Abstract
Recent studies have established that autotaxin (ATX), also known as phosphodiesterase Iα/autotaxin (PD-Iα/ATX) or (ecto)nucleotide pyrophosphatase/phosphodiesterase 2 [(E)NPP2], represents a multi-functional and multi-modular protein. ATX was initially thought to function exclusively as a phosphodiesterase/pyrophosphatase. However, it has become apparent that this enzymatically active site, which is ultimately responsible for ATX’s originally discovered property of tumor cell motility stimulation, mediates the conversion of lysophosphatidylcholine (LPC) to lysophosphatidic acid (LPA). In addition, a separate functionally active domain, here referred to as the Modulator of Oligodendrocyte Remodeling and Focal adhesion Organization (MORFO) domain, was discovered in studies analyzing the role of ATX during the differentiation of myelinating cells of the central nervous system (CNS), namely oligodendrocytes. This novel domain was found to mediate anti-adhesive, i.e. matricellular, properties and to promote morphological maturation of oligodendrocytes. In this review, we summarize our current understanding of ATX’s structure-function domains and discuss their contribution to the presently known main functional roles of ATX.
Keywords: autotaxin, lysoPLD, phosphodiesterase-Iα, matricellular, tumor progression, vascular development, oligodendrocyte development
1. Introduction
Autotaxin (ATX), also designated phosphodiesterase Ia/autotaxin (PD-Iα/ATX) or (ecto)nucleotide pyrophosphatase/phosphodiesterase 2 [(E)NPP2], was originally discovered as an autocrine motility stimulating factor released by human melanoma cells [1]. This functional property was the foundation for its name autotaxin, which remains the most commonly used designation. cDNA cloning, identified a brain-specific alternatively spliced isoform that based on the protein’s enzymatic phosphodiesterase activity was designated phosphodiesterase Iα (PD-Iα) [2, 3]. Possessing enzymatic activity, ATX was subsequently included in the family of nucleotide pyrophosphatase/phosphodiesterase (NPP)-type ectophosphodiesterases and additionally termed (E)NPP2 [4–6]. To date, at least three splice variants/isoforms of ATX have been identified in both human and mouse, and they have been recently referred to as autotaxin α, β and γ, with autotaxin γ being identical to PD-Iα [7–10]. The known isoforms of ATX display characteristic expression patterns but do not appear to exert major differences in the substrate specificity of the enzymatically active site, and to our knowledge, no major functional isoform differences have been identified so far [8, 10, 11]. In this review, we will refer to all isoforms collectively as ATX.
ATX has been found to be released by a large variety of tumor cells and to be expressed within a number of different tissues during normal development and in the adult [2, 3, 8, 10–14]. Furthermore, high protein levels of ATX have been observed in biological fluids, i.e. plasma and cerebrospinal fluid (CSF) [15–17]. Despite this rather broad expression pattern, a major focus in the research related to ATX has long remained in tumor cell biology. More recently, ATX has gained new attention since it was discovered to act as the major extracellular enzyme generating the bioactive lipid signaling molecule LPA, i.e. as an extracellular lysophopholipaseD (lysoPLD) [18, 19]. In addition, current studies investigating ATX’s role during CNS development uncovered a novel functionally active site and thus highlighted a multi-functional and multi-modular nature of ATX [20–23]. We, therefore, consider it timely to provide an updated overview over the distinct properties that are known to be mediated by ATX and its functionally active sites.
2. ATX: A Historical Perspective
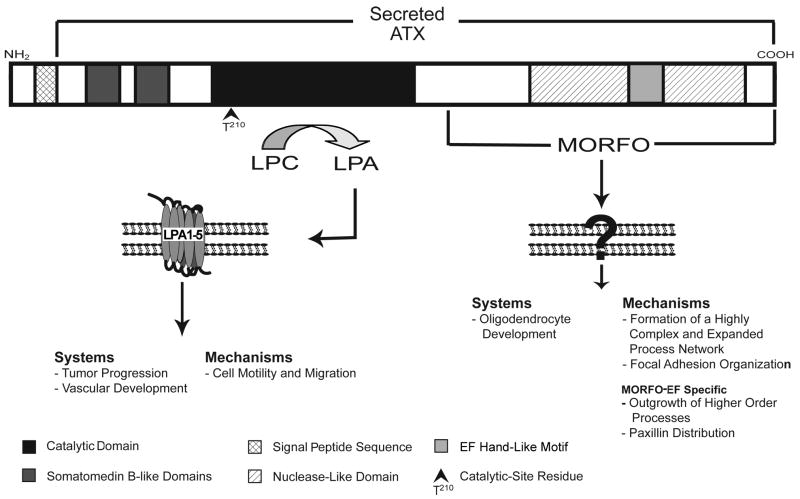
The first mentioning of ATX in the literature dates to the early 1990s, when it was discovered as part of a search for active cellular motility stimulating factors involved in tumor cell invasion [1, 24]. Isolation of a cDNA encoding this 125 kDa glycoprotein and subsequent sequencing revealed that ATX did not exhibit any significant homologies to previously characterized motility and growth factors. However, ATX was found to contain a domain that displayed high homology with the enzymatically active domain of proteins known to possess nucleotide phosphodiesterase and pyrophosphatase activity, in particular bovine intestinal 5′ nucleotide phosphodiesterase and plasma cell glycoprotein-1 (PC-1) [7, 25–27]. Furthermore, it was found that the homology between ATX and PC-1 was not restricted to the enzymatically active site but extended through the entire length of their extracellular portions. Thus, both proteins shared additional properties and domains, namely two adjacent somatomedin B-like domains, a nuclease-like domain and a single EF hand-like motif (see Fig. 1). Due to the homology to PC-1, ATX’s N-terminus was originally thought to represent a transmembrane region thus rendering ATX a type II transmembrane protein. However, it is now clear that ATX is synthesized as a pre-pro-enzyme and secreted upon removal of the N-terminal signal peptide and further trimming by a furine-type protease [28–31]. In the years following ATX’s first cDNA isolation and sequencing, proteins with similar structure-function domains were identified, leading to the creation of a novel protein family, namely the (E)NPP family, which currently consists of seven known members [4–6, 32, 33].
