Abstract
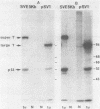
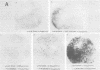
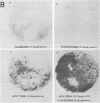
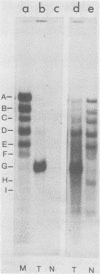
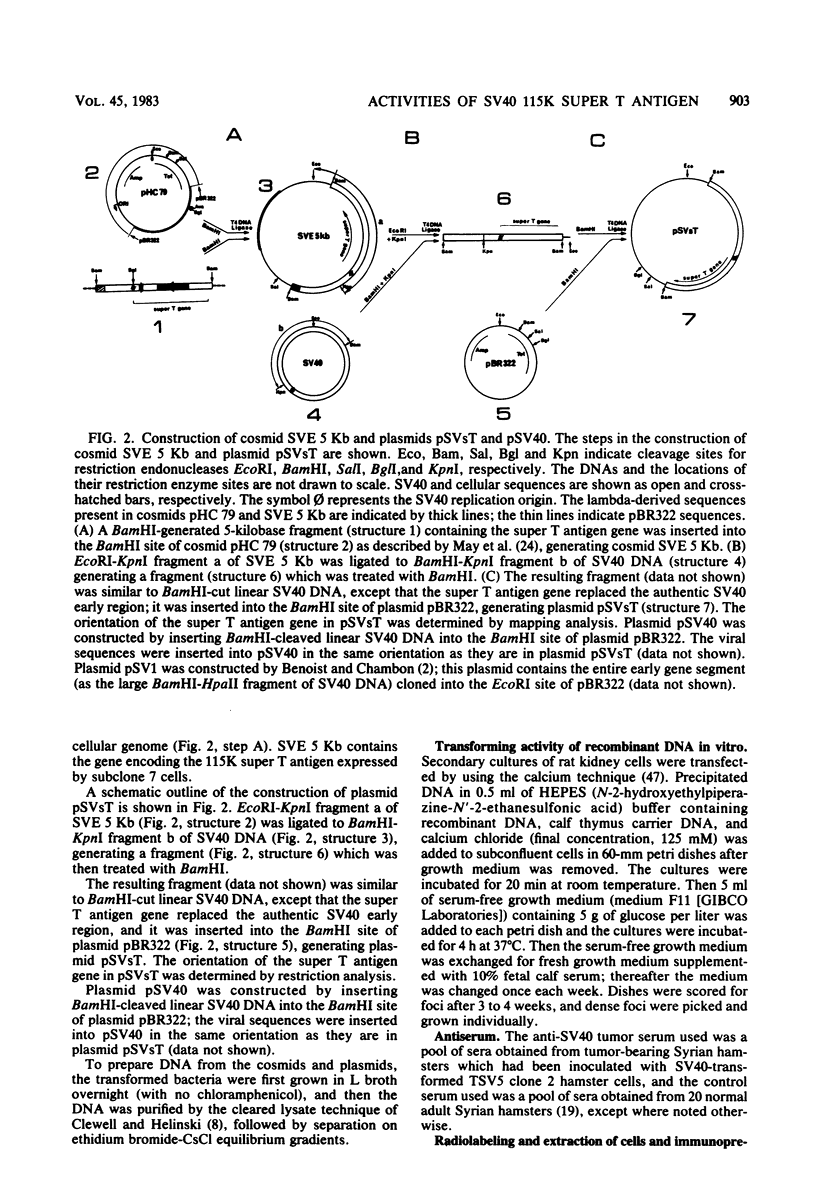
Simian virus 40 (SV40) transformed V 11 F 1 clone 1 subclone 7 rat cells (subclone 7) do not synthesize normal-size large T antigen (Mr, 90,000); instead, they produce a 115,000 Mr super T antigen (115K super T antigen). This super T antigen is SV40 virus coded, and its synthesis results from rearrangement and amplification of integrated viral DNA sequences in subclone 7 (May et al., Nucleic Acids Res. 9:4111-4128, 1981). In this study the functional activities of 115K super T antigen were compared with the functional activities of SV40 large T antigen. Transfection experiments were performed with (i) cosmid SVE 5 Kb and plasmid pSVsT, both containing the super T antigen gene and (ii) plasmids pSV1 and pSV40, both containing the large T antigen gene. Transfection of pSVsT DNA or SVE 5 Kb DNA into secondary cultures of rat kidney cells induced the formation of transformed cell foci with an efficiency that was about 50% of the efficiency of pSV1 DNA or pSV40 DNA. Concomitant with the transforming activity, two other activities were also retained by super T antigen, namely, the ability to enhance the level of host cellular protein p53 and the capacity to bind to p53. In contrast, pSVsT and SVE 5 Kb DNAs were markedly deficient in the capacity to support tsA58 DNA replication in CV1-P cells at a nonpermissive temperature (41°C), as shown by cotransfection experiments. The yield of virus produced in these experiments was 400-fold less than the yield obtained in parallel experiments with pSV40 or pSV1. However, SVE 5 Kb and pSVsT have a functional SV40 replication origin, as shown by their efficient replication in COS 1 cells which provided functional large T antigen. Super T antigen also possesses a specific affinity for sequences of SV40 viral origin. Our results suggest that under certain conditions, evolutionary changes in T antigen take place and that these changes could be restricted to the phenotypic requirement of maintaining a structure that is able to induce cell transformation, to form a complex with p53, and to enhance the cellular level of p53. Therefore, there appears to be a close relationship among the activities of T antigen involved in transforming cells, in binding to p53, and in enhancing the p53 cellular level. Moreover, this set of activities appears to be separable from the replicative ability of T antigen, based on the observation that 115K super T antigen is markedly defective for initiating viral DNA synthesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender M. A., Brockman W. W. Rearrangement of integrated viral DNA sequences in mouse cells transformed by simian virus 40. J Virol. 1981 Jun;38(3):872–879. doi: 10.1128/jvi.38.3.872-879.