Abstract
Although urine analysis remains the standard for detection of drugs of abuse, sweat patches provide a convenient alternative that avoids some of the problems with drug testing such as violations of privacy in observed urination, possibility of disease transmission, and transport of noxious fluids. This study examined minimum length of wear necessary to detect recent or concurrent cocaine use in a convenience sample of active cocaine users and also differences in analyte concentrations with increasing longer-term wear. Twenty-seven subjects (22 active drug users and 5 comparison subjects who did not use drugs) wore short-term (½ h, 1 h, 1½ h, and 2 h), then long-term patches (1, 3, 7, and 14 day). Short- and long-term patches were identical except for duration of wear. The predominant analyte found was cocaine, followed by benzoylecgonine, then ecgonine methylester. The minimum duration that patches must be worn to detect recent or concurrent cocaine use in this sample is more than 2 h and less than or equal to 1 day. Analyte concentrations increase significantly with increasing lengths of wear. However, increases between the one-week and two-week patches were significant for benzoylecgonine only.
Introduction
Urine analysis has become the standard for detection of drugs (1,2). However, urinalysis is not without problems including violations of privacy in observed specimen collection or alternatively the possibility of dilution or substitution of specimens, possibility of disease transmission, and transport of noxious fluid. Therefore, research has continued to focus on alternative biological matrices for testing. These include hair, saliva, sweat, and others (2-7).
The present study employs an advanced statistical technique called multilevel modeling (8), or hierarchical linear modeling (9), to examine how cocaine (COC) analytes increase in sweat patches as a function of how long the sweat patch is worn. Detail is provided on how the data are coded, and results are interpreted for readers unfamiliar with this technique.
In the field of drug testing for substances of abuse, researchers typically dichotomize analyte levels (levels of the parent drug and its metabolites) to determine use versus non-use (2,6,10). They do this for two reasons. First, at the level of the individual case, the ultimate goal of the inquiry is determination of use or non-use which can have legal consequences in criminal justice settings or therapeutic consequences in drug-treatment programs. Second, the wide degree of intra-individual variability of analyte concentrations has led researchers and toxicologists to be conservative and to limit themselves to dichotomous treatments of data. Of greatest concern is the occurrence of false positives, cases where tests appear positive when in fact the subject has not used the drug. False positives can result in unjust legal penalties or inappropriate sanctions from a drug-treatment program. However, when data are used for research purposes and all individual identifiers have been properly removed, these concerns are diminished.
There are advantages to not dichotomizing data. From a functional perspective, it would be useful to distinguish low doses at which subjects may be slightly impaired from high doses at which they are likely to be dysfunctional. Moreover, from a statistical perspective, dichotomizing analyte concentrations leads to restriction of range and loss of information. Again the focus of this paper is the relationship between analyte concentrations and length of wear in a dose-uncontrolled sample of cocaine users.
Because each subject in this study wore more than one sweat patch, there are repeated or multiple measures on each subject. The advantage of multilevel modeling over classical repeated measures for these data is that subjects do not need to have the same number of observations and therefore no cases need to be excluded. This increases the power of the analysis.
Sweat Patches
Early efforts to systematically collect sweat specimens for analysis by various means are documented by Sunshine and Sutliff (11). These efforts included developing sweat collection devices. These early devices were occlusive, trapping the whole specimen including fluids against the skin. In the early 1990's a low-cost, non-occlusive device was developed making sweat testing practical and economical. The sweat patch can be used to detect most drugs of abuse (3). However, our discussion is limited to the detection of cocaine analytes because that was the focus of this research project.
Laboratory tests have shown the efficacy of the sweat patch in detecting cocaine use. In controlled-dose clinical trials, the sweat patch showed a clear, dose-response relationship. Cone et al. (12) found that administrating as little as 1–5 mg. of cocaine would produce detectable levels of cocaine and/or metabolites in sweat. Employing doses which ranged from 1 to 25 mg of cocaine hydrochloride, COC appeared in the patches after 1–2 h, ecgonine methylester (EME) after 2–4 h, and benzoylecgonine (BE) only at doses of 25 mg after 8 h using gas chromatography–mass spectrometry (GC–MS) testing. They report on a second study which employed three doses and modes of administration: smoked (42 mg), injected (25 mg), and snorted intranasally (32 mg). In this study, duplicate patches were applied prior to cocaine administration and were removed 24, 48, and 72 h later. They found that most of the analytes in the patch achieved in 72 h appeared within the first 24 h. In other words, analyte levels were near their asymptote at 24 h with only small increases after that. They concluded that the patch shows promise as a drug monitoring tool.
