Abstract
Efforts to improve bone response to biomaterials have focused on ligands that bind α5β1 integrins. However, antibodies to α5β1 reduce osteoblast proliferation but do not affect differentiation when cells are grown on titanium (Ti). β1-silencing blocks the differentiation stimulus of Ti microtopography, suggesting that other β1 partners are important. Stably α2-silenced MG63 human osteoblast-like cells were used to test whether α2β1 specifically mediates osteoblast response to Ti surface micron-scale structure and energy. WT and α2-silenced MG63 cells were cultured on tissue culture polystyrene (TCPS) and Ti disks with different surface microtopographies: machined pretreatment (PT) surfaces [mean peak to valley roughness (Ra) < 0.02 μm], PT surfaces that were grit-blasted and acid-etched (SLA; Ra = 4 μm), and SLA with high surface energy (modSLA). Alkaline phosphatase (ALP), α2 and β1 mRNA, but not α5, αv, β3, type-I collagen, or osteocalcin, increased on SLA and modSLA at 6 days. α2 increased at 8 days on TCPS and PT, but remained unchanged on SLA and modSLA. α2-protein was reduced 70% in α2-siRNA cells, whereas α5-mRNA and protein were unaffected. α2-knockdown blocked surface-dependent increases in β1 and osteocalcin and decreases in cell number and increases in ALP and local factors typical of MG63 cells grown on SLA and modSLA [e.g., prostaglandin E2, osteoprotegerin, latent and active TGF-β1, and stimulatory effects of 1α,25(OH)2D3 on these parameters]. This finding indicates that α2β1 signaling is required for osteoblastic differentiation caused by Ti microstructure and surface energy, suggesting that conclusions based on cell behavior on TCPS are not predictive of behavior on other substrates or the mechanisms involved.
Keywords: α-2 integrin siRNA, MG63 human osteoblasts, titanium surface roughness
Titanium (Ti) and Ti alloys are commonly used as biomaterials because their surface properties provide a biocompatible interface with peri-implant tissues. Strategies for modifying the nature of this interface frequently involve changes to the surface, thereby affecting protein adsorption, cell–substrate interactions, and tissue development (1). A common modification has been to create micron-scale and submicron scale roughness. Preclinical and clinical studies (2–12) show that these surfaces support greater bone-to-implant contact than smooth surfaces.
How surface microstructure promotes an osteogenic response is an important question, because bone-forming osteoblasts preferentially colonize bone surfaces that have been preconditioned by bone-resorbing osteoclasts (13), resulting in complex micron-scale and submicron-scale morphologies (14). In vitro experiments using model surfaces indicate that migration, growth, and colony morphology of rat bone marrow cells (15) and osteoblasts (16–18) are sensitive to microstructure. These observations suggest that structural elements can modulate the spatial organization of cells and their ECM.
The topography of osteoclast resorption pits in bone can be modeled by using Ti substrates that have been grit-blasted and acid-etched (13). Osteoblasts exhibit a more differentiated phenotype when grown on such surfaces (see refs. 19 and 20 for reviews), resulting in a complex osteoblast/ECM/biomaterial interface that exhibits greater adhesion power than is seen on smoother surfaces (21). Enhanced osteoblast differentiation is also seen on electron micromachined substrates that have both micron scale and submicron scale structural elements (22, 23). In addition, cells on microstructured surfaces produce increased levels of factors that inhibit osteoclast activity, including TGF-β1 and osteoprotegerin (OPG) (24, 25), suggesting that increased bone formation seen in vivo is caused not only by enhanced osteoblastic activity but also by decreased bone resorption.
Surface chemistry and energy also play roles (26). Greater bone formation is found around microstructured implant surfaces that have been modified to have high surface energy (modSLA) than around implants with the same topography but with a more hydrophobic surface (SLA) (27). In vitro, osteoblasts are more differentiated when grown on modSLA than on SLA and there is a marked increase in the prostaglandin E2 (PGE2), TGF-β1, and OPG content of the conditioned media. Response to systemic hormones is also affected by surface topography and surface energy. The vitamin D metabolite 1,25(OH)2D3 increases osteocalcin production by osteoblasts cultured on tissue culture polystyrene (TCPS), but the effect of the hormone is greater when cells are grown on smooth Ti disks, greater yet on SLA substrates, and even more pronounced on modSLA (28).
These studies indicate that osteoblast behavior is sensitive to surface properties and that this can translate into improved performance in vivo, but they do not explain why these responses occur. Differences in surface chemistry and energy can affect adsorption of serum proteins (29), including fibronectin (30), which can alter cell attachment (31, 32). Microtopography also alters osteoblast attachment to a substrate (33), although surface chemistry may be a more critical variable for many materials (34). Although initial attachment can influence the number of cells that can occupy a given surface, it does not appear to be correlated with the long-term adhesion of osteoblasts to the surface once they produce their ECM (35). Moreover, there appear to be surface-specific differences in ECM organization and mineralization (36, 37), suggesting that different properties mediate initial attachment and adhesion, proliferation, and ultimately, differentiation.
Osteoblasts interact with their substrate via integrin binding to ECM proteins, leading investigators to use specific peptide motifs to increase attachment and adhesion of cells to implants and tissue engineering scaffolds based on the behavior of these cells when grown on TCPS (38, 39). However, integrin expression is substrate-sensitive (40, 41); thus assumptions about cell behavior based on TCPS may not be relevant for cells on implant materials (37, 42). Osteoblasts express primarily α5β1 when grown on TCPS, but they shift to expression of α2β1 when grown on Ti and Ti-6Al-4V (43, 44). The consequences of this shift to the cell are not well understood, nor is it known whether integrin expression is sensitive to surface morphology or surface energy. Although α5β1, which binds the RGD motif in fibronectin (45), is involved in differentiation of osteoblasts on TCPS (46), it may not play as important a role in determining cell response when cells are grown on more clinically relevant biomaterials like Ti and may promote cell attachment and proliferation at the expense of osteoblast differentiation (47).
