Abstract
A half-century ago, Fischer and colleagues found correlations between food preference and genetic markers of taste [propylthiouracil (PROP), quinine]. Recently, a number of studies report differences in sweet liking/disliking with taste phenotype or genotype. Here we modeled optimal liking for milk/sugar mixtures using the response surface method among 79 mostly normal weight adults (36 women) who reported low dietary restraint. Two non-overlapping phenotype analyses were performed: a) discordance in PROP versus quinine bitterness and b) number of fungiform papillae (FP, taste papillae on the tongue tip). Although all phenotype groups liked highly sweet and creamy sensations (in liking by sensation models), the fat and sugar levels for hedonic optima varied (in liking by concentration models). Males generally liked higher fat (20 to 40%) and sugar levels, with females disliking unsweetened cream. In quinine/PROP groups, liking peaked at 30% fat/15% sucrose for men and women who tasted 0.32mM quinine more bitter than 3.2mM PROP (n=15); a group previously shown to have highest sugar intakes (Duffy et al, 2003). Those tasting PROP more bitter than quinine (n=14) reported greater creamy/sweet sensations, with peak liking at lower fat and sweet levels (3.3% fat/10% sucrose). Generally, those in the high FP group perceived more creamy/sweet sensations with level of liking more influenced by sugar level, especially among high FP females. At high sugar/high fat levels low-FP males and females retained this liking while liking fell off for those in the high FP group. In summary, although most liked sweet/creamy sensations, perceptual differences in these sensations varied with oral phenotype, explaining some of the differences in the amount of sugar and fat required to reach hedonic optima. A high affinity for high sugar/high fat mixtures among oral phenotype subgroups has relevance for energy consumption and could explain the link previously observed between oral sensation and body weight.
A half-century ago, Fischer and colleagues found correlations between food preference and genetic markers of taste [6-n-propylthiouracil (PROP), quinine]. Recently, a number of studies report differences in sweet liking/disliking with taste phenotype or emerging genes. Here we modeled optimal liking for milk/sugar mixtures using the response surface method among 79 mostly normal weight adults (36 women) who reported low dietary restraint. Two non-overlapping phenotype analyses were performed: a) discordance in PROP versus quinine bitterness and b) number of fungiform papillae (FP, taste papillae on the tongue tip). Although all phenotype groups liked highly sweet and creamy sensations (in liking by sensation models), the fat and sugar levels for hedonic optima varied (in liking by concentration models). Males generally liked higher fat (20 to 40%) and sugar levels, with females disliking unsweetened cream. In quinine/PROP groups, liking peaked at 30% fat/15% sucrose for men and women who tasted 0.32mM quinine more bitter than 3.2mM PROP (n=15); a group previously shown to have highest sugar intakes (Duffy et al, 2003). Those tasting PROP more bitter than quinine (n=14) reported greater creamy/sweet sensations, with peak liking at lower fat and sweet levels (3.3% fat/10% sucrose). Generally, those in the high FP group perceived more creamy/sweet sensations with level of liking more influenced by sugar level, especially among high FP females. At high sugar/high fat levels low-FP males and females retained this liking while liking fell off for those in the high FP group. In summary, although most liked sweet/creamy sensations, perceptual differences in these sensations varied with oral phenotype, explaining some of the differences in the amount of sugar and fat required to reach hedonic optima. A high affinity for high sugar/high fat mixtures among oral phenotype subgroups has relevance for energy consumption and could explain the link previously observed between oral sensation and body weight.
Keywords: sweet, creamy, taste, genetics, response surface model, hedonics, food preferences, dietary fats, dietary carbohydrates, fungiform papillae, propylthiouracil, quinine, bitter
1. Introduction
How much a food is liked or disliked has long been deemed a major determinant of intake [1]. Elevated sweet preference associates with greater intake of added sugars and consumption of sweet foods [2,3] and vice versa [4]. Increased sweet affinity likely results from a genetic predisposition [2,5,6], variation in oral sensation associated with taste-related pathologies [2] and habitual level of intake [7,8]. Although many reports fail to link “a sweet tooth” with being overweight or obese, recent advances in assessing hedonic responses suggest sweet liking differs across normal and obese individuals [9]. More established is the fat liking-adiposity link. Heightened fat liking associates with increased intake [10] and adiposity in normal weight adults [11] and overweight/obese men [12]. Longitudinal data from an obesity prone population track greater liking for sugar-fat mixtures with greater weight gain [13]. Moreover, the relationships between sweetness, fat level and liking are influenced by taste phenotype [14,15], sex [14,16] and age [16].