Burns and Baselt (13) gave 18 male subjects either 50 mg then 126 mg of cocaine snorted intranasally or reversed the order of doses with the starting condition randomized. Subjects wore 14 patches that were removed at intervals. In this crossover design following a week of rest, subjects were then re run in the alternate condition. Again, cocaine appeared in patches 1–2 h after use reaching near maximum levels at 24 h and asymptote at 72 h. There was a clear indication of dose-response relationship with significantly greater concentrations in the high dose (126 mg) condition. However, the authors concluded that the degree of intra- and intersubject variability was too great for quantitative use of the patch. Qualitative (positive/negative) use was recommended .
A large-scale field trial of the sweat patch was performed by PharmChem Laboratories in Michigan for the Michigan Department of Corrections (14). One thousand, fifty-four prison inmates or other individuals under Department of Corrections supervision wore patches for approximately 1 or 2 weeks; 2885 patches and 10,080 urine specimens were collected. Random urine testing was supplemented with a sample collected at time of patch application and removal. “For purposes of comparing patch and urine results, urine specimens collected during the period starting two days prior to patch application and ending two days after patch removal were considered to be associated with the patch.” (14). It was found that 3.4% of patches and 5.8% of subjects were positive for cocaine, but only 0.2% of specimens and 1.4% of subjects were found to be cocaine positive by urine analysis. The authors conclude that these data demonstrate the greater sensitivity of sweat patch testing over urine testing.
Fogerson et al. (15) have documented the efficacy of the sweat patch for cocaine detection by comparing the patch with EMIT urines obtaining 93% agreement. They validated the EIA procedure against GC–MS obtaining 94% specificity and 97% sensitivity.
Method
Description of patch
The PharmChek Sweat Patch™ is a nonocclusive appliance in that it permits water vapor and other volatile components in sweat to evaporate through small pores. However, solid components of drugs of abuse are composed of much larger molecules which are trapped in the patch. The PharmChek Sweat Patch has three components: the absorption pad, a release liner, and a polyurethane/adhesive layer. The absorption pad is approximately 3-cm wide, 5-cm long, and consists of inert, medical grade cellulose that retains the nonvolatile components of sweat (including drugs of abuse and their metabolites) collected from the surface of the skin. The release liner allows removal of the collection pad from the adhesive layer after patch use. The outer polyurethane adhesive layer is identical to that used in 3M's Tegaderm™ 1625 transparent dressing.
The patch has three distinctive features useful for drug testing. First, because it is nonocclusive, it does not alter the transport properties of the skin and water is not trapped against the skin, minimizing skin irritation (11). Additionally, the patch itself is relatively impervious to environmental contamination. Moderate amounts of cocaine powder applied externally under normal environmental conditions will not penetrate the protective layer. However, under extreme laboratory conditions and very high external doses not normally found in the environment, the patch's protective cover can be penetrated (16). Second, 3M developed the adhesive so that once removed, the patch cannot be reapplied. Exfoliated stratum corneum skin cells stick to the adhesive upon removal of the patch preventing readhesion. Also, the light-gauge polyurethane adhesion layer typically deforms when it is peeled, making attempts at reapplication obvious. Third, each patch has a unique, nine-digit number printed underneath the polyurethane layer that is visible through a window while the patch is being worn. This number serves as an aid in maintaining chain-of-custody control of the patch and prevents deceptive replacement of patches.
Patches used in the study were identical regardless of length of wear, but are described and referred to by their intended period of wear. Patches were worn for the following times during this study: ½ h, 1 h, 1½ h, 2 h, 1 day, 1 day (random), 3 days, 1 week, and 2 weeks.
Random patches
Each subject wore two random daily patches: One in the first week of the study, and one in the second. A random number table was used to select on which 2 study days, 1–14 excluding weekends, random patches would be applied. These patches were planned to serve two purposes. First, to simulate the conditions of random drug testing, and second to allow for a reliability check on the daily patches because on these two days, the subject would be wearing two identical daily patches.