Targeted knockdown of the β1 integrin subunit in osteoblasts indicates that integrin subunits that partner with β1 might be involved in the response of osteoblasts to Ti surface microstructure (48). Specific antibody inhibition of α5β1 binding reduced cell attachment but did not block osteoblast differentiation (49). In contrast, antibodies to α2β1 reduced osteoblast differentiation, suggesting a role for α2. Moreover, β1 and α2 mRNAs were increased when osteoblasts were grown on Ti substrates rather than TCPS, whereas α5 expression was unaffected (43). 1α,25(OH)2D3 further increased α2 and β1 mRNAs in cells cultured on Ti, but had no effect on α5. Others have shown that α2 is required for activation of the transcription factor RUNX2, subsequent expression of the osteoblast markers osteocalcin and bone sialoprotein in osteoblasts cultured on TCPS (50), and ECM mineralization (15), further supporting a role for α2β1. The present study tested the hypothesis that α2 expression is regulated by surface structure and surface energy and is required for the effects of these surfaces on osteoblast differentiation.
Results
Cell Culture Model.
MG63 cells (American Type Culture Collection) were used for this study. They are a well characterized osteoblast-like cell culture model for assessing responses to Ti surface microstructure (24, 51) and surface energy (28). Observations using MG63 cells have been confirmed by using normal human osteoblasts (52), normal mouse calvarial osteoblasts, fetal rat calvarial cells, and other osteoblast cell lines (53), and the results correlate with clinical performance in animals and humans (3–5, 7, 8).
For the experiments described below, cells were cultured on Ti substrates (15-mm diameter) that were fabricated by Institut Straumann AG (28). The pretreatment (PT) surface has a mean peak to valley roughness (Ra) of 0.2 μm. PT surfaces were sand-blasted and acid-etched to produce SLA surfaces (Ra = 3.2 μm). Before use, PT and SLA surfaces were washed in an ultrasonic cleaner and sterilized in an oxygen plasma (PDC-32G; Harrick Plasma). After SLA processing, modSLA disks were kept in an N2 atmosphere and stored in sealed glass tubes containing isotonic NaCl, retaining surface hydrophilicity. These sealed disks were sterilized by gamma irradiation at 25 kGy overnight.
Surface topography and cell morphology on the surface have been published (17). SLA and modSLA surfaces have identical morphologies consisting of overlapping craters (100-μm diameter) overlaid with small pits (1- to 3-μm diameter). The acid-etch produces submicron scale spikes that are ≈700 nm in height, but because of the underlying craters the overall roughness is micron scale. Structural elements of the SLA and modSLA surfaces and the surface chemistry, including x-ray photoelectron spectroscopy (XPS) and Auger analyses, have also been published (54). SLA surface energy is hydrophobic, whereas modSLA approaches zero.
Integrin Expression.
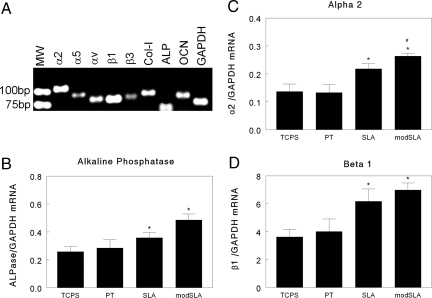
Cells were harvested at 6 days (confluence) and 8 days (postconfluence) to assess effects of time on mRNA expression. RT-PCR of mRNA from confluent cultures of MG63 cells demonstrated expression of genes for α2, α5, αv, β1, and β3 integrin subunits and alkaline phosphatase (ALP), osteocalcin, and type I collagen (Fig. 1A). Real-time PCR of mRNA from day-6 cultures showed that ALP expression was increased on SLA and further increased on modSLA (Fig. 1B). In contrast, type I collagen and osteocalcin mRNAs were comparable on all surfaces (data not shown). Only α2 and β1 exhibited surface-dependent differences in expression. α2 was increased on SLA and further increased on modSLA (Fig. 1C); β1 was increased on SLA, but no further increase was observed on modSLA (Fig. 1D).
Fig. 1.
Effects of surface microstructure and energy on mRNA expression in osteoblast-like MG63 cells cultured for 6 days on TCPS, PT, SLA, and modSLA. (A) RT-PCR of mRNA isolated from cells grown on TCPS demonstrating expression of integrin subunits α2, α5, αv, β1, and β3 and collagen type I (Col-I), ALP, osteocalcin (OCN), and glyceraldehyde phosphate dehydrogenase (GAP). (B) ALP mRNAs normalized to GAPDH. (C) α2 normalized to GAPDH. (D) β1 normalized to GAPDH. *, P < 0.05, Ti surface vs. TCPS. #, P < 0.05, modSLA vs. PT.
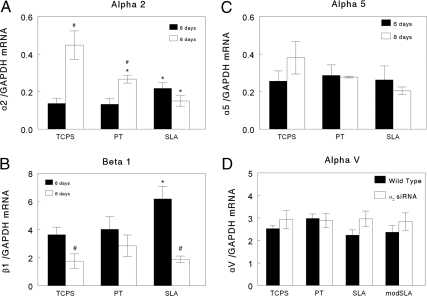
Integrin expression varied as a function of time in a surface-dependent manner. At 8 days α2 mRNA increased when cells were grown on TCPS and to a lesser extent on PT (Fig. 2A). However, α2 mRNAs did not change with time on SLA, resulting in lower levels at day 8 in comparison with cells grown on the smooth TCPS and Ti surfaces. β1 mRNA was reduced on all surfaces at day 8 in comparison to levels at day 6 (Fig. 2B). α5, αv [supporting information (SI) Fig. S1], and β3 (data not shown) did not change with time.