Fig. 1.
Scheme of the structure-function domains of ATX. The N-terminal hydrophobic sequence of ATX is a signal peptide, thus resulting in the secretion of the protein. Two somatomedin B-like domains are located at the N-terminal end of the protein. So far, no functional properties have been assigned to these domains. The catalytic domain of ATX functions as lysoPLD thus generating LPA, which exerts its effects through binding to G-protein-coupled receptors (LPA1–5). Catalytic activity is dependent on the catalytic site residue T210. At the C-terminal end, the Modulator of Oligodendrocyte Remodeling and Focal adhesion Organization (MORFO) domain entails the nuclease-like domain, which is probably enzymatically inactive. The functional properties of the MORFO domain are thought to be mediated via binding to a yet-to-be-identified cell surface receptor. The EF-hand like motif located at the far C-terminal end of the protein was found to contribute to the function of the MORFO domain. The most intensively characterized biologically systems (Systems) and cellular molecular mechanisms (Mechanisms) for each of the functionally active sites of ATX are listed. Individual structure-function domains are depicted according to the gray scale scheme shown at the bottom.
With a detailed structure-function characterization and a known functional property in hand, it was a logical step to ask which of the structural domains may be responsible for ATX’s known functional role. Using mutant recombinant forms of ATX, Lee et al. [34] identified, shortly after ATX’s sequence had been identified, that it is the catalytic domain that is crucial for motility stimulation. This finding naturally prompted a more detailed characterization of the catalytic activity of ATX and its relationship to the catalytic activities of the other (E)NPP family members. In the early studies, all (E)NPPs were described to hydrolyze pyrophosphate or phosphodiester bonds in (di)nucleotides and their derivatives [4]. However, their actual physiological substrates still remained largely unidentified. What has increasingly becoming clear though is that even though (E)NPPs have a structurally related catalytic domain, they largely differ in their enzymatic substrate specificities [6, 35, 36].
With regard to ATX, an important clue for its most likely physiological substrate came from research related to the lipid signaling molecule LPA. The discovery of an extracellular enzyme catalyzing the conversion from LPC to LPA, i.e. an extracellular lysoPLD, revealed somewhat surprisingly that ATX is molecularly identical to this extracellular lysoPLD [15, 16, 37, 38]. Moreover, ATX’s affinity for LPC was found to be significantly higher than its affinity for nucleotides, thus suggesting a preference for the enzymatic conversion of LPC over the conversion of nucleotides [15, 16]. This finding that ATX may exert various biological effects through its lysoPLD activity clearly marked a turning point in research related to ATX [39].
3. ATX: Enzymatic activity via the catalytic lysoPLD active site
Once the molecular identity of ATX with lysoPLD had been identified, the characterization of ATX’s enzymatic activities was revisited. ATX’s NPP activity had long been known to be dependent on a single threonine residue located within the catalytic domain [40]. Mutational analyses showed that the same residue is critical for ATX’s lysoPLD activity and that lysoPLD activity shares a common reaction mechanism with the original described 5′-nucleotide phosphodiesterase activity [41, 42]. However, the lysoPLD activity appears unique to ATX within the (E)NPP protein family, and it seems to require in addition to the critical threonine residue, sequences within the N- and C-terminal domains of ATX [6, 36, 41]. Recently, it was proposed that the substrate specificity of ATX‘s lysoPLD activity extends to the phosphosphingolipid sphingosine phosphorylcholine (SPC), thus suggesting that ATX not only generates LPA but also sphingosine 1-phosphate (S1P) [43]. While this ability of ATX has been well demonstrated in vitro, its physiological relevance still needs to be demonstrated [44]. Plasma ATX is constitutively active and LPC is abundant in plasma and serum [45]. However, LPA levels are known to be low, suggesting that ATX’s enzymatic activity is negatively regulated in vivo [46–48]. Indeed ATX’s lysoPLD products, LPA and S1P, were shown to inhibit enzymatic activity at biologically relevant concentrations [28]. On the other hand, positive regulatory mechanisms likely also exist since serum LPA levels gradually increase following platelet activation [44].
Physiological actions of LPA are mediated through the binding and activation of specific plasma membrane receptors belonging to the superfamily of G protein-coupled receptors (GPCRs) [49–55]. To date, there are five known LPA receptors. The three originally identified receptors belong to the endothelial differentiation gene (Edg) family: LPA1/Edg-2/vzg-1, LPA2/Edg-4, and LPA3/Edg-7. More recently identified receptors are only distantly related to the Edg family of receptors (20–24% sequence homology) and are more closely related to the purinergic family of receptors: GPR23/P2Y9 (LPA4) and GPR92 (LPA5) [56–58]. Further complexity to the mechanism of LPA signaling is contributed by the discovery that PPARγ can function as intracellular LPA receptor [59].
Before discussing the most prominent biological processes that ATX has been found involved in, we would like to emphasize that biochemical pathways other than the conversion of LPC via lysoPLD activity have been identified to lead to the generation of LPA [38, 60, 61]. Thus, biological actions of LPA are not restricted to those mentioned below and involving ATX.
ATX’s enzymatic lysoPLD activity has been implicated in a large variety of biological processes during normal development and under pathological conditions [5]. Developmental roles include adipogenesis, intestinal cell motility and neurite morphology [17, 62, 63], while a contribution to disease has been described for Alzheimer’s disease, chronic Hepatitis C, Multiple Sclerosis, neuropathic pain, obesity and rheumatoid arthritis [37, 64–72]. The functional importance of ATX’s lysoPLD active site has, however, been investigated most intensively during tumor progression and vascular development. We will thus discuss these two areas in more detail.