Subjects
Data collection for this study was conducted by National Development and Research Institutes (NDRI) staff at a store front in East Harlem. The sample of 27 subjects consisted of 22 active drug users recruited from the neighborhood, and the outreach workers and other store front staff served as the 5 comparison group subjects who did not use drugs. Outreach workers from this project are known in the community and maintain contact with many different types of drug users.
The mean age of the sample was 40 years (SD 8 years); 59% of subjects were Hispanic, 37% were African-American, and 4% were white; and 63% of the sample was male. All subjects in the experimental group reported recent cocaine use on entrance into the study, and 86% of these subjects reported using cocaine during the study. There was no subject attrition from this study. All subjects completed the study, despite several major blizzards during the collection phase. This was probably because of the high and steady incentives, described here, paid to subjects.
Experimental procedures
When subjects arrived at the store front site, the interviewer read and discussed an informed consent with the subject; reviewed the Sweat Patch Data Collection Calendar with the subject which described the schedule of patch application and removal, urine specimens to be collected, and explained the attendance requirements and payment schedule for each visit. If, following this explanation, the subject agreed to participate, a signed consent was obtained, and a urine sample was collected.
On the next day, four identical short - term patches (½ h, 1 h, 1½ h, and 2 h) were applied and removed at half-hour intervals. These patches were applied two to each biceps. As soon as the patches were in place, a urine specimen was collected, and while the patches were being worn and periodically removed, detailed self-reports were obtained on drug consumption. (The urinalysis and self-report data will be analyzed in another paper.) After the fourth patch, the 2-h patch was removed, and the four long-term patches were applied (1 day, 3 day, 7 day, and 14 day).
On each week day over the next 14 days, subjects arrived, donated a urine specimen, gave a brief self-report on drug use since their previous visit, and one to four sweat patches were swapped depending on the day. This schedule resulted in each subject contributing 10–11 1-day patches, 4 or 5 3-day patches, 2 7-day patches, and 1 14-day patch. Variation in the number of patches each subject wore resulted from starting subjects on different days, and the field site was closed on weekends.
Subjects were paid approximately $205 in participation fees. They were paid $10 per day for most days. However, they were paid $30 for the second day when numerous patches were applied and removed and extensive self-report interviews were completed, $30 for the last day when all patches were removed, and a $25 completion bonus on the last day if they had not missed a single day.
Patch application and removal procedures
Patches were applied to the exterior of subjects' left and right arms at the biceps. The area was scrubbed prior to application with a Baxter NO.4458 Pharmaseal surgical scrub, rinsed free of soap with tap water in a spray bottle, and finally cleaned again with an alcohol wipe. The interviewer wore clean, new, disposable rubber gloves during patch application and removal. During patch removal, subjects were asked to peel back the edge of the patch being careful not to touch the absorbent pad. When the patch was half uncovered, the interviewer lifted the pad out with disposable tweezers. New tweezers were used for each patch. The pad was then placed in a self-sealing specimen bag for which the sealing glue was stronger than the plastic bag itself. Therefore, any attempt to access the patch after it was placed in the bag would deform the bag and be apparent.
Subjects wore two patches on each bicep at all times. When patches were replaced, the area was cleaned twice with alcohol wipes and the new patch was applied in exactly the same place. Removal of the patch most likely removed a small amount of skin cells. However, because the collection area is in the center of the patch, away form the adhesive, the removal of cells would not affect results. Cleaning the application area again prevented a patch from appearing positive simply because the prior one was.
Chain of custody
Each patch was uniquely identified by a number that was visible through a window when the patch was applied. The interviewer wrote this number on a chain of custody form at the time of application and matched it at time of patch removal.
Laboratory procedures
Drugs from the absorbent pads of sweat patches were extracted using 2.5 mL of 0.2M acetate/methanol (25:75) buffer (pH 5.0). All patch specimens were then tested by GC–MS for the presence of COC or its chief metabolites, BE and EME. The assay was performed using a Hewlett-Packard 5890/5971 GC–MS. SIM was used to acquire data on COC (182, 272, 303 amu) BE (318, 334, 439 amu), and EME (182, 314, 345 amu) and on the internal standards COC-d3 (185, 306 amu) BE-d3 (321, 442 amu), and EME-d3 (185, 348 amu). The samples were derivatized using HFIP and PFPA. The limits of quantitation (LOQ) were 4 ng/mL for COC, 2 ng/mL for BE, and 2 ng/mL for EME.