The belief that differences in preference are influenced by phenotypic variation in oral sensation was reported by Fischer and colleagues in the 1960’s [17]. The best-characterized phenotypic marker of genetic variation in oral sensation is the bitterness of 6-n-propylthiouracil (PROP). Those tasting PROP as less bitter typically report less tactile sensations from fat [18–21]. A weaker oral signal potentially explains why those tasting PROP as less bitter report greater preference for high-fat foods [14,22,23] – a greater absolute amount is required to elicit the same hedonic response. PROP also associates with sweetness of sucrose in solution, sweet foods and qualitatively complex beverages (eg [2,24]). An individual minimally responsive to PROP requires almost twice the sucrose concentration to achieve the same level of sweetness as an individual for whom PROP is highly bitter [21]. When split into likers and dislikers (eg positive or negative slope with increasing concentration), disliking associates with PROP bitterness ratings [25] and thresholds [26], although differences in sweet intensity cannot completely explain liker/disliker classification [27]. Assessed via questionnaire, liking of sweet foods negatively associates with PROP bitterness in women but not men, who show a flat relationship [14]; PROP may interact with number of fungiform papillae (FP), another marker of variation in taste [28], to influence sweet liking [25,27]. Although PROP bitterness and FP number are correlated, they capture separate but overlapping sources of variation in oral sensation [29].
Quinine response is a heritable phenotype [30] that is linked to ingestive behaviors like smoking [31] and eating [32]. Notably, food preference and liking are highly correlated for monozygotic but not dizygotic twins [33,34], a relationship that may be mediated via taste genetics. This early work also found lower quinine sensitivity was associated with increased preference for strongly flavored foods [33] while recent work supports various quinine measures as PROP independent predictors of vegetable liking [35], alcohol intake [36], sucrose intensity and liking of sampled sweet foods [2]. Although PROP and quinine bitterness are typically correlated in a population [37,38], some individuals are discordant in bitterness of these compounds and differ in liking of sweet foods, frequency of consuming sweet foods, and alcohol intake [39]. Why quinine relates to dietary behavior remains unclear – it may reflect overall taste responsiveness [37] as it covaries with the intensity of other tastants [40,41], even in the absence of cross-adaptation [40]. Alternatively, applied to specific regions of the tongue, it can serve as a marker of exposure to taste-related pathology [35,42]. While the receptor for quinine is unknown, hT2R7 was recently implicated as the receptor for two related antimalarials – quinacrine and chloroquine [43].
The present study defined orosensory phenotype in two ways—by PROP/quinine discordance as well as by FP number in men and women —and used the Response Surface Method (RSM) to examine differences in amount of sugar and fat required for optimal liking (physicohedonic functions) and to study sweet/fat sensation related to optimal liking (psychohedonic functions). The relationship between pleasantness and intensity was noted as a single peaked inverted U shape by Joseph Priestley in 1775 and Wilhelm Wundt in 1874 [44], empirically tested by Saidullah and Engel in the 1920s [45], and later by Pfaffmann [46] and Moskowitz [47]. In a uni-dimensional system, the liking function can be modeled with a quadratic best fit line (a univariate second order polynomial). In a two dimensional system, a parabolic surface (a bivariate second order polynomial) can describe how changes in either dimension influence liking and provides the basis of RSM [48]. The RSM contour plots allow easy visualization of synergy (ie, the sum is greater than would be predicted from the individual parts) and deviation from additivity. The study of individual differences in oral sensation has shown that concentration is not synonymous with sensation. Yet, the need for discrete factors in techniques like ANOVA precludes asking about the psychohedonic function; in contrast, RSM provides information about liking as a function of concentration or sensation.
RSM models have been used to study liking of sugar-fat mixtures in normal [49], and overweight [50] adults, individuals with eating disorders [51], and the elderly [52]. Although, the link between oral phenotype and liking in these mixtures has been tested previously [53], RSM models have never been used to study groups who differ in oral phenotype. We address this knowledge gap by providing response surfaces that describe the hedonic optima of liquid sugar-fat mixtures in a sample of primarily normal weight adults.
2. Methods
Intensity and hedonic testing were conducted in a laboratory setting in three sessions, typically one week apart. Participants were characterized phenotypically for the discordance between PROP and quinine bitterness and for fungiform papillae number via videomicroscopy [28] as described previously [54].