Since its first description as a tumor cell motility stimulating factor for human melanoma cells, ATX has been found expressed by a large variety of tumor cells, including breast cancer, glioblastoma, hepatocellular carcinoma, neuroblastoma, non-small-cell lung cancer, prostate cancer, renal cell carcinoma and thyroid carcinoma [9, 73–80]. Active tumor cell motility is involved in many stages of the metastatic cascade and it is thought that over expression of motility factors, such as ATX, is required for tumor cell penetration [24]. Thus, the expression of ATX was rapidly emerging as a factor involved in tumor progression. Furthermore, the observation that over expression of ATX in ras-transformed NIH3T3 cells stimulated tumorigenesis and metastatic potential promoted the idea that increased motility (stimulated by ATX’s enzymatic activity) may also support tumor invasiveness and aggressiveness [81]. Indeed, metastatic capability and tumor aggressiveness has been correlated with high ATX expression for at least some types of tumors [9, 75, 82]. It is worthwhile noting here that over or ectopic expression of ATX alone does not appear to be sufficient to induce cell transformation [81, 83].
ATX was found to stimulate both random and directed motility in human melanoma cells, and ATX-dependent cell motility has since been demonstrated for many other tumor cell types [1, 9, 75, 79]. In the search for the molecular mechanism responsible for the tumor cell motility stimulating effect of ATX, a link with its enzymatic activity and the involvement of G-protein-coupled receptors was uncovered early on [1, 34, 84]. However, it took the identification of ATX as a lysoPLD to unravel that the above effects are mediated, at least in part, via the conversion of LPC to LPA and via binding of LPA to one of its high affinity receptors [15, 16, 42, 44, 79, 85, 86]. The above described involvement of ATX and its lysoPLD active site in tumor progression is further supported by the following findings: 1) The substrate LPC is released in significant amounts by several tumor cell lines [16], 2) ATX expression is up-regulated by the v-Jun oncogene [83] and 3) ATX expression is down-regulated by the candidate tumor suppressor CST6 [87].
Studies investigating ATX’s role in tumorigenesis indicated as an additional functional role of ATX a pro-angiogenic effect [81]. In support of such a function, a chromosomal region including the ATX gene had been associated with angiogenic responsiveness to FGF2 [88]. However, an essential role of ATX for vascular development was only revealed through the generation of ATX null mice [18, 19]. ATX-deficient mice were found to die at midgestation (E9.5–E11.5) and to harbor severe vascular defects that were in particular noticeable in the yolk sac. Further analysis of this phenotype showed that endothelial, smooth muscle and blood cell differentiation was not significantly impaired. Thus, it appears that during development initial blood vessel formation occurs properly in the absence of ATX. However, newly formed blood vessels fail to develop into mature vessels. Using an allantois explant culture system, it could be demonstrated that it is ATX’s lysoPLD active site that under normal conditions prevents disassembly of newly formed vessels [18]. Further support for the concept that the role of ATX for vascular development may be solely dependent on its lysoPLD active site came from recent transgenic studies. Notably, in a mouse line expressing only a lysoPLD inactivated ATX gene product embryonic lethality was observed to be similar to the one seen in the complete absence of ATX [89].
4. ATX: Matricellular properties via the MORFO domain
Studies in our laboratory characterized ATX, which we referred to as PD-Iα/ATX, as an extracellular factor involved in the differentiation oligodendrocytes [20–23]. Most interestingly, these studies revealed a novel functionally active domain, the MORFO domain, that 1) is located at the C-terminal end of the protein, 2) entails the nuclease-like domain not thought to be catalytically active and 3) functions independently of ATX’s enzymatic activity (see Fig. 1 and [6, 20–23, 35]). Early studies uncovered that the MORFO domain antagonizes adhesion of oligodendrocytes to naturally occurring extracellular matrix (ECM) molecules such as fibronectin in an active and pertussis toxin-sensitive fashion [23]. This finding classifies ATX as a matricellular protein, i.e. a protein that mediates an intermediate adhesive state, and it suggests a supportive role of ATX, via its MORFO domain, on cellular remodeling [90].
In agreement with the above, recent data have demonstrated that ATX’s MORFO domain facilitates morphological maturation of post-migratory, premyelinating oligodendrocytes [20]. During development, oligodendrocytes undergo discrete steps of maturation that are characterized by distinct morphological features and gene expression profiles [91, 92]. In particular, post-migratory, premyelinating oligodendrocytes mature from cells that extend a few processes to cells that generate a highly complex process network, and it is this transition that is facilitated by ATX’s MORFO domain [20]. The role of ATX in process outgrowth was found to be mediated in part via the EF hand-like motif located at the far C-terminal end of the MORFO domain. It is thought that the MORFO-EF site, in contrast to classical EF hand motifs, exerts its function via interaction with a cell surface receptor and not solely by binding to divalent cations [20, 93]. The fact that the MORFO-EF site was found to only partially mimic the effect of ATX’s MORFO domain on process outgrowth raises the possibility that an additional functionally active site may be located within the MORFO domain [20]. Interestingly, ATX’s enzymatic activity and ability to stimulate tumor cell motility apparently does not require the EF hand-like motif [93].
In an attempt to characterize the molecular basis for the effects of ATX’s MORFO domain on process outgrowth, this domain was found to mediate a reorganized assembly of focal adhesions [20, 22]. In particular, the MORFO domain was found to stimulate dephosphorylation at the tyrosine 925 residue of focal adhesion kinase and to promote redistribution of paxillin and a-actinin away from focal adhesion complexes. The effect on paxillin distribution can be fully mimicked by a peptide representing the EF hand loop region, suggesting that the EF hand-like motif is sufficient and necessary for this effect of ATX’s MORFO domain [20].