Fig. 2.
Effect of culture age on integrin expression in MG63 cells grown for 6 and 8 days on TCPS and Ti substrates (PT, SLA, modSLA) as a function of microtopography and surface energy. (A) α2. (B) β1. (C) α5. (D) αv. *, P < 0.05, Ti vs. TCPS; #, P < 0.05, SLA vs. modSLA.
Surface Effects Require α2.
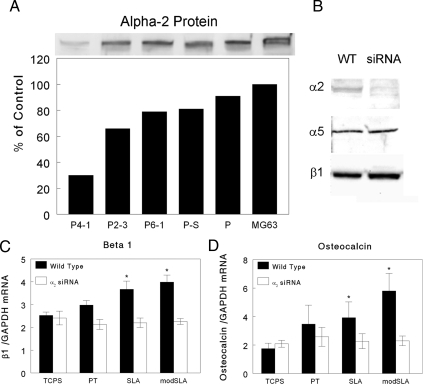
The siRNA strategy was successful and generated plasmids that reduced levels of α2 protein in the MG63 cells (Fig. 3A). Transfection using an empty vector reduced α2 protein levels <10% and plasmids containing the scrambled siRNA reduced α2 protein <20%. Of the three siRNA plasmids tested, plasmid P4–1 caused the greatest reduction in α2 protein (70%). P4–1 had no effect on α5 or β1 protein based on Western blots (Fig. 3B). P4–1-treated cells exhibited reduced adhesion to collagen-coated TCPS in a centrifugation assay, indicating that the α2 knockdown was effective (Fig. S2). As the collagen concentration was increased, adhesion of WT cells increased and this adhesion was blocked by antibodies to α2. Only 4% of the α2-silenced cells remained adherent regardless of collagen concentration and this adherence was further reduced by antibodies to α2. Based on these results, adherence–1 was selected for subsequent studies.
Fig. 3.
Effect of α2 siRNA on integrin subunit protein levels and substrate-dependent mRNA expression in MG63 cells. (A) MG63 cells were transfected with one of three plasmids containing siRNA for α2 (P4–1, P2–3, and P6–1), scrambled siRNA (P-S) or plasmid alone (P), and α2 protein levels were determined by Western blot. Data are expressed as a percent of α2 in nontransfected MG63 cells. (B) Western blots showing α2, α5, and β1 protein levels in the P4–1 stably transfected cell line and untransfected MG63 cells. (C) β1 mRNA in WT and α2-silenced MG63 cells cultured for 7 days on each substrate. (D) Effect of α2 silencing on osteocalcin mRNA. *, P < 0.05, Ti surface vs. TCPS.
To examine effects of α2 silencing, cells were cultured for 7 days to correspond to the experiments assessing the effects of 1α,25(OH)2D3 treatment. α2-silenced cells did not exhibit the surface-dependent increases in β1 RNA seen in WT cells (Fig. 3C). mRNAs for α5 and αv were comparable to WT MG63 cells regardless of substrate (Fig. S3). β1 mRNA levels in WT cells were higher on SLA and modSLA compared with either TCPS or PT. β3 mRNAs were variable from experiment to experiment, but overall, no change as a function of surface or siRNA was observed (Fig. S3). Type I collagen mRNA was comparable on all substrates and was unaffected by the presence of the α2 siRNA (Fig. S3). In contrast, the surface-dependent increase in osteocalcin mRNA seen in WT cells was lost in silenced cells (Fig. 3D).
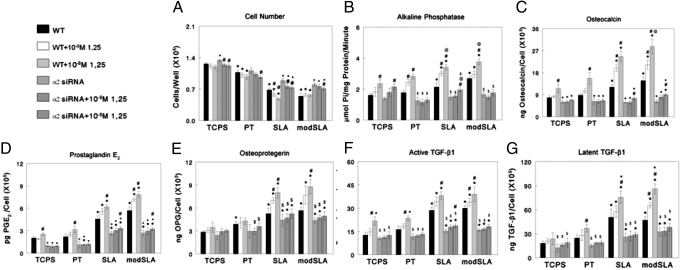
Cell number was substrate-dependent (Fig. 4A), with fewer cells on all Ti surfaces than on TCPS (TCPS > PT > SLA > modSLA). α2 silencing increased cell numbers on all surfaces, including TCPS, which was greatest on SLA and modSLA. Inhibitory effects of 1α,25(OH)2D3 on cell number were evident in α2 knockdown cells on all surfaces.
Fig. 4.
Effect of α2 knockdown on response to surface microstructure and surface energy. At day 6, MG63 cells were treated for 24 h with media containing vehicle or 10−9 or 10−8 1α,25(OH)2D3. Cell number (A), ALP in cell layer lysates (B), osteocalcin (C), PGE2 (D), OPG (E), active TGF-β1 (F), and latent TGF-β1 (G) were determined. *, P < 0.05, Ti vs. TCPS; ●, P < 0.05, α2 siRNA vs. WT on each substrate; #, P < 0.05, with 1α,25(OH)2D3 vs. no 1α,25(OH)2D3.
Effects on mRNA were reflected in phenotypic expression. WT MG63 cells had increased ALP-specific activity on SLA and modSLA, and 1α,25(OH)2D3 caused a dose-dependent increase on all surfaces (Fig. 4B). α2-silenced cells behaved like WT cells when grown on TCPS, but when grown on Ti activity was reduced compared with that seen in WT cells on the same surface. Moreover, response to 1α,25(OH)2D3 was blocked. Osteocalcin was affected in a similar manner (Fig. 4C). At the highest concentration of 1α,25(OH)2D3 in α2-silenced cells, there was a small increase in osteocalcin on SLA and modSLA, but levels remained below that seen in WT cells without 1α,25(OH)2D3.