Statistical analysis
The values presented in Table I were transformed for statistical analysis employing the function ln(1 + x) both to linearize the data simplifying analysis (17) and because analyte excretion in sweat often follows an exponential function over time (18). Because the mean analyte levels and variances of these levels for short - term patches (½ h, l h, 1½ h, and 2 h) and for control group patches were zero or near zero, these patches were excluded from the multilevel regression analysis which follows because there was insufficient variation for significance tests.
Table I.
Levels of Cocaine and Chief Metabolites Classified by Length of Wear of Patches
| Drug-Using Experimental Group* |
Non-Using Comparison Group† |
|||||
|---|---|---|---|---|---|---|
| Analyte | Mean‡ | SD | N§ | Mean | SD | N |
| Cocaine | ||||||
| ½ h | 0.00 | 0.00 | 22 | 0.00 | 0.00 | 4 |
| 1 h | 0.17 | 0.83 | 22 | 0.00 | 0.00 | 5 |
| 1½ h | 0.31 | 1.47 | 22 | 0.00 | 0.00 | 5 |
| 2 h | 0.36 | 1.01 | 22 | 0.00 | 0.00 | 5 |
| 1 day | 325.83 | 644.40 | 201 | 0.20 | 0.86 | 50 |
| 1 day (random) | 384.91 | 812.41 | 44 | 0.00 | 0.00 | 10 |
| 3 day | 816.93 | 1027.82 | 101 | 0.20 | 0.98 | 24 |
| 1 week | 1195.00 | 338.13 | 43 | 0.00 | 0.00 | 10 |
| 2 week | 1664.31 | 2330.26 | 19 | 5.83 | 11.45 | 4.00 |
| Ecgonine methyl ester | ||||||
| ½ h | 0.00 | 0.00 | 22 | 0.00 | 0.00 | 4 |
| 1 h | 0.00 | 0.00 | 22 | 0.00 | 0.00 | 5 |
| 1½ h | 0.00 | 0.00 | 22 | 0.00 | 0.00 | 5 |
| 2 h | 0.00 | 0.00 | 22 | 0.00 | 0.00 | 5 |
| 1 day | 29.91 | 63.94 | 201 | 0.00 | 0.00 | 50 |
| 1 day (random) | 33.72 | 66.03 | 44 | 0.00 | 0.00 | 10 |
| 3 day | 83.11 | 115.28 | 101 | 0.00 | 0.00 | 24 |
| 1 week | 116.99 | 131.51 | 43 | 0.00 | 0.00 | 10 |
| 2 week | 210.96 | 279.52 | 19 | 0.00 | 0.00 | 4 |
| Benzoylecgonine | ||||||
| ½ h | 0.00 | 0.00 | 22 | 0.00 | 0.00 | 4 |
| 1 h | 0.00 | 0.00 | 22 | 0.00 | 0.00 | 5 |
| 1½ h | 0.00 | 0.00 | 22 | 0.00 | 0.00 | 5 |
| 2 h | 0.00 | 0.00 | 22 | 0.00 | 0.00 | 5 |
| 1 day | 59.22 | 370.30 | 200 | 0.00 | 0.00 | 50 |
| 1 day (random) | 25.51 | 50.10 | 44 | 0.00 | 0.00 | 10 |
| 3 day | 168.16 | 461.69 | 101 | 0.00 | 0.00 | 24 |
| 1 week | 181.90 | 348.16 | 43 | 0.00 | 0.00 | 10 |
| 2 week | 723.61 | 748.63 | 19 | 0.00 | 0.00 | 4 |
22 subjects.
5 subjects.
Analyte concentrations in nanograms per milliliter of sweat eluate.
Number of patches.
Multilevel statistical models (8), also called hierarchical linear models (9), are particularly well suited for biological data of this nature. Because there are multiple observations on each subject, multilevel modeling can increase the power of the analysis, much as repeated measures designs and covariance analysis do, but without the restrictive assumptions that these other techniques require. In classical repeated measures analysis, individual cases cannot have missing data. If a datum is missing, that case must be excluded from conventional analyses or imputed. Multilevel modeling tolerates missing data because multilevel techniques employ random effects models in which each observation is only a sample of possible levels of an independent variable, and measurement intervals or times of measurement do not have to be the same for all subjects, because, again, the points in time at which measurements occur are viewed as a subset of possible time points under the random effects model (19).