Focal adhesions are multi-molecular signaling complexes that establish a link between the actin cytoskeleton and the ECM [94, 95]. For controlled cellular remodeling, focal adhesions have to undergo constant high rate disassembly/turnover, which leads to a reduction in the efficiency of the ECM-cytoskeletal linkage [96]. Thus, it is tempting to speculate that ATX, via its MORFO domain and in part via the EF hand-like motif, regulates focal adhesion dynamics, thereby enabling the establishment of a complex and expanded process network by post-migratory, premyelinating oligodendrocytes.
5. Future Perspectives
Since its discovery as a tumor cell motility stimulating factor, ATX has been implicated in a variety of biological processes during normal development and under pathological conditions. Importantly, ATX has been found to represent a multi-functional and multi-modular protein exerting distinct functional effects via at least two different functionally active sites, namely the lysoPLD active site and the MORFO domain.
Clearly, our understanding of ATX’s functional roles has significantly advanced. However, many questions remain. So far, the functions of the lysoPLD active site and the MORFO domain have been investigated in different cell types. Data from our laboratory suggest that the known MORFO domain effects are not ubiquitous but restricted to receptive cell types [23]. No extensive survey of MORFO responsive/unresponsive cell types has been undertaken. Thus, MORFO effects are currently only characterized for maturing oligodendrocytes [23]. Interestingly, recent studies indicate that ATX may exert a dis-adhesive effect toward glioma cells [86]. However, it remains to be elucidated if there are other ATX-MORFO responsive cell types and how similar the effects of ATX’s MORFO domain are for these different cell types.
Maturing oligodendrocytes are also known to express LPA receptors, and LPA has been shown to exert signaling effects in these cells [97–100]. The above raises the question whether ATX’s lysoPLD active site may be involved in the regulation of oligodendrocyte maturation and if so, in what relation to the MORFO domain it may act. In the case of the severe vascular phenotype seen in ATX null mice, ATX’s lysoPLD active site may be solely responsible [89]. However, future studies will be necessary to dissect the exact roles of the different functionally active sites of ATX and their contribution to ATX’s biological effects.
Acknowledgments
The authors would like to thank Christopher Waggener for assistance in preparing the figure. This work was supported by grants from the National Institute of Health (B.F) and the National Multiple Sclerosis Society (B.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stracke ML, Kruttzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, Liotta LA. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524–2529. [PubMed] [Google Scholar]
- 2.Narita M, Goji J, Nakamura H, Sano K. Molecular Cloning, Expression, and Localization of a Brain-specific Phosphodiesterae I/Nucleotide Pyrophosphatase (PD-Ia) from Rat Brain. J Biol Chem. 1994;269:28235–28242. [PubMed] [Google Scholar]
- 3.Kawagoe H, Soma O, Goji J, Nishimura N, Narita M, Inazawa J, Nakamura H, Sano K. Molecular cloning and chromosomal assignment of the human brain-type phosphodiesterase I/nucleotide pyrophosphatase gene (PDNP2) Genomics. 1995;30:380–384. doi: 10.1006/geno.1995.0036. [DOI] [PubMed] [Google Scholar]
- 4.Bollen M, Gijsbers R, Caulemans H, Stalmans W, Stefan C. Nucleotide pyrophosphatases/phosphodiesterases on the move. Crit Rev Biochem Mol Biol. 2000;35:393–432. doi: 10.1080/10409230091169249. [DOI] [PubMed] [Google Scholar]
- 5.Goding JW, Grobben B, Slegers H. Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim Biophys Acta. 2003;1638:1–19. doi: 10.1016/s0925-4439(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 6.Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci. 2005;30:542–550. doi: 10.1016/j.tibs.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Murata J, Lee HW, Clair T, Krutzch HC, Arestad AA, Sobel ME, Liotta LA, Stracke ML. cDNA cloning of the human tumor motility-stimulating protein, autotaxin, reveals a homology with phosphodiesterases. J Biol Chem. 1994;269:30479–30484. [PubMed] [Google Scholar]
- 8.Lee HW, Murata J, Clair T, Polmeropoulos M, Torres R, Manrow R, Liotta LA, Stracke ML. Cloning, chromosomal localization and tissue expression of autotaxin from human tetracarcinoma cells. Biochem Biophys Res Commun. 1996;218:714–719. doi: 10.1006/bbrc.1996.0127. [DOI] [PubMed] [Google Scholar]
- 9.Kawagoe H, Stracke ML, Nakamura H, Sano K. Expression and transcriptional regulation of the PD-Ialpha/autotaxin gene in neuroblastoma. Cancer Res. 1997;57:2516–2521. [PubMed] [Google Scholar]
- 10.Giganti A, Rodriguez M, Fould B, Moulharat N, Cogé F, Chomarat P, Galizzi JP, Valet P, Saulnier-Blache JS, Boutin JA, Ferry G. Murine and human autotaxin alpha, beta, gamma isoforms: Gene organization, tissue distribution and biochemical characterization. J Biol Chem. 2008 doi: 10.1074/jbc.M708705200. [DOI] [PubMed] [Google Scholar]