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., Chambon P. Deletions covering the putative promoter region of early mRNAs of simian virus 40 do not abolish T-antigen expression. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3865–3869. doi: 10.1073/pnas.77.7.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Simmons D. T., Martin M. A., Mora P. T. Identification and partial characterization of new antigens from simian virus 40-transformed mouse cells. J Virol. 1979 Aug;31(2):463–471. doi: 10.1128/jvi.31.2.463-471.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Verderame M., Lo A., Pollack R. Nonlytic simian virus 40-specific 100K phosphoprotein is associated with anchorage-independent growth in simian virus 40-transformed and revertant mouse cell lines. Mol Cell Biol. 1981 Nov;1(11):994–1006. doi: 10.1128/mcb.1.11.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Avila J., Martin R. G. Viral DNA synthesis in cells infected by temperature-sensitive mutants of simian virus 40. J Virol. 1974 Jul;14(1):116–124. doi: 10.1128/jvi.14.1.116-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E., Lovett M., Rigby P. W. Functional analysis of a simian virus 40 super T-antigen. J Virol. 1982 Dec;44(3):974–982. doi: 10.1128/jvi.44.3.974-982.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E., Rigby P. W. Cloning and characterization of the integrated viral DNA from three lines of SV40-transformed mouse cells. Cell. 1981 Aug;25(2):547–559. doi: 10.1016/0092-8674(81)90073-8. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni V., Dailey L., Basilico C. Amplification of integrated viral DNA sequences in polyoma virus-transformed cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3850–3854. doi: 10.1073/pnas.77.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya-Grosjean L., Lasne C., Nardeux P., Chouroulinkov I., Monier R. Oncogenic transformation of rat lung epitheloid cells by SV 40 DNA and restriction enzyme fragments. Arch Virol. 1979;62(2):87–100. doi: 10.1007/BF01318062. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Davison J., Oren M., Winocour E. Properties of permissive monkey cells transformed by UV-irradiated simian virus 40. J Virol. 1977 May;22(2):256–266. doi: 10.1128/jvi.22.2.256-266.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Greenspan D. S., Carroll R. B. Complex of simian virus 40 large tumor antigen and 48,000-dalton host tumor antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):105–109. doi: 10.1073/pnas.78.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Pim D. C., Crawford L. V. Complex of simian virus 40 large-T antigen and host 53,000-molecular-weight protein in monkey cells. J Virol. 1981 Feb;37(2):564–573. doi: 10.1128/jvi.37.2.564-573.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday A., Gandini-Attardi D., Fried M. Detection in situ of foreign DNA in eukaryotic cells. Gene. 1981 Oct;15(1):53–65. doi: 10.1016/0378-1119(81)90104-9. [DOI] [PubMed] [Google Scholar]