PGE2 was increased on SLA and modSLA compared with TCPS or PT, and the stimulatory effect of 1α,25(OH)2D3 was greater (Fig. 4D). α2 siRNA reduced PGE2 on all surfaces, abrogated the stimulatory effect of 1α,25(OH)2D3 on TCPS and PT, and reduced the effect of 1α,25(OH)2D3 on SLA and modSLA. Similar results were seen when measuring levels of OPG (Fig. 4E), latent TGF-β1 (Fig. 4F), and active TGF-β1 (Fig. 4G).
Discussion
Previously we showed that β1 is required for osteoblastic differentiation on Ti substrates with micron-scale roughness (48). Studies examining cells on TCPS (49, 55, 56) identified α5 as the integrin partner for β1 in signaling osteoblasts to differentiate. However, antibodies to α5β1 did not affect differentiation of MG63 cells grown on Ti with rough microtopographies (48), suggesting an alternate partner was involved. The present study demonstrates clearly the importance of α2 integrin subunits in mediating the differentiation of osteoblasts in response to Ti surface microstructure and surface energy.
We previously noted that MG63 cells exhibit increased expression of α2 and β1 but not α5 when cultured on Ti compared with TCPS (43). Here, we report that microstructure can modulate α2 and β1 expression and that surface energy also plays a regulatory role. Although mRNAs for α2 and its partner β1 were increased in confluent MG63 cells on microstructured SLA and modSLA, expression of α5, αv, and β3 were unaffected. The importance of α2β1 is underscored by the observation that knockdown of α2 had only minor effects when cells were grown on TCPS or smooth PT, primarily on the stimulatory effect of 1α,25(OH)2D3 on osteocalcin, active TGF-β1, and PGE2, but silencing blocked the effects of surface roughness on all parameters.
Our results also show that the apparent effects of surface properties on α2β1 integrin expression are time-dependent and that α2β1 is required for differentiation on all surfaces in a time-dependent manner. α2 mRNA levels were increased on SLA at 6 days and on TCPS and PT at 8 days, suggesting that its role in differentiation was comparable, but delayed on the smooth substrates. In support of this finding, ALP mRNAs were elevated to a greater extent at 6 days on SLA and modSLA but no surface-dependent differences in osteocalcin mRNAs were noted, suggesting that the cells were at an early stage of osteoblast differentiation, particularly evident in cells grown on TCPS and PT. Similarly, at 7 days, osteocalcin mRNA was elevated in the SLA and modSLA cultures, indicating that cells grown on those substrates were now at a later state of osteoblast differentiation. We did not specifically address changes in mRNA levels between days 6 and 7 and 8, but collectively our results support the hypothesis that α2 is important as MG63 cells transition to a more differentiated phenotype. Moreover, the structural and chemical properties of the SLA and modSLA substrates cause this transition to occur more rapidly, potentially by affecting the cytoskeleton and downstream gene transcription. No evidence of a surface-dependent difference in α2 mRNA was seen in 7-day cultures, supporting the reduced levels in α2 mRNA observed at 8 days.
Cell proliferation did not depend on α2β1, although the number of cells present at 7 days was increased in the α2 knockdown cells on all substrates. One possibility is that the reduction in α2 freed β1 to partner with α5. Interestingly, expression of α5 was not sensitive to surface properties, but knockdown of α2 reduced the surface-dependent increase in β1 mRNA to levels typical of cells grown on TCPS or PT. Thus, although β1 was reduced, there was still a sufficient amount of the integrin subunit to partner with α5, particularly in the relative absence of α2. We previously showed that attachment of MG63 cells to both PT and SLA, and the activation of focal adhesion kinase were mediated by α5β1 (49). In the present study, we saw the greatest siRNA-dependent increase in cell number on modSLA surfaces. It is likely that this increase was a result of greater involvement of α5β1 caused by enhanced adsorption of fibronectin to the modSLA surface as a function of its higher surface energy (55).
Cell attachment to type I collagen mediated by α2β1 may have been a factor in determining cell response to Ti microstructure. Antibodies to α2 blocked initial adhesion to type I collagen-coated TCPS surfaces as did knockdown of α2 protein, confirming that this integrin was functional. Expression of α2 and β1 increased as the microstructure of the Ti surface became more complex. Why this was the case is not known. ECM production is increased on rougher Ti surfaces, and at least some of this increase is caused by an increase in collagen synthesis (51). Others have shown that ECM organization and adhesion strength of osteoblast colonies to their substrate are increased when cells are grown on grit-blasted Ti surfaces (35, 37, 57). Thus, the cells may use α2β1 to anchor to the collagen-rich matrix, resulting in a more stable construct in vitro and in vivo. This hypothesis is supported by studies demonstrating enhanced osteoblastic differentiation of MC-3T3-E1 cells grown on TCPS coated with a collagen peptide consisting of the α2β1 binding motif, GFOGER (58) and enhanced peri-implant bone formation associated with Ti implants coated with GFOGER (59).
α2 may not be required for sustaining the differentiation cascade. By 8 days in culture, α2 integrin expression was already reduced on Ti surfaces, particularly on SLA. Moreover, β1 expression decreased to levels on SLA that were comparable to levels on TCPS and PT. In contrast, α2 integrin mRNAs were increased in cells grown on TCPS for 8 days, consistent with previous observations showing that differentiation is delayed or reduced in cultures grown on traditional cell culture materials (51). α2 knockdown experiments support this idea. Loss of α2 resulted in loss of the enhanced differentiation observed on SLA and modSLA. Moreover, reduced α2 resulted in loss of the release of growth factors associated with growth on these substrates and in reduced responsiveness to 1α,25(OH)2D3, which reflects the lower state of phenotypic maturation, more typical of cells grown on TCPS.