- 11.Fuss B, Baba H, Phan T, Tuohy VK, Macklin WB. Phosphodiesterase I, A Novel Adhesion Molecule and/or Cytokine Involved in Oligodendrocyte Function. J Neurosci. 1997;17:9095–9203. doi: 10.1523/JNEUROSCI.17-23-09095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachner D, Ahrens M, Betat N, Schroder D, Gross G. Developmental expression analysis of murine autotaxin (ATX) Mech Dev. 1999;84:121–125. doi: 10.1016/s0925-4773(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 13.Ohuchi H, Hayashibara Y, Matsuda H, Onoi M, Mitsumori M, Tanaka M, Aoki J, Arai H, Noji S. Diversified expression patterns of autotaxin, a gene for phospholipid-generating enzyme during mouse and chicken development. Dev Dyn. 2007;236:1134–1143. doi: 10.1002/dvdy.21119. [DOI] [PubMed] [Google Scholar]
- 14.Savaskan SE, Rocha L, Kotter MR, Baer A, Lubec G, van Meeteren LA, Kishi Y, Aoki J, Moolenaar WH, Nitsch R, Bräuer AU. Autotaxin (NPP-2) in the brain: cell type-specific expression and regulation during development and after neurotrauma. Cell Mol Life Sci. 2006;64:230–243. doi: 10.1007/s00018-006-6412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tokumura A. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- 16.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H. Autotaxin has lysophospholipase activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato K, Malchinkhuu E, Muraki T, Ishikawa K, Hayashi K, Tosaka M, Mochiduki A, Inoue K, Tomura H, Mogi C, Nochi H, Tamoto K, Okajima F. Identification of autotaxin as a neurite retraction-inducing factor of PC12 cells in cerebrospinal fluid and its possible sources. J Neurochem. 2005;92:904–914. doi: 10.1111/j.1471-4159.2004.02933.x. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, Noji S, Yatomi Y, Aoki J, Arai H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822–25830. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 19.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, Rooijen MAv, Pradère JP, Pettit TR, Wakelam MJO, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. Autotaxin, a Secreted Lysophospholipase D, Is Essential for Blood Vessel Formation during Development. Mol Cell Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis J, White MA, Forrest AD, Yuelling LM, Nogaroli L, Afshari FS, Fox MA, Fuss B. Phosphodiesterase-Ialpha/autotaxin’s MORFO domain regulates oligodendroglial process network formation and focal adhesion organization. Mol Cell Neurosci. 2008;37:412–424. doi: 10.1016/j.mcn.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennis J, Nogaroli L, Fuss B. Phosphodiesterase-Ialpha/autotaxin (PD-Ialpha/ATX): a multifunctional protein involved in central nervous system development and disease. J Neurosci Res. 2005;82:737–742. doi: 10.1002/jnr.20686. [DOI] [PubMed] [Google Scholar]
- 22.Fox MA, Alexander JK, Afshari FS, Colello RJ, Fuss B. Phosphodiesterase-Ia/autotaxin controls cytoskeletal organization and FAK phosphorylation during myelination. Mol Cell Neurosci. 2004;27:140–150. doi: 10.1016/j.mcn.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Fox MA, Colello RJ, Macklin WB, Fuss B. Phosphodiesterase-Ia/autotaxin: a counteradhesive protein expressed by oligodendrocytes during onset of myelination. Mol Cell Neurosci. 2003;23:507–519. doi: 10.1016/s1044-7431(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 24.Stracke M, Liotta LA, Schiffmann E. The role of autotaxin and other motility stimulating factors in the regulation of tumor cell motility. Symp Soc Exp Biol. 1993;47:197–214. [PubMed] [Google Scholar]
- 25.Culp JS, Blytt HJ, Hermodson M, Butler LG. Amino acid sequence of the active site peptide of bovine intestinal 5′-nucleotide phosphodiesterase and identification of the active site residue as threonine. J Biol Chem. 1985;260:8320–8324. [PubMed] [Google Scholar]
- 26.van Driel IR, Goding JW. Plasma cell membrane glycoprotein PC-1. Primary structure deduced from cDNA clones. J Biol Chem. 1987;262:4882–4887. [PubMed] [Google Scholar]
- 27.Buckley MF, Loveland KA, McKinstry WJ, Garson OM, Goding JW. Plasma cell membrane glycoprotein PC-1. cDNA cloning of the human molecule, amino acid sequence, and chromosomal location. J Biol Chem. 1990;265:17506–17511. [PubMed] [Google Scholar]
- 28.van Meeteren LA, Ruurs P, Christodoulou E, Goding JW, Takakusa H, Kikuchi K, Perrakis A, Nagano T, Moolenaar WH. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J Biol Chem. 2005;280:21155–21161. doi: 10.1074/jbc.M413183200. [DOI] [PubMed] [Google Scholar]
- 29.Jansen S, Stefan C, Creemers J, Waelkens E, Van Eynde A, Stalmans W, Bollen M. Proteolytic maturation and activation of autotaxin (NPP2), a secreted metastasis-enhancing lysophopsholipase D. J Cell Sci. 2005;118:3081–3089. doi: 10.1242/jcs.02438. [DOI] [PubMed] [Google Scholar]
- 30.Koike S, Keino-Masu K, Ohto T, Masu M. The N-terminal hydrophobic sequence of autotaxin (ENPP2) functions as a signal peptide. Genes Cells. 2006;11:133–142. doi: 10.1111/j.1365-2443.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 31.Pradere JP, Tarnus E, Gres S, Valet P, Saulnier-Blache JS. Secretion and lysophospholipase D activity of autotaxin by adipocytes are controlled by N-glycosylation and signal peptidase. Biochim Biophys Acta. 2007;1771:93–102. doi: 10.1016/j.bbalip.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Goding JW, Terkeltaub R, Maurice M, Deterre P, Sali A, Belli SI. Ecto-phosphodiesterase/pyrophosphatase of lymphocytes and non-lymphoid cells: structure and function of the PC-1 family. Immunol Rev. 1998;161:11–26. doi: 10.1111/j.1600-065x.1998.tb01568.x. [DOI] [PubMed] [Google Scholar]