For each type of analyte (COC, BE, and EME), two separate multilevel linear regression models were computed on the same data. For these analyses, the Level One units were patches and the Level Two units were subjects. This means that for each subject there is a separate Level One equation. The parameters in these equations are estimated in the Level Two equations which average parameters across individuals. Both models employed standard dummy coding but used different reference groups to allow for different comparisons. By employing two different regression models on the same data, each type of patch could be compared with each other, except for the short - term patches which, as noted, were excluded from the analyses because their variances were close to zero.
The first model (model #1) computed for each analyte employed standard dummy coding for the Level One model where the 1-day patch was the reference group, and zero-one codes were employed for each other patch (i.e., 1-day random, 3 day, 1 week, and 2 week). These dummy codes were one if the given observation was the particular type of patch, otherwise they were zero. Therefore, the model tested was
where X1 (0 = No, 1 = Yes) = 1-day random patch, X2 = 3-day patch, X3 = 1-week patch, and X4 = 2-week patch. In this model, the b0 term represented the mean analyte level of the excluded or reference group, (the 1-day patch), and each other b term represented the difference between the mean level for that group and the mean level of the 1-day patch. The j terms indicated that there are 1 to j = 22 Level One equations, one for each subject. The Level Two equations were as follows
The lamba terms (λ) were the maximum likelihood, Bayesian estimates of the Level One parameters, and the u terms reflected the Level Two or between-person variation around these parameters. Results of these regressions for COC, EME, and BE are shown in Table II and will be discussed here .
Table II.
Effect of Duration of Wear of Sweat Patches on Analyte Concentrations†
| Model #1 |
Model #2 |
||||||
|---|---|---|---|---|---|---|---|
| Analyte | Variable | Patch duration |
Parameter | Standard error |
Patch duration |
Parameter | Standard error |
| Cocaine | |||||||
| b0 | 1 day | 3.858 | .498*** | 1 week | 5.436 | .568*** | |
| b1 | 1 day random | −0.078 | .183 | 1 day | −1.601 | .235*** | |
| b2 | 3 days | 1.108 | .183*** | 1 day random | −1.656 | .334*** | |
| b3 | 1 week | 1.574 | .226*** | 3 days | −0.469 | .198* | |
| b4 | 2 weeks | 1.854 | .403*** | 2 weeks | 0.116 | .301 | |
| Ecgonine methyl ester | |||||||
| b0 | 1 day | 2.099 | .339*** | 1 week | 3.463 | .445*** | |
| b1 | 1 day random | −0.028 | .153 | 1 day | −1.366 | .196*** | |
| b2 | 3 days | 0.930 | .149*** | 1 day random | −1.392 | .257*** | |
| b3 | 1 week | 1.362 | .180*** | 3 days | −0.440 | .166** | |
| b4 | 2 weeks | 1.807 | .331*** | 2 weeks | 0.284 | .252 | |
| Benzoylecgonine | |||||||
| b0 | 1 day | 1.954 | .350*** | 1 week | 3.702 | .482*** | |
| b1 | 1 day random | −0.103 | .163 | 1 day | −1.757 | .223*** | |
| b2 | 3 days | 1.289 | .160*** | 1 day random | −1.850 | .272*** | |
| b3 | 1 week | 1.734 | .242*** | 3 days | −0.478 | .179** | |
| b4 | 2 weeks | 3.175 | .352*** | 2 weeks | 1.300 | .271*** | |
Note: Significance test of b0 term evaluates whether that coded group (the reference group) is significantly different from zero. Significance tests of b1–b4 evaluate whether the difference between the mean of that group and the reference group is significantly different.