Certainly, integrins other than α2β1 are involved and may modulate the end result through cross-talk (60, 61). Brugge et al. (62) reported a shift in integrin expression in osteoblasts that were cultured on a variety of substrates at 7 and 8 days postseeding. Whether one or more of these participated in the response of osteoblasts to surface microstructure or chemistry is not known. mRNA levels for αv and β3, which partner to bind the ECM protein vitronectin, were unaffected by substrate surface or time, suggesting that they do not mediate the surface-dependent effects on osteoblast differentiation, and others have shown that bone mineralization and osteoblast differentiation are negatively modulated by αvβ3 (63).
In summary, this study demonstrates that the α2β1 integrin plays an important role in determining osteoblast behavior on Ti implants and that this role increases as the surface micron-scale and submicron-scale structure becomes more complex. Integrin binding initiates the differentiation cascade, but once the cascade is begun, high levels of α2 may not be required. Cross-talk between the α2β1 signaling cascade and signaling induced by 1α,25(OH)2D3 further enhance phenotypic differentiation. Loss of α2 blocks this cross-talk, most likely by reducing osteogenic maturation, resulting in cells that are less sensitive to this vitamin D metabolite.
These observations suggest that tissue engineering strategies for peri-implant bone formation that focus on the α5β1 integrin via binding to RGD motifs (64, 65) may not yield optimal results, particularly when used in combination with microrough topographies. Recently, the GFOGER peptide present in type I collagen, which binds α2β1 integrins (66), was shown to be effective at enhancing peri-implant osteogenesis in vitro and in vivo (67, 68), supporting the hypothesis that this α2β1 signaling is an important target for stimulating an osteogenic response. The present study suggests that enhanced osteogenesis via α2β1 signaling can also be accomplished by optimizing surface topography and chemistry.
Methods
Cells were seeded at 15,000 cells per well and cultured in DMEM containing 10% FBS and 1% penicillin and streptomycin at 37°C in an atmosphere of 5% CO2 and 100% humidity. Osteoblasts do not conform to the surface but anchor to the surface via cytoplasmic extensions across rough regions (22, 23); thus we did not correct for differences in surface area.
Assessment of Integrin mRNA Levels.
RNA was extracted by using Qiagen's RNeasy mini kit and reverse-transcribed by using the Qiagen-Omniscript RTkit as per the manufacturer's directions. RT-PCR and real-time PCR were performed for osteocalcin [National Center for Biotechnology Information (NCBI) accession no. NM_000711], ALP (NCBI accession no. NM_000478), collagen type I mRNA (NCBI accession no. NM_000088), α2 (NCBI accession no. NM_002203), α5 (NCBI accession no. NM_002205), αv (NCBI accession no. NM_002210), β1 (NCBI accession no. NM_002211), and β3 (NCBI accession no. NM_000212). α2β1 specifically binds collagen I; α5β1 binds fibronectin; and αvβ3 binds vitronectin (32). Optimal oligonucleotide primers were designed by using Primer Express 2.0 software and purchased from Sigma–Genosys. Agarose gels (1%) demonstrated the presence of the genes of interest. Real-time PCR was performed by using ABI Prism 7000 (Agentek; Applied Biosystem Laboratories) and ABI Prism 7000 SDS version 1.1 software. Data were normalized to the endogenous reference gene GAPDH (NCBI accession no. NM_002046).
siRNA Knockdown of α2.
Coding sequences were determined empirically, and candidate sequences were analyzed by Blast search to avoid significant sequence homologies with other genes. The α2 integrin siRNA targets 21 bases starting at base 3406 of the α2 gene (NCBI accession no. NM_002203.3). Thus, the antisense sequence for α2 integrin siRNA was: ACA AGG AAG TTA GCA CGT GCC TAA GCC ACG TGC TAA CTT CCT TGT AAA AAG ATC. Scrambled α2 sequences served as a negative control. A pSuppressorNeo vector containing a U6 promoter with a GeneSupressorTM system (IMGENEX) was used per the manufacturer's directions.
Plasmids were screened as a function of α2 protein production based on Western blots of homogenates of silenced cells (30 μg protein per lane) by using antibodies to α2 (Millipore). MG63 cells were transfected with one of three plasmids containing the α2 siRNA template. Controls included cells treated with empty vector and plasmid containing scrambled siRNA. Based on these results, two cell lines were selected for these studies: MG63-α2 cells, transfected with plasmid P4–1, and MG63-α2S cells, which contained the scrambled siRNA plasmid and exhibited the same α2 levels as nontransfected MG63 cells and MG63 cells treated with empty plasmid or the transfection medium. Therefore in subsequent studies we compared the effects of silencing with the P4–1 plasmid to WT cells directly. Permanent cell lines were established by using the antibiotic G418 (Invitrogen). To verify the effectiveness of the knockdown strategy, a centrifugation assay was used to assess the ability of the α2-silenced cells to adhere to type I collagen (58). Detailed methods and data are provided in Fig. S2.
Effects of α2 Knockdown on Osteoblast Response to Ti Substrates.
To assess substrate-dependent effects of α2 knockdown on integrin expression, Western blots were probed with antibodies to α2 (Millipore), α5, and β1 (Santa Cruz Biotechnology) integrin subunits. mRNAs for α5, αv, β1, and β3 and collagen type I and osteocalcin were determined by real-time PCR for WT and α2 knockdown cells. Effects on cell response were determined by treating confluent (6 days) MG63 cells and MG63-α2 cells for 24 h with 10−9 M or 10−8 M 1α,25(OH)2D3 (Biomol International). Cells were harvested 24 h later by two sequential trypsin digestions, which release all cells from the Ti substrates (51), and the total number of cells on each disk was determined. ALP-specific activity was measured in cell lysates (51). Conditioned media were examined for osteocalcin (Human Osteocalcin RIA Kit; Biomedical Technologies), active and latent TGF-β1 (G7591 TGF-β1 Emax Immunoassay System; Promega), PGE2 (NEK020A Prostaglandin E2 RIA kit; PerkinElmer), and OPG (DY805 Osteoprotegerin DuoSet; R&D Systems) as described (24, 25).