- 33.Zimmermann H. Two novel families of ectonucleotidases: molecular structures, catalytic properties and a search for function. Trends Pharmacol Sci. 1999;20:231–236. doi: 10.1016/s0165-6147(99)01293-6. [DOI] [PubMed] [Google Scholar]
- 34.Lee HY, Clair T, Mulvaney PT, Woodhouse EC, Aznavoorian S, Liotta LA, Stracke ML. Stimulation of tumor cell motility linked to phosphodiesterase catalytic site of autotaxin. J Biol Chem. 1996;271:24408–24412. doi: 10.1074/jbc.271.40.24408. [DOI] [PubMed] [Google Scholar]
- 35.Gijsbers R, Ceulemans H, Stalmans W, Bollen M. Structural and catalytic similarities between nucleotide pyrophosphatases/phosphodiesterases and alkaline phosphatases. J Biol Chem. 2001;276:1361–1368. doi: 10.1074/jbc.M007552200. [DOI] [PubMed] [Google Scholar]
- 36.Cimpean A, Stefan C, Gijsbers R, Stalmans W, Bollen M. Substrate-specifying determinants of the nucleotide pyrophosphatases/phosphodiesterases NPP1 and NPP2. Biochem J. 2004;381:71–77. doi: 10.1042/BJ20040465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferry G, Tellier E, Try A, Grés S, Naime I, Simon MF, Rodriguez M, Boucher J, Tack I, Gesta S, Chomarat P, Dieu M, Raes M, Galizzi JP, Valet P, Boutin JA, Saulnier-Blache JS. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J Biol Chem. 2003;278:18162–18169. doi: 10.1074/jbc.M301158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie Y, Meier KE. Lysophospholipase D and its role in LPA production. Cell Signal. 2004;16:975–981. doi: 10.1016/j.cellsig.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Moolenaar WH. Lysophospholipids in the limelight: autotaxin takes center stage. J Cell Biol. 2002;158:197–199. doi: 10.1083/jcb.200206094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clair T, Lee HW, Liotta LA, Stracke ML. Autotaxin is an exoenzyme possessing 5′-nucleotide phosphodiesterase/ATP pyrophosphatase and ATPase activities. J Biol Chem. 1997;272:996–1001. doi: 10.1074/jbc.272.2.996. [DOI] [PubMed] [Google Scholar]
- 41.Gijsbers R, Aoki J, Arai H, Bollen M. The hydrolysis of lysophospholipids and nucleotides by autotaxin (NPP2) involves a single catalytic site. FEBS. 2003;538 doi: 10.1016/s0014-5793(03)00133-9. [DOI] [PubMed] [Google Scholar]
- 42.Koh E, Clair T, Woodhouse EC, Schiffmann E, Liotta L, Stracke M. Site-directed Mutations in the Tumor-associated Cytokine, Autotaxin, Eliminate Nucleotide Phosphodiesterase, Lysophospholipase D, and Motogenic Activities. Cancer Res. 2003;63:2042–2045. [PubMed] [Google Scholar]
- 43.Clair T, Aoki J, Koh E, Bandle RW, Nam SW, Ptaszynska MM, Mills GB, Schiffmann E, Liotta LA, Stracke ML. Autotaxin hydrolyzes sphingosylphosphorylcholine to produce the regulator of migration, sphingosine-1-phosphate. Cancer Res. 2003;63:5446–5453. [PubMed] [Google Scholar]
- 44.van Meeteren LA, Moolenaar WH. Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res. 2007;46:145–160. doi: 10.1016/j.plipres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Croset M, Bossard N, Polette A, Lagarde M. Characterization of plasma unsaturated lysophospatidlycholines in human and rat. Biochem J. 2000;345:61–67. [PMC free article] [PubMed] [Google Scholar]
- 46.Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291:677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker DL, Morrison P, Miller B, Riely CA, Tolley B, Westermann AM, Bonfrer JM, Bais E, Moolenaar WH, Tigyi G. Plasma lysophosphatidic acid concentration and ovarian cancer. JAMA. 2002;287:3081–3082. doi: 10.1001/jama.287.23.3081. [DOI] [PubMed] [Google Scholar]
- 48.Sano T, Baker DL, Virag T, Wada A, Yatomi Y, Kobayashi T, Igarashi Y, Tigyi GJ. Multiple mechanisms linked to platelet activation result in lysophospahtidic acid and sphingosine-1-phosphate generation in blood. J Biol Chem. 2002;277:21197–21206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- 49.Moolenaar WH. Lysophosphatidic acid signaling. Curr Opin Cell Biol. 1995;7:203–210. doi: 10.1016/0955-0674(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 50.Moolenaar WH. Bioactive lysophospholipis and their G protein-coupled receptors. Exp Cell Res. 1999;253:230–238. doi: 10.1006/excr.1999.4702. [DOI] [PubMed] [Google Scholar]
- 51.Chun J, Goetzi EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 52.Takuwa Y, Takuwa N, Sugimoto N. The Edg family G protein-coupled receptors for lysophospholipids: their signaling properties and biological activities. J Biochem. 2002;131:767–771. doi: 10.1093/oxfordjournals.jbchem.a003163. [DOI] [PubMed] [Google Scholar]
- 53.Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol. 2004;15:457–465. doi: 10.1016/j.semcdb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Fukushima N. LPA in neural cell development. J Cell Biochem. 2004;92:993–1003. doi: 10.1002/jcb.20093. [DOI] [PubMed] [Google Scholar]