P < 0.05,
P < 0.01,
P < 0.001
Model #2 employed identical equations; however, the coding of the Xs was changed to provide different comparisons. The excluded or reference group was the 1-week patch. X1 was the 1-day patch; X2 was the 1-day random patch; X3 was the 3-day patch; and X4 was the 2-week patch. Therefore, λ1 measured the difference between the 1-day and the 1-week patches. (Because the 1-day patch, on average, had lower values than the 1-week patch, it was anticipated that this value would be negative.) Similarly, λ2 measured the difference between the 1-day random patch and the 1-week patch, λ3 measured the difference between the 3-day patch and the 1-week patch, and finally, λ4 measured the difference between the 1-week and the 2-week patches. By examining the λs for model #1 and model #2 we determined what meaningful differences in analyte levels exist between patches of different lengths of intended wear.
Results
Comparison of experimental and control groups
Mean concentrations and standard deviations of cocaine analytes found in patches for each length of wear are shown in Table I. In the experimental group of active users, all patches worn less than one day contained extremely low concentrations of COC and zero concentrations of cocaine metabolites, BE and EME.
The nonzero average values for the comparison group COC levels for 1-day, 3-day, and 2-week patches, seen in Table I, requires some discussion. All positive patches were for a single subject and for COC only, not for EME or BE. This subject had three consecutive positive 1-day patches (actual values were from 1.7 to 4.8 ng/mL for the individual patches) but none of these daily patches exceeded the 10 ng/mL threshold typically used to consider a patch as positive. A single 3-day patch (Monday–Thursday) was positive below threshold, and the subject's 2-week patch was positive (22.9 ng/mL). Both 1-week patches were negative. All urine EMIT tests for this subject were negative. In standard, applied drug testing, except for the 2-week patch this subject would have appeared negative. These individual values that were greater than zero caused the average values for the control group in Table II for the 1-day, 3-day, and 2-week patches to be greater than zero. It should be noted that in routine laboratory procedures at PharmChem, no patch is reported as positive unless metabolites are found as well as cocaine. The policy is that unless metabolites are found, the analyst cannot be certain that cocaine was ingested. This rule is applied to protect against scoring a patch as positive in the rare case where external contamination has occurred.
The presence of COC in the absence of metabolites is unusual for users of cocaine, but it is not impossible because COC is the most prevalent analyte found in sweat of active cocaine users and therefore could be reflective of very recent use. We felt the pattern of these data suggested another possibility. Control subjects were outreach workers who routinely entered locations called shooting galleries and crack houses where drug users can be found. In shooting galleries where the main activity is the injection of illicit drugs, cocaine powder is available and routinely mixed with heroin (“speedball”) to be snorted or injected. Workers may accidentally come in contact with cocaine powder, which may settle on their clothes or skin. Crack houses are similar indoor locations where crack cocaine is smoked and often shared among users. In these enclosed environments crack smoke pervades the air, settling on hair and clothing, and may have been inhaled by the outreach worker. The patch itself is relatively impervious to environmental contamination. Cocaine powder applied externally will generally not penetrate the protective layer of the patch. If properly applied, the edges of the patch are tightly sealed and cocaine powder cannot get under the patch seal.
However, in environments where drugs are used, low-level ingestion of cocaine is possible. Therefore, we believe that this outreach worker and control subject unintentionally ingested cocaine either through powder lying on skin and clothes or through the inhalation of cocaine powder or crack smoke. These possibilities can be viewed as occupational hazards for outreach workers.
Predominant analyte found
COC was the predominant analyte found in patches of all durations of wear followed by BE and then EME. These results can be seen in Figure 1.
Figure 1.
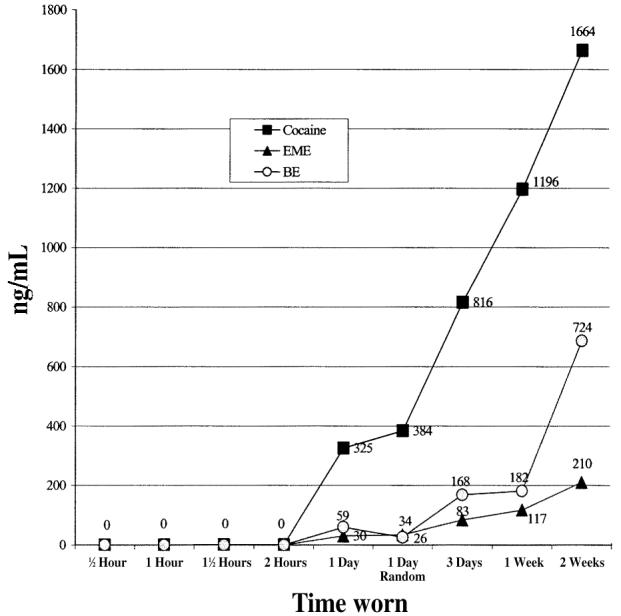
Cocaine analyte levels by length of patch wear.