- Hiscott J. B., Murphy D., Defendi V. Instability of integrated viral DNA in mouse cells transformed by simian virus 40. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1736–1740. doi: 10.1073/pnas.78.3.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J., Murphy D., Defendi V. Amplification and rearrangement of integrated SV40 DNA sequences accompany the selection of anchorage-independent transformed mouse cells. Cell. 1980 Nov;22(2 Pt 2):535–543. doi: 10.1016/0092-8674(80)90363-3. [DOI] [PubMed] [Google Scholar]
- Kress M., May E., Cassingena R., May P. Simian virus 40-transformed cells express new species of proteins precipitable by anti-simian virus 40 tumor serum. J Virol. 1979 Aug;31(2):472–483. doi: 10.1128/jvi.31.2.472-483.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Maltzman W., Levine A. J. The SV40 A gene product is required for the production of a 54,000 MW cellular tumor antigen. Virology. 1979 Oct 30;98(2):308–318. doi: 10.1016/0042-6822(79)90554-3. [DOI] [PubMed] [Google Scholar]
- Lovett M., Clayton C. E., Murphy D., Rigby P. W., Smith A. E., Chaudry F. Structure and synthesis of a simian virus 40 super T-antigen. J Virol. 1982 Dec;44(3):963–973. doi: 10.1128/jvi.44.3.963-973.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
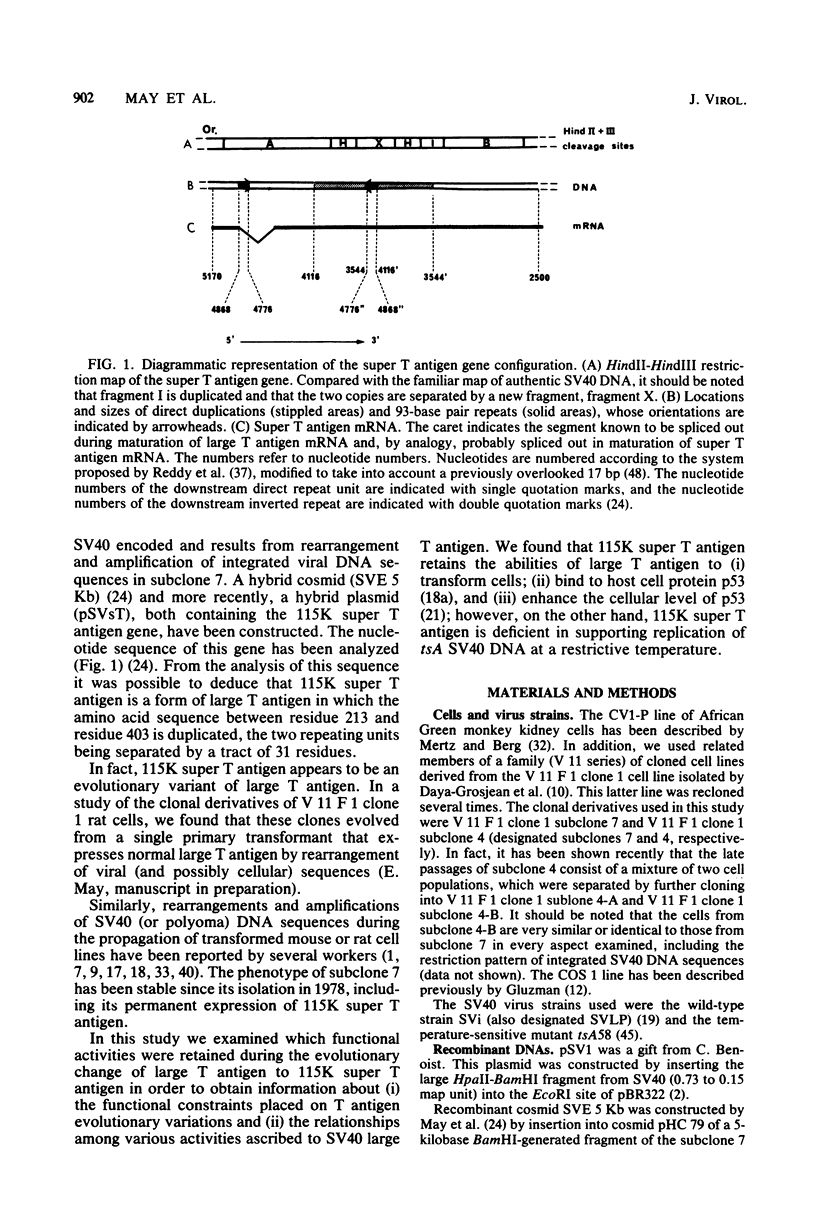
- May E., Jeltsch J. M., Gannon F. Characterization of a gene encoding a 115 K super T antigen expressed by a SV40-transformed rat cell line. Nucleic Acids Res. 1981 Aug 25;9(16):4111–4128. doi: 10.1093/nar/9.16.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E., Kress M., Daya-Grosjean L., Monier R., May P. Mapping of the viral mRNA encoding a super-T antigen of 115,000 daltons expressed in simian virus 40-transformed rat cell lines. J Virol. 1981 Jan;37(1):24–35. doi: 10.1128/jvi.37.1.24-35.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P., Kress M., Lange M., May E. New genetic information expressed in SV40-transformed cells: characterization of the 55K proteins and evidence for unusual SV40 mRNAs. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):189–200. doi: 10.1101/sqb.1980.044.01.022. [DOI] [PubMed] [Google Scholar]
- McCormick F., Chaudry F., Harvey R., Smith R., Rigby P. W., Paucha E., Smith A. E. T antigens of SV40-transformed cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):171–178. doi: 10.1101/sqb.1980.044.01.020. [DOI] [PubMed] [Google Scholar]
- McCormick F., Clark R., Harlow E., Tjian R. SV40 T antigen binds specifically to a cellular 53 K protein in vitro. Nature. 1981 Jul 2;292(5818):63–65. doi: 10.1038/292063a0. [DOI] [PubMed] [Google Scholar]
- McCormick F., Harlow E. Association of a murine 53,000-dalton phosphoprotein with simian virus 40 large-T antigen in transformed cells. J Virol. 1980 Apr;34(1):213–224. doi: 10.1128/jvi.34.1.213-224.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- McKay R. D. Binding of a simian virus 40 T antigen-related protein to DNA. J Mol Biol. 1981 Jan 25;145(3):471–488. doi: 10.1016/0022-2836(81)90540-4. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Defective simian virus 40 genomes: isolation and growth of individual clones. Virology. 1974 Nov;62(1):112–124. doi: 10.1016/0042-6822(74)90307-9. [DOI] [PubMed] [Google Scholar]