- 55.Valentine WJ, Fujiwara Y, Tsukahara R, Tigyi G. Lysophospholipid signaling: Beyond the EDGs. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbagen.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 57.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- 58.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 59.McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, Hinshaw JC, Zimmerman GA, Hama K, Aoki J, Arai H, Prestwich GD. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci U S A. 2003;100:131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pagès C, Simon MF, Valet P, Saulnier-Blache JS. Lysophosphatidic acid synthesis and release. Prostaglandins. 2001;64:1–20. doi: 10.1016/s0090-6980(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 61.Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, Mizuno K, Sakull K, Taguchi R, Arai H. Serum Lysophosphatidic Acid Is Produced through Diverse Phospholipase Pathways. J Biol Chem. 2002;277:48737–48744. doi: 10.1074/jbc.M206812200. [DOI] [PubMed] [Google Scholar]
- 62.Simon MF, Daviaud D, Pradere JP, Gres S, Guigne C, Wabitsch M, Chun J, Valet P, Saulnier-Blache JS. Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor gamma2. J Biol Chem. 2005;280:14656–14662. doi: 10.1074/jbc.M412585200. [DOI] [PubMed] [Google Scholar]
- 63.Khurana S, Tomar A, George SP, Wang Y, Siddiqui MR, Guo H, Tigyi G, Mathew S. Autotaxin and lysophosphatidic acid stimulate intestinal cell motility by redistribution of the actin modifying protein villin to the developing lamellipodia. Exp Cell Res. 2008;314:530–542. doi: 10.1016/j.yexcr.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao C, Fernandes MJ, Prestwich GD, Turgeon M, Di Battista J, Clair T, Poubelle PE, Bourgoin SG. Regulation of lysophosphatidic Acid receptor expression and function in human synoviocytes: implications for rheumatoid arthritis? Mol Pharmacol. 2008;73:587–600. doi: 10.1124/mol.107.038216. [DOI] [PubMed] [Google Scholar]
- 65.Inoue M, Xie W, Matsushita Y, Chun J, Aoki J, Ueda H. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neurosci. 2008 doi: 10.1016/j.neuroscience.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 66.Inoue M, Ma L, Aoki J, Chun J, Ueda H. Autotaxin, a synthetic enzyme of lysophosphatidic acid (LPA), mediates the induction of nerve-injured neuropathic pain. Mol Pain. 2008;4 doi: 10.1186/1744-8069-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Aoki J, Hama K, Okudaira S, Tanaka M, Tomiya T, Yanase M, Tejima K, Nishikawa T, Arai M, Arai H, Omata M, Fujiwara K, Yatomi Y. Both Plasma Lysophosphatidic Acid and Serum Autotaxin Levels are Increased in Chronic Hepatitis C. J Clin Gastroenterol. 2007;41:616–623. doi: 10.1097/01.mcg.0000225642.90898.0e. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Tomiya T, Tejima K, Nishikawa T, Arai M, Yanase M, Aoki J, Arai H, Omata M, Fujiwara K, Yatomi Y. Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity. Life Sci. 2007;81:1009–1015. doi: 10.1016/j.lfs.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Umemura K, Yamashita N, Yu X, Arima K, Asada T, Makifuchi T, Murayama S, Saito Y, Kanamaru K, Goto Y, Kohsaka S, Kanazawa I, Kimura H. Autotaxin expression is enhanced in frontal cortex of Alzheimer-type dementia patients. Neurosci Lett. 2006;400:97–100. doi: 10.1016/j.neulet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Boucher J, Quilliot D, Pradère JP, Simon MF, Grès S, Guigné C, Prévot D, Ferry G, Boutin JA, Carpéné C, Valet P, Saulnier-Blache JS. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetologia. 2005;48:569–577. doi: 10.1007/s00125-004-1660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hammack BN, Fung KY, Hunsucker SW, Duncan MW, Burgoon MP, Owens GP, Gilden DH. Proteomic analysis of multiple sclerosis cerebrospinal fluid. Mult Scler. 2004;10:245–260. doi: 10.1191/1352458504ms1023oa. [DOI] [PubMed] [Google Scholar]
- 72.Santos AN, Riemann D, Kehlen A, Thiele K, Langner J. Treatment of fibroblast-like synoviocytes with IFN-gamma results in the down-regulation of autotaxin mRNA. Biochem Biophys Res Commun. 1996;229:419–424. doi: 10.1006/bbrc.1996.1819. [DOI] [PubMed] [Google Scholar]
- 73.Yang Y, Mou LJ, Liu N, Tsao MS. Autotaxin expression in non-small-cell lung cancer. Am J Respir Cell Mol Biol. 1999;21:216–222. doi: 10.1165/ajrcmb.21.2.3667. [DOI] [PubMed] [Google Scholar]
- 74.Zhang G, Zhao Z, Xu S, Ni L, Wang X. Expression of autotaxin mRNA in human hepatocellular carcinoma. Chin Med J. 1999;112:330–332. [PubMed] [Google Scholar]
- 75.Yang SY, Lee J, Park CG, Kim S, Hong S, Chung HC, Min SK, Han JW, Lee HW, Lee HY. Expression of autotaxin (NPP-2) is closely linked to invasiveness of breast cancer cells. Clin Exp Metastasis. 2002;19:603–608. doi: 10.1023/a:1020950420196. [DOI] [PubMed] [Google Scholar]
- 76.Stassar MJ, Devitt G, Brosius M, Rinnab L, Prang J, Schradin T, Simon J, Petersen S, Kopp-Schneider A, Zöller M. Identification of human renal cell carcinoma associated genes by suppression subtractive hybridization. Br J Cancer. 2001;85:1372–1382. doi: 10.1054/bjoc.2001.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kehlen A, Englert N, Seifert A, Klonisch T, Dralle H, Langner J, Hoang-Vu C. Expression, regulation and function of autotaxin in thyroid carcinomas. Int J Cancer. 2004;109:833–838. doi: 10.1002/ijc.20022. [DOI] [PubMed] [Google Scholar]
