenuates the behavioral effects of D-amphetamine in humans. J Clin Psychopharm. 2004;24:410–20. doi: 10.1097/01.jcp.0000130553.55630.ad. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Bíró É, Gardi J, Vecsernyés M, Julesz J, Telegdy G. Alterations of corticotropin-releasing factor-like immunoreactivity in different brain regions after acute cocaine administration in rats. Brain Res. 1993;616:315–9. doi: 10.1016/0006-8993(93)90224-b. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–43. [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology. 1998;137:184–9. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Goeders NE. Ketoconazole enhances cocaine-induced dopamine release in the medial prefrontal cortex (Mpfc) in rats. Neurosci Abstr. 2000;26:1822. [Google Scholar]
- Smagin GN, Goeders NE. Effects of acute and chronic ketoconazole administration on hypothalamo-pituitary-adrenal axis activity and brain corticotropin-releasing hormone. Psychoneuroendocrinology. 2004;29:1223–28. doi: 10.1016/j.psyneuen.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Kosten TR. Novel approaches to the treatment of cocaine addiction. CNS Drugs. 2005;19:13–25. doi: 10.2165/00023210-200519010-00002. [DOI] [PubMed] [Google Scholar]
- Sonino N. The use of ketoconazole as an inhibitor of steroid production. New England J Med. 1987;317:812–8. doi: 10.1056/NEJM198709243171307. [DOI] [PubMed] [Google Scholar]
- Spark RF. Simplified assessment of pituitary-adrenal reserve. Measurement of serum 11-deoxycortisol and cortisol after metyrapone. Ann Intern Med. 1971;75:717–23. doi: 10.7326/0003-4819-75-5-717. [DOI] [PubMed] [Google Scholar]
- Thienpont D, Van Cutsem J, Van Gerven F, Heeres J, Janssen PAJ. Ketoconazole -- a new broad spectrum orally active antimycotic. Experientia. 1979;35:606–7. doi: 10.1007/BF01960348. [DOI] [PubMed] [Google Scholar]
- Torpy DJ, Grice JE, Hockings GI, Walters MM, Crosbie GV, Jackson RV. Alprazolam blocks the naloxone-stimulated hypothalamo-pituitary-adrenal axis in man. J Clin Endocrinol Metab. 1993;76:388–91. doi: 10.1210/jcem.76.2.8381800. [DOI] [PubMed] [Google Scholar]
- Uhlenhuth EH, Balter MB, Ban TA, Yang K. International study of expert judgment on therapeutic use of benzodiazepines and other psychotherapeutic medications: II. Pharmacotherapy of anxiety disorders. J Affect Disord. 1995;35:153–62. doi: 10.1016/0165-0327(95)00064-x. [DOI] [PubMed] [Google Scholar]
- Van Vugt DA, Piercy J, Farley AE, Reid RL, Rivest S. Luteinizing hormone secretion and corticotropin-releasing factor gene expression in the paraventricular nucleus of rhesus monkeys following cortisol synthesis inhibition. Endocrinology. 1997;138:2249–58. doi: 10.1210/endo.138.6.5171. [DOI] [PubMed] [Google Scholar]
- Ward AS, Collins ED, Haney M, Foltin RW, Fischman MW. Ketoconazole attenuates the cortisol response but not the subjective effects of smoked cocaine in humans. Behav Pharmacol. 1998;9:577–86. doi: 10.1097/00008877-199811000-00013. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Froestl W, Griffiths RR. Effects of GABAergic modulators on food and cocaine self-administration in baboons. Drug Alcohol Depend. 2005;80:369–76. doi: 10.1016/j.drugalcdep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Smith DE. Cocaine: Treatment perspectives. In: Kozel NJ, Adams NJ, editors. Cocaine Use in America: Epidemiologic and Clinical Perspectives. Washington, DC: National Institute on Drug Abuse Research Monograph 61., DHHS Pub. No. (ADM)85-1414. Supt of Docs, US Govt Printing Office; 1985. pp. 193–202. [Google Scholar]
- Winhusen T, Somoza E. The HPA axis in cocaine use: implications for pharmacotherapy. J Addict Dis. 2001;20:105–19. doi: 10.1300/J069v20n03_09. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Somoza E, Harrer JM, Moore E, Ussery T, Kropp F, Singal B, Elkashef A, Mojsiak J. Metyrapone and cocaine: A double-blind, placebo-controlled drug interaction study. Pharmacol Biochem Behav. 2005;80:631–8. doi: 10.1016/j.pbb.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: Neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–50. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. Corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during “binge”-pattern cocaine administration and withdrawal. J Pharmacol Exp Ther. 1996;279:351–8. [PubMed] [Google Scholar]