Statistical Analysis.
For experiments examining phenotype (cell number, ALP, and media OPG, osteocalcin, PGE2, and active and latent TGF-β1) or cell adhesion, each data point represents the means ± SE for six separate cultures. For experiments assessing changes in integrin expression, total RNA was extracted from the combined cells from three cultures, and two separate replicate real-time PCR runs were performed for each of these samples. Six samples were analyzed for each surface (3 disks/sample × 6 samples = 18 disks per variable). All experiments were repeated to ensure validity of the results. Data were first analyzed by ANOVA; when statistical differences were detected, Student's t test for multiple comparisons using Bonferroni's modification was used. P ≤ 05 was considered to be significant.
Supplementary Material
Acknowledgments.
This research was supported by Public Health Service Grant AR052102, National Science Foundation Grant EEC 9731643, the ITI Foundation (Basel, Switzerland), and Children's Healthcare of Atlanta. Institut Straumann AG provided the Ti disks used in this study.
Footnotes
Conflict of interest statement: M.W. is an employee of Institut Straumann AG, which supplied the titanium disks used in this study.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805420105/DCSupplemental.
References
- 1.Sousa SR, Lamghari M, Sampaio P, Moradas-Ferreira P, Barbosa MA. Osteoblast adhesion and morphology on TiO2 depends on the competitive preadsorption of albumin and fibronectin. J Biomed Mater Res A. 2008;84:281–290. doi: 10.1002/jbm.a.31201. [DOI] [PubMed] [Google Scholar]
- 2.Albrektsson T, Branemark PI, Hansson HA, Lindstrom J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52:155–170. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- 3.Cochran DL, et al. The use of reduced healing times on ITI implants with a sandblasted and acid-etched (SLA) surface: Early results from clinical trials on ITI SLA implants. Clin Oral Implants Res. 2002;13:144–153. doi: 10.1034/j.1600-0501.2002.130204.x. [DOI] [PubMed] [Google Scholar]
- 4.Roccuzzo M, Bunino M, Prioglio F, Bianchi SD. Early loading of sandblasted and acid-etched (SLA) implants: A prospective split-mouth comparative study. Clin Oral Implants Res. 2001;12:572–578. doi: 10.1034/j.1600-0501.2001.120604.x. [DOI] [PubMed] [Google Scholar]
- 5.Buser D, et al. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25:889–902. doi: 10.1002/jbm.820250708. [DOI] [PubMed] [Google Scholar]
- 6.Li D, et al. Biomechanical comparison of the sandblasted and acid-etched and the machined and acid-etched titanium surface for dental implants. J Biomed Mater Res. 2002;60:325–332. doi: 10.1002/jbm.10063. [DOI] [PubMed] [Google Scholar]
- 7.Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: A histometric study in the canine mandible. J Biomed Mater Res. 1998;40:1–11. doi: 10.1002/(sici)1097-4636(199804)40:1<1::aid-jbm1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.Buser D, et al. Interface shear strength of titanium implants with a sandblasted and acid-etched surface: A biomechanical study in the maxilla of miniature pigs. J Biomed Mater Res. 1999;45:75–83. doi: 10.1002/(sici)1097-4636(199905)45:2<75::aid-jbm1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.McDermott NE, Chuang SK, Woo VV, Dodson TB. Maxillary sinus augmentation as a risk factor for implant failure. Int J Oral Maxillofac Implants. 2006;21:366–374. [PubMed] [Google Scholar]
- 10.Graziani F, Donos N, Needleman I, Gabriele M, Tonetti M. Comparison of implant survival following sinus floor augmentation procedures with implants placed in pristine posterior maxillary bone: A systematic review. Clin Oral Implants Res. 2004;15:677–682. doi: 10.1111/j.1600-0501.2004.01116.x. [DOI] [PubMed] [Google Scholar]
- 11.Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dental implants: A systematic review. Ann Periodontol. 2003;8:328–343. doi: 10.1902/annals.2003.8.1.328. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz Z, et al. Micron-scale roughness on the surface of Ti6Al4V pedicle screws enhances osteoblast differentiation in vitro and osteointegration in sheep spine in vivo. J Bone Joint Surg Am. 2008 doi: 10.2106/JBJS.G.00499. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyan BD, et al. Pretreatment of bone with osteoclasts affects phenotypic expression of osteoblast-like cells. J Orthop Res. 2003;4:638–647. doi: 10.1016/S0736-0266(02)00261-9. [DOI] [PubMed] [Google Scholar]
- 14.Davies JE. Understanding peri-implant endosseous healing. J Dent Educ. 2003;67:932–949. [PubMed] [Google Scholar]
- 15.Ricci JL, Grew JC, Alexander H. Connective-tissue responses to defined biomaterial surfaces. I. Growth of rat fibroblast and bone marrow cell colonies on microgrooved substrates. J Biomed Mater Res A. 2008;85:313–325. doi: 10.1002/jbm.a.31379. [DOI] [PubMed] [Google Scholar]
- 16.Sader MS, Balduino A, Soares GA, Borojevic R. Effect of three distinct treatments of titanium surface on osteoblast attachment, proliferation, and differentiation. Clin Oral Implants Res. 2005;16:667–675. doi: 10.1111/j.1600-0501.2005.01135.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Raines AL, Wieland M, Schwartz Z, Boyan BD. Requirement for both micron- and submicron-scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials. 2007;28:2821–2829. doi: 10.1016/j.biomaterials.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lumbikanonda N, Sammons R. Bone cell attachment to dental implants of different surface characteristics. Int J Oral Maxillofac Implants. 2001;16:627–636. [PubMed] [Google Scholar]