- 78.Hoelzinger DB, Mariani L, Weis J, Woyke T, Berens TJ, McDonough WS, Sloan A, Coons SW, Berens ME. Gene expression profile of glioblastoma multiforme invasive phenotype points to new therapeutic targets. Neoplasia. 2005;7:7–16. doi: 10.1593/neo.04535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kishi Y, Okudaira S, Tanaka M, Hama K, Shida D, Kitayama J, Yamori T, Aoki J, Fujimaki T, Arai H. Autotaxin is overexpressed in glioblastoma multiforme and contributes to cell motility of glioblastoma by converting lysophosphatidylcholine to lysophosphatidic acid. J Biol Chem. 2006;281:17492–17500. doi: 10.1074/jbc.M601803200. [DOI] [PubMed] [Google Scholar]
- 80.Zhao H, Ramos CF, Brooks JD, Peehl DM. Distinctive gene expression of prostatic stromal cells cultured from diseased versus normal tissues. J Cell Physiol. 2006;210:111–121. doi: 10.1002/jcp.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nam SW, Clair T, Kim YS, McMarlin A, Schiffmann E, Liotta LA, Stracke ML. Autotaxin (NPP-2), a metastasis-enhancing motogen, is an angiogenic factor. Cancer Res. 2001;61:6938–6944. [PubMed] [Google Scholar]
- 82.Hoelzinger DB, Nakada M, Demuth T, Rosensteel T, Reavie LB, Berens ME. Autotaxin: a secreted autocrine/paracrine factor that promotes glioma invasion. J Neurooncol. 2008;86:297–309. doi: 10.1007/s11060-007-9480-6. [DOI] [PubMed] [Google Scholar]
- 83.Black EJ, Clair T, Delrow J, Neiman P, Gillespie DA. Microarray analysis identifies Autotaxin, a tumour cell motility and angiogenic factor with lysophospholipase D activity, as a specific target of cell transformation by v-Jun. Oncogene. 2004;23:2357–2366. doi: 10.1038/sj.onc.1207377. [DOI] [PubMed] [Google Scholar]
- 84.Lee HY, Bae GU, Jung ID, Lee JS, Kim YK, Noh SH, Stracke ML, Park CG, Lee HW, Han JW. Autotaxin promotes motility via G protein-coupled phosphoinositide 3-kinase in human melanoma cells. FEBS Lett. 2002;515:137–140. doi: 10.1016/s0014-5793(02)02457-2. [DOI] [PubMed] [Google Scholar]
- 85.Hama K, Aoki J, Fukaya M, Kishi Y, Sakai T, Suzuki R, Ohta H, Yamori T, Watanabe M, Chun J, Arai H. Lysophosphatidic Acid and Autotaxin Stimulate Cell Motility of Neoplastic and Non-neoplastic Cells through LPA1. J Biol Chem. 2004;279:17634–17639. doi: 10.1074/jbc.M313927200. [DOI] [PubMed] [Google Scholar]
- 86.Hoelzinger DB, Nakada M, Demuth T, Rosensteel T, Reavie LB, Berens ME. Autotaxin: a secreted autocrine/paracrine factor that promotes glioma invasion. J Neurooncol. 2008;86:297–309. doi: 10.1007/s11060-007-9480-6. [DOI] [PubMed] [Google Scholar]
- 87.Song J, Jie C, Polk P, Shridhar R, Clair T, Zhang J, Yin L, Keppler D. The candidate tumor suppressor CST6 alters the gene expression profile of human breast carcinoma cells: down-regulation of the potent mitogenic, motogenic, and angiogenic factor autotaxin. Biochem Biophys Res Commun. 2006;340:175–182. doi: 10.1016/j.bbrc.2005.11.171. [DOI] [PubMed] [Google Scholar]
- 88.Rogers MS, Rohan RM, Birsner AE, D’Amato RJ. Genetic loci that control the angiogenic response to basic fibroblast growth factor. FASEB J. 2004;18:1050–1059. doi: 10.1096/fj.03-1241com. [DOI] [PubMed] [Google Scholar]
- 89.Ferry G, Giganti A, Coge F, Bertaux F, Thiam K, Boutin JA. Functional inactivation of the autotaxin gene by a single amino acid mutation in mouse is lethal. FEBS Lett. 2007;581:3572–3578. doi: 10.1016/j.febslet.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 90.Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- 92.Dugas JC, Tai YC, Speed TP, Ngai J, Barres BA. Functional genomic analysis of oligodendrocyte differentiation. J Neurosci. 2006;26:10967–10983. doi: 10.1523/JNEUROSCI.2572-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee J, Jung ID, Nam SW, Clair T, Jeong EM, Hong SY, Han JW, Lee HW, Stracke ML, Lee HY. Enzymatic activation of autotaxin by divalent cations without EF-hand loop region involvement. Biochem Pharmacol. 2001;62:219–224. doi: 10.1016/s0006-2952(01)00658-x. [DOI] [PubMed] [Google Scholar]
- 94.Zamir E, Geiger B. Components of cell-matrix adhesions. J Cell Sci. 2001;114:3577–3579. doi: 10.1242/jcs.114.20.3577. [DOI] [PubMed] [Google Scholar]
- 95.Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- 96.Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells -- over and over and over again. Nat Cell Biol. 2002;4:E97–100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- 97.Weiner JA, Hecht JH, Chun J. Lysophosphatidic acid receptor gene vzg-1/lpa1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J Comp Neurol. 1998;398:587–598. [PubMed] [Google Scholar]
- 98.Moller T, Musante DB, Ransom BR. Lysophosphatidic acid-induced calcium signals in cultured rat oligodendrocytes. NeuroReport. 1999;10:2929–2932. doi: 10.1097/00001756-199909290-00010. [DOI] [PubMed] [Google Scholar]
- 99.Stankoff B, Barron S, Allard J, Barbin G, Noel F, Aigrot MS, Premont J, Sokoloff P, Zalc B, Lubetzki C. Oligodendroglial expression of edg-2 receptor: Developmental analysis and pharmacological responses to lysophosphatidic acid. Mol Cell Neurosci. 2002;20:415–428. doi: 10.1006/mcne.2002.1129. [DOI] [PubMed] [Google Scholar]
- 100.Yu N, Lariosa-Willingham KD, Lin FF, Webb M, Rao TS. Characterization of lysophosphatidic acid and sphingosine 1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia. 2004;45:17–27. doi: 10.1002/glia.10297. [DOI] [PubMed] [Google Scholar]