2.1 Subjects
The 79 subjects, described previously [2,21], participated in an Institutional Review Board-approved procedure, provided written consent, and were paid for their time. Using body mass index (BMI; kg/m2) calculated from weights and heights measured in the laboratory, 57 were normal weight (18.5≤BMI<25) with one underweight (BMI<18.5), 18 overweight (25≤BMI<30), and 3 obese (BMI≥30) subjects. The men were more likely (χ2(1)=8.1, p<0.01) to be overweight or obese. All had low levels of dietary restraint, a common construct [55] measured here with two instruments; potential participants were screened with the concern for dieting subscale of the Restraint Scale [56,57] and then administered the Three Factor Eating Questionnaire (TFEQ) [58] during the first laboratory visit. Individuals with low ‘cognitive restraint of eating’–defined as a TFEQ-R score of 13 or below [59] – were included in the study. Men and women had restraint scores of 5.1±3.28 and 6.41±3.37, which are below collegiate norms of 6.1 and 10.2.
2.2 Stimuli
Participants tasted 15 mixtures – heavy cream (36% fat), whole milk (3.5%), skim milk (>0.5%) and water (0% fat) that varied in added sucrose (0, 5, 10, 20% w/v); data from the sucrose-water mixtures was excluded from analysis to avoid any odor effects when shifting from water to milk [21]. Samples were served cold (5° C) in duplicate; participants rated degree of liking, sweetness and creaminess within a trial. They rinsed between each sample with room temperature deionized (>15 MΩ) water.
The participants rated the bitterness of 0.32mM quinine monohydrochloride (Pfaltz & Bauer, Waterbury, CT) within a battery of prototypical tastants during the first day. In a protocol described previously [2,35,60], PROP solutions – 3.2, 1, 0.32, 0.1, 0.032 mM 6-n-propthiouracil (Sigma, St. Louis, MO) – were presented randomly in blocks along with 1khz tones (50–98 dB in 12-dB steps) and NaCl solutions (.01, .032, .1, .32. 1M). This was done at the end of the third testing session to minimize contrast and range effects [61,62] that may vary non-randomly with PROP response. Raw PROP data were used; tone normalized and raw data produce comparable PROP functions and relationships with sensory and diet variables [54].
2.3 Data Collection
2.3.1 Measuring Intensity and Liking
Adults were instructed to use the general Labeled Magnitude Scale (gLMS) [63,64] to report the intensity and degree of liking/disliking of the samples. For intensity, the gLMS ranges from ‘no sensation’ at the bottom (0) to ‘the strongest imaginable sensation of any kind’ at the top (100) with intermediate labels at ‘barely detectable’ (1.4), ‘weak’ (6), ‘moderate’ (17), ‘strong’ (35), and ‘very strong’ (53). This scale generalizes the Labeled Magnitude Scale [65,66] by broadening the context from oral sensations to all sensations of any kind. Changing the top anchor is critical because individuals do not use adjective labels to denote the same perceived intensities [63]. The flawed assumption that subjects use adjective labels in a similar manner can attenuate, obfuscate or even reverse intensity effects [67]. For hedonic ratings of milk samples, subjects were instructed to anchor the top of the scale to either ‘strongest imaginable liking’ (+100) or the ‘strongest imaginable disliking’ (−100) with neutral being zero, as reported previously [35,68].
2.3.2 Fungiform Papillae Number
Mean FP number in a 6mm circular area averaged across the right and left tongue tip was ascertained by staining the tongue blue and viewing the recorded image collected via videomicroscope, as described elsewhere [54,60]. Using the overall median (23.5 FP), we formed low and high groups. Men tended to fall into the low group: 26 of 43 men vs. 15 of 36 women (χ2(1)=2.77, p=0.10), although means were not different across sex (t79=1.543, p=0.13). Since previous work indicates men and women react to fat cues differently [14], and women may have more FP than men [29,60], we examined FP effects separately for men and women. The proportion of overweight/obese subjects (BMI≥25) did not differ by FP Group (χ2(1) =0.00, p=0.96).