- Mougneau E., Birg F., Rassoulzadegan M., Cuzin F. Integration sites and sequence arrangement of SV40 DNA in a homogeneous series of transformed rat fibroblast lines. Cell. 1980 Dec;22(3):917–927. doi: 10.1016/0092-8674(80)90569-3. [DOI] [PubMed] [Google Scholar]
- Oren M., Maltzman W., Levine A. J. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981 Feb;1(2):101–110. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintel D., Bouck N., di Mayorca G. Separation of lytic and transforming functions of the simian virus 40 A region: two mutants which are temperature sensitive for lytic functions have opposite effects on transformation. J Virol. 1981 May;38(2):518–528. doi: 10.1128/jvi.38.2.518-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Gilboa E., Revel M., Winocour E. Cell-free translation of simian virus 40 early messenger RNA coding for viral T-antigen. Proc Natl Acad Sci U S A. 1977 Feb;74(2):457–461. doi: 10.1073/pnas.74.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Piatak M., Weissman S. M. Simian virus 40 early mRNA's. I. Genomic localization of 3' and 5' termini and two major splices in mRNA from transformed and lytically infected cells. J Virol. 1979 Apr;30(1):279–296. doi: 10.1128/jvi.30.1.279-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Ferguson J., Davis R. W., Stark G. R. T antigen binds to simian virus 40 DNA at the origin of replication. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1605–1609. doi: 10.1073/pnas.72.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Howell N. Genomic rearrangements in a mouse cell line containing integrated SV40 DNA. Cell. 1981 Jan;23(1):41–50. doi: 10.1016/0092-8674(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Scheller A., Covey L., Barnet B., Prives C. A small subclass of SV40 T antigen binds to the viral origin of replication. Cell. 1982 Jun;29(2):375–383. doi: 10.1016/0092-8674(82)90154-4. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Paucha E. Characterization of different tumor antigens present in cells transformed by simian virus 40. Cell. 1979 Oct;18(2):335–346. doi: 10.1016/0092-8674(79)90053-9. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. Protein-DNA interactions at the origin of simian virus 40 DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):655–661. doi: 10.1101/sqb.1979.043.01.073. [DOI] [PubMed] [Google Scholar]
- Van Heuverswyn H., Fiers W. Nucleotide sequence of the Hind-C fragment of simian virus 40 DNA. Comparison of the 5'-untranslated region of wild-type virus and of some deletion Mutants. Eur J Biochem. 1979 Oct;100(1):51–60. doi: 10.1111/j.1432-1033.1979.tb02032.x. [DOI] [PubMed] [Google Scholar]
- van der Eb A. J., Graham F. L. Assay of transforming activity of tumor virus DNA. Methods Enzymol. 1980;65(1):826–839. doi: 10.1016/s0076-6879(80)65077-0. [DOI] [PubMed] [Google Scholar]