Minimum length of wear
What is the minimum length of time that a patch can be worn under actual field conditions and effectively detect prior or concurrent cocaine use? This is a question of both scientific importance and practical significance. First, short-term patches of varying time periods (as described) were applied in parallel (½-h, 1-h, 1½-h, and 2-h patches). Immediately after these short-term patches were removed, longer term patches were applied in parallel (1-day, 3-day, 1-week, 2-week). Comparison of analyte concentrations in these patches provided an assessment of minimum necessary length of wear to detect cocaine. Table I displays the average analyte concentrations for COC, BE, and EME for each type of patch for both the Drug-Using Experimental Group and the Non-Using Comparison Group.
In Table II model #1, mean analyte levels for all patches for COC, EME, and BE were significantly different from the one day patch levels except, as anticipated, levels for the 1-day random patch. These results indicated that longer lengths of intended wear (3-day, 1-week, and 2-week) result in higher analyte levels than that found in the 1-day patch. Examination of the b0 or intercept term in model #1 is particularly important. Whereas the b0 term often has no substantive interpretation, in this particular instance it is very important. The significance test shows that the mean analyte levels of the 1-day patch are significantly different from zero, and therefore, by implication, significantly different from the short - term patches (½ h, 1 h, 1½ h, and 2 h) which were excluded from the analysis because their means and variances were close to zero. This result suggests that the minimum length of wear needed to detect concurrent cocaine use is greater than 2 h and less than or equal to 1 day in this cocaine-using sample.
Longer length of wear
It should be noted that the parameters and standard errors which are reported in Table II have no substantive metric interpretation in nanograms per milliliter. Indeed, the actual analyte values have been log transformed [x = ln(analyte ng/mL +1)] and these log transformed values have been entered into multilevel regression models and have generated parameters (b0 – b4) that are similar to beta weights seen in a variety of regression techniques.
In both model #1 and model #2 the 1-day and 1-week patch are significantly different from each other (Table II). However, in model #2, the 3-day patch also has significantly lower analyte levels than the 1-week patch. Comparing the analyte levels of the 2-week patch with the 1-week patch, only for BE is this patch significantly different from the 1-week patch (Parameter = 1.3, S.E. = 2.71). Figure 1 illustrates these relationships.
Summary
In summary, for all three analytes, the 1-day patch has higher analyte levels than the excluded short - term patches (model #1, b0). The 3-day patch has higher analyte levels than the 1-day patch (model #1, b2). The 1-week patch has higher levels than the 3-day patch (model #2, b3). For BE only, the 2-week patch has higher analyte levels than the 1-week (model #2, b4), and no significant difference was shown for COC or EME.
Limitations
The major limitation of these findings is that the study was a dose-uncontrolled field trial. As such, these findings are dependent on the average cocaine usage levels of this particular sample of cocaine and crack users and the purity of cocaine available on the street when the study was conducted.
On the other hand, there are advantages to testing sweat patches on actual users rather than limiting research to laboratory controlled dosing studies. Doses, modes of administration, and consumption patterns are more likely to match the applied settings in which sweat patches will be used.
Discussion
This article reports on a field trial of sweat patches as an alternative to urinalysis in testing for drugs of abuse, particularly cocaine. It is possible that sweat patches might be used in two ways: for short periods (hours or days) in a spot fashion very similar to urinalysis, or for longer periods (weeks), during which it is hoped that they will have a deterrent effect through long-term monitoring. Spot use might be employed in place of random urinalysis in the workplace if the minimum length of wear is short enough where a patch could be applied to a worker on arrival and removed at departure.
Analysis of patches of varying lengths of wear show that 1. cocaine is the dominant analyte found in sweat regardless of length of wear with BE second and EME third. 2. As length of wear increases, analyte levels increase up to one week. Only levels of BE increased significantly between one week and two weeks of wear, which is consistent with the metabolism of cocaine and subsequent secretion of BE in the sweat. 3. Because patches worn for 2 h or less had no detectable analyte levels, and patches worn for one day were significantly different from zero, the minimum length of wear of sweat patches in an active cocaine using sample is greater than 2 h and less than or equal to one day. Additional research is needed to narrow this window and to determine if parameters found in this sample are similar to those from other cocaine-use samples.