- 19.Boyan BD, et al. Mechanisms involved in osteoblast response to implant surface morphology. Annu Rev Mater Res. 2001;31:357–371. [Google Scholar]
- 20.Boyan BD, et al. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur Cell Mater. 2003;6:22–27. doi: 10.22203/ecm.v006a03. [DOI] [PubMed] [Google Scholar]
- 21.Anselme K, Bigerelle M. Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomater. 2005;1:211–222. doi: 10.1016/j.actbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Zinger O, et al. Differential regulation of osteoblasts by substrate microstructural features. Biomaterials. 2005;26:1837–1847. doi: 10.1016/j.biomaterials.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Zhao G, et al. Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin Oral Implants Res. 2006;17:258–264. doi: 10.1111/j.1600-0501.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 24.Kieswetter K, et al. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J Biomed Mater Res. 1996;32:55–63. doi: 10.1002/(SICI)1097-4636(199609)32:1<55::AID-JBM7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 25.Lossdorfer S, et al. Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J Biomed Mater Res A. 2004;70:361–369. doi: 10.1002/jbm.a.30025. [DOI] [PubMed] [Google Scholar]
- 26.Kilpadi DV, Lemons JE. Surface energy characterization of unalloyed titanium implants. J Biomed Mater Res. 1994;28:1419–1425. doi: 10.1002/jbm.820281206. [DOI] [PubMed] [Google Scholar]
- 27.Buser D, et al. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83:529–533. doi: 10.1177/154405910408300704. [DOI] [PubMed] [Google Scholar]
- 28.Zhao G, et al. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005;74:49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]
- 29.Rupp F, Axmann D, Ziegler C, Geis-Gerstorfer J. Adsorption/desorption phenomena on pure and Teflon AF-coated titania surfaces studied by dynamic contact angle analysis. J Biomed Mater Res. 2002;62:567–578. doi: 10.1002/jbm.10198. [DOI] [PubMed] [Google Scholar]
- 30.Michael KE, et al. Adsorption-induced conformational changes in fibronectin due to interactions with well defined surface chemistries. Langmuir. 2003;19:8033–8040. [Google Scholar]
- 31.Moursi AM, et al. Fibronectin regulates calvarial osteoblast differentiation. J Cell Sci. 1996;109:1369–1380. doi: 10.1242/jcs.109.6.1369. [DOI] [PubMed] [Google Scholar]
- 32.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667–681. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 33.Sader MS, Balduino A, Soares GA, Borojevic R. Effect of three distinct treatments of titanium surface on osteoblast attachment, proliferation, and differentiation. Clin Oral Implants Res. 2005;16:667–675. doi: 10.1111/j.1600-0501.2005.01135.x. [DOI] [PubMed] [Google Scholar]
- 34.Anselme K, Bigerelle M. Statistical demonstration of the relative effect of surface chemistry and roughness on human osteoblast short-term adhesion. J Mater Sci Mater Med. 2006;17:471–479. doi: 10.1007/s10856-006-8475-8. [DOI] [PubMed] [Google Scholar]
- 35.Bigerelle M, Anselme K. Bootstrap analysis of the relation between initial adhesive events and long-term cellular functions of human osteoblasts cultured on biocompatible metallic substrates. Acta Biomater. 2005;1:499–510. doi: 10.1016/j.actbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Cooper LF, Masuda T, Whitson SW, Yliheikkila P, Felton DA. Formation of mineralizing osteoblast cultures on machined, titanium oxide grit-blasted, and plasma-sprayed titanium surfaces. Int J Oral Maxillofac Impl. 1999;14:37–47. [PubMed] [Google Scholar]
- 37.Saruwatari L, et al. Osteoblasts generate harder, stiffer, and more delamination-resistant mineralized tissue on titanium than on polystyrene, associated with distinct tissue micro- and ultrastructure. J Bone Miner Res. 2005;20:2002–2016. doi: 10.1359/JBMR.050703. [DOI] [PubMed] [Google Scholar]
- 38.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 39.Shibata Y, Hosaka M, Kawai H, Miyazaki T. Glow discharge plasma treatment of titanium plates enhances adhesion of osteoblast-like cells to the plates through the integrin-mediated mechanism. Int J Oral Maxillofac Implants. 2002;17:771–777. [PubMed] [Google Scholar]
- 40.Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–798. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taite LJ, et al. Bioactive hydrogel substrates: Probing leukocyte receptor-ligand interactions in parallel plate flow chamber studies. Ann Biomed Eng. 2006;34:1705–1711. doi: 10.1007/s10439-006-9173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmad M, McCarthy MB, Gronowicz G. An in vitro model for mineralization of human osteoblast-like cells on implant materials. Biomaterials. 1999;20:211–220. doi: 10.1016/s0142-9612(98)00152-5. [DOI] [PubMed] [Google Scholar]
- 43.Raz P, et al. 1α,25(OH)2D3 regulation of integrin expression is substrate dependent. J Biomed Mater Res A. 2004;71:217–225. doi: 10.1002/jbm.a.30134. [DOI] [PubMed] [Google Scholar]
- 44.Siebers MC, ter Brugge PJ, Walboomers XF, Jansen JA. Integrins as linker proteins between osteoblasts and bone replacing materials: A critical review. Biomaterials. 2005;26:137–146. doi: 10.1016/j.biomaterials.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 45.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 46.Garcia AJ, Keselowsky BG. Biomimetic surfaces for control of cell adhesion to facilitate bone formation. Crit Rev Eukaryotic Gene Expression. 2002;12:151–162. doi: 10.1615/critreveukaryotgeneexpr.v12.i2.50. [DOI] [PubMed] [Google Scholar]