2.3.3 Discordance and FP number are Separate Phenotypes
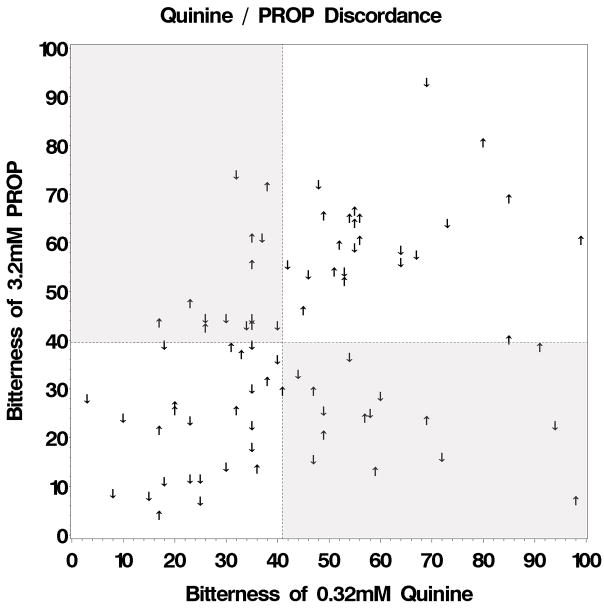
The study sample showed variability in quinine and PROP bitterness and FP number; all three measures were positively correlated with each other (r’s 0.34 to 0.36, p’s <0.01). In spite of the positive correlation, 1 of 3 individuals were discordant between 0.32mM quinine and 3.2mM PROP bitterness [2]. Using median splits for bitterness of 0.32mM quinine (median=41.0) and 3.2mM PROP (median=39.5), matched responses [low/low (n=26) or high/high (n=24)] were considered concordant and unmatched responses [low/high (n=15) or high/low (n=14)] were considered discordant (shaded regions of Figure 1). The proportion of males and females did not differ across the four quinine/PROP groups (χ2(3)=0.23, p=0.97), or within the concordant (χ2(1)=0.06, p=0.80) or discordant (χ2(1)=0.03, p=0.86) groups. The proportion of overweight/obese subjects (BMI>25) did not differ for the discordant (Fisher’s exact p=0.42) or concordant (χ2(1)=0.25, p=0.61) groups. Because of sensory differences in creamy ratings (described below) and previous data on sweet food liking and intake [2], we focused primarily on the two discordant groups; the results for the concordant groups are described briefly.
Figure 1.
Scatterplot of the relationship between quinine and PROP bitterness (measured on the generalized Labeled Magnitude Scale (gLMS)). Arrows indicate membership in the high (↑) or low (↓) fungiform papillae density group and shaded regions indicate quinine/propylthiouracil discordance.
The classification of Quinine/PROP discordance was independent from FP classification. The distribution of FP did not differ (χ2(3)=2.01, p=0.57) across all groups (Figure 1), or within just the concordant (χ2(1)=1.97, p=0.16) or discordant (χ2(1)=0.03, p=0.86) groups.
2.4 Analysis
Statistical analyses were conducted using SAS Release 9.1.3 (SAS, Cary, NC). To test for differences across quinine/PROP groups, replicated sweet and creamy ratings were averaged and tested for fat or sucrose concentration effects via repeated measures ANOVA using PROC MIXED. Subjects and concentration were treated as random, and within subjects factors (the repeated measures) were allowed to covary [69]. Overall F-values were not generated because PROC MIXED uses maximum likelihood estimation; denominator degrees of freedom were estimated using the Satterthwaite approximation. Higher order interactions were interpreted first, followed by less complicated interactions if the higher order interaction was not significant [70].
Response surface models [71] were used to determine intensity and liking for the 12 milk samples. Normal probability plots and skew values indicate the liking, creamy and sweet ratings were approximately normal. The amount of sucrose and fat eliciting the peak response (liking, sweetness, creaminess) was estimated with a bivariate second order polynomial surface: y-hat = β0 + β1x1 + β2x2 + β3(x1)2 + β4(x2)2 + β5x1x2 +ε. Fat levels were log transformed to maintain a roughly equal spacing of stimuli in the response field. The surfaces were estimated using PROC RSREG using unaveraged replicates for each subject. Following the recommendations of Freund and Little [72], the models were evaluated statistically on the basis of whether: a) the entire model explained the response variable (variance explained and overall fit); b) each factor was needed (ie, all combinations of x1 or x2); c) any quadratic term was needed (ie, (x1)2 or (x2)2); and d) the cross-product [(x1x2)] term was needed. Surfaces were visualized as three-dimensional surface plots (via PROC G3D) or two-dimensional contour plots (via PROC CONTOUR). Consistent with prior work (eg [50]), plots for each group were assessed in terms of the shape of the function, the location of peak liking, and height of maximal response as well as compared qualitatively. ANOVA was used to test specific group differences revealed by the physicohedonic plots. Synergy was defined as a deflection toward