Acknowledgments
This research was supported by grant R41 DA09175 from the National Institute on Drug Abuse. Thanks to David Rindskopf for technical support on the use of the statistical technique called multilevel modeling.
References
- 1.Visher C. A Comparison of Urinalysis Technologies for Drug Testing in Criminal Justice. National Institute of Justice; Washington, D.C.: 1991. [Google Scholar]
- 2.Wolff K, Farrell M, Marsden J, Cone MG, Ali R, Welch S, Strang J. A review of biological indicators of illicit drug use, practical considerations and clinical usefulness. Addiction. 1999;94(9):1279–1298. doi: 10.1046/j.1360-0443.1999.94912792.x. [DOI] [PubMed] [Google Scholar]
- 3.Cone EJ. New developments in biological measures of drug prevalence. NIDA Res. Monogr. 1997;167:108–129. [PubMed] [Google Scholar]
- 4.Kintz P. Drug Testing in Hair. CRC Press; New York, NY: 1996. [Google Scholar]
- 5.Mieczkowski T. Drug Testing Technology. CRC Press; New York, NY: 1999. [Google Scholar]
- 6.Richter L, Cone PB. Current methods of assessing substance use: a review of strengths, problems, and developments. J. Drug Issues. 2001;31(4):809–832. [Google Scholar]
- 7.Wong SHY, Sunshine I, editors. The Handbook of Analytical Therapeutic Drug Monitoring and Toxicology. CRC Press; New York, NY: 1997. [Google Scholar]
- 8.Kreft I, DeLeeuw J. Introducing Multilevel Modeling. Sage Publications; Newbury Park, CA: 1998. [Google Scholar]
- 9.Bryk AS, Cone SW. Hierarchical Linear Models: Applications and Data Analysis Methods. Sage Publications; Newbury Park, CA: 1992. [Google Scholar]
- 10.Cone HC. Evaluating Medical Tests. Sage Publications; Newbury Park, CA: 1992. [Google Scholar]
- 11.Sunshine I, Cone JP. Sweat it out. In: Wong SHY, Sunshine I, editors. In The Handbook of Analytical Therapeutic Drug Monitoring and Toxicology. CRC Press; New York, NY: 1997. pp. 253–264. [Google Scholar]
- 12.Cone EJ, Hillsgrove M, Cone WD. Simultaneous measurement of cocaine, cocaethylene, their metabolites, and “crack” pyrolysis products by gas chromatography–mass spectrometry. Clin. Chem. 1994;40:1299–1305. [PubMed] [Google Scholar]
- 14.PharmChem Laboratories, Inc. Sweat Patch Drug Testing Pilot Program. PharmChem Laboratories; Menlo Park, CA: 1995. [Google Scholar]
- 15.Fogerson R, Fortner N, Warren M, Sutliff J, Cone HJ, Johnson B. Detection of cocaine and its metabolites using the PharmChek Sweat Patch; Paper presented at Society of Forensic Toxicologists Annual Meeting; Albuquerque, NM. 1996. [Google Scholar]
- 16.Kidwell D, Cone FP. Susceptibility of PharmChek drugs of abuse patch to environmental contamination. Forensic Sci. Int. 2001;116(23):89–106. doi: 10.1016/s0379-0738(00)00353-4. [DOI] [PubMed] [Google Scholar]
- 17.Cone BJ, Cone DR, Cone KM. Statistical Princi ples in Experimental Design. McGraw-Hill, Inc.; New York, NY: 1991. pp. 400–401. [Google Scholar]
- 18.Ambre J. The urinary excretion of cocaine and metabolites in humans: a kinetic analysis of published data. J. Anal. Toxicol. 1985;9:241–245. doi: 10.1093/jat/9.6.241. [DOI] [PubMed] [Google Scholar]
- 19.Cone RD, Hedeker D, Elkin I, Waternaux C, Cone HC, Cone JB, Shea T, Cone SD, Cone SM, Cone JT. Arch. Gen. Psychiatry. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]