- 47.Triplett RG, Frohberg U, Sykaras N, Woody RD. Implant materials, design, and surface topographies: Their influence on osseointegration of dental implants. J Long Term Eff Med Implants. 2003;13:485–501. doi: 10.1615/jlongtermeffmedimplants.v13.i6.50. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, et al. Integrin β1 silencing in osteoblasts alters substrate-dependent responses to 1,25-dihydroxy vitamin D3. Biomaterials. 2006;27:3716–3725. doi: 10.1016/j.biomaterials.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 49.Keselowsky BG, Wang L, Schwartz Z, Garcia AJ, Boyan BD. Integrin α5 controls osteoblastic proliferation and differentiation responses to titanium substrates presenting different roughness characteristics in a roughness-independent manner. J Biomed Mater Res A. 2007;80:700–710. doi: 10.1002/jbm.a.30898. [DOI] [PubMed] [Google Scholar]
- 50.Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the α2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273:32988–32994. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 51.Martin JY, et al. Effect of titanium surface-roughness on proliferation, differentiation, and protein-synthesis of human osteoblast-like cells (MG63) J Biomed Mater Res. 1995;29:389–401. doi: 10.1002/jbm.820290314. [DOI] [PubMed] [Google Scholar]
- 52.Lohmann CH, et al. Response of normal female human osteoblasts (NHOst) to 17 β-estradiol is modulated by implant surface morphology. J Biomed Mater Res. 2002;62:204–213. doi: 10.1002/jbm.10290. [DOI] [PubMed] [Google Scholar]
- 53.Lohmann CH, et al. Maturation state determines the response of osteogenic cells to surface roughness and 1,25-dihydroxyvitamin D3. J Bone Min Res. 2000;15:1169–1180. doi: 10.1359/jbmr.2000.15.6.1169. [DOI] [PubMed] [Google Scholar]
- 54.Rupp F, et al. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006;7:323–334. doi: 10.1002/jbm.a.30518. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, Pantschenko AG, McCarthy MB, Gronowicz G. Bone-targeted overexpression of Bcl-2 increases osteoblast adhesion and differentiation and inhibits mineralization in vitro. Calcif Tissue Int. 2007;80:111–122. doi: 10.1007/s00223-006-0168-2. [DOI] [PubMed] [Google Scholar]
- 56.Cutler SM, Garcia AJ. Engineering cell adhesive surfaces that direct integrin α5β1 binding using a recombinant fragment of fibronectin. Biomaterials. 2003;24:1759–1770. doi: 10.1016/s0142-9612(02)00570-7. [DOI] [PubMed] [Google Scholar]
- 57.Cooper LF, Masuda T, Whitson SW, Yliheikkila P, Felton DA. Formation of mineralizing osteoblast cultures on machined, titanium oxide grit-blasted, and plasma-sprayed titanium surfaces. Int J Oral Maxillofac Implants. 1999;14:37–47. [PubMed] [Google Scholar]
- 58.Reyes CD, Garcia AJ. α2β1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. J Biomed Mater Res A. 2004;69:591–600. doi: 10.1002/jbm.a.30034. [DOI] [PubMed] [Google Scholar]
- 59.Reyes CD, Petrie TA, Burns KL, Schwartz Z, Garcia AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28:3228–3235. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blystone SD, Slater SE, Williams MP, Crow MT, Brown EJ. A molecular mechanism of integrin crosstalk: αvβ3 suppression of calcium/calmodulin-dependent protein kinase II regulates α5β1 function. J Cell Biol. 1999;145:889–897. doi: 10.1083/jcb.145.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zreiqat H, et al. The effect of surface chemistry modification of titanium alloy on signaling pathways in human osteoblasts. Biomaterials. 2005;26:7579–7586. doi: 10.1016/j.biomaterials.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 62.ter Brugge PJ, Torensma R, de Ruijter JE, Figdor CG, Jansen JA. Modulation of integrin expression on rat bone marrow cells by substrates with different surface characteristics. Tissue Eng. 2002;8:615–626. doi: 10.1089/107632702760240535. [DOI] [PubMed] [Google Scholar]
- 63.Cheng SL, Lai CF, Blystone SD, Avioli LV. Bone mineralization and osteoblast differentiation are negatively modulated by integrin αvβ3. J Bone Miner Res. 2001;16:277–288. doi: 10.1359/jbmr.2001.16.2.277. [DOI] [PubMed] [Google Scholar]
- 64.Kilpadi KL, Sawyer AA, Prince CW, Chang PL, Bellis SL. Primary human marrow stromal cells and Saos-2 osteosarcoma cells use different mechanisms to adhere to hydroxylapatite. J Biomed Mater Res A. 2004;68:273–285. doi: 10.1002/jbm.a.20043. [DOI] [PubMed] [Google Scholar]
- 65.Matsuura T, Hosokawa R, Okamoto K, Kimoto T, Akagawa Y. Diverse mechanisms of osteoblast spreading on hydroxyapatite and titanium. Biomaterials. 2000;21:1121–1127. doi: 10.1016/s0142-9612(99)00264-1. [DOI] [PubMed] [Google Scholar]
- 66.Kim JK, et al. A novel binding site in collagen type III for integrins α1β1 and α2β1. J Biol Chem. 2005;280:32512–32520. doi: 10.1074/jbc.M502431200. [DOI] [PubMed] [Google Scholar]
- 67.Reyes CD, Garcia AJ. α2β1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. J Biomed Mater Res A. 2004;69:591–600. doi: 10.1002/jbm.a.30034. [DOI] [PubMed] [Google Scholar]
- 68.Reyes CD, Petrie TA, Burns KL, Schwartz Z, Garcia AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28:3228–3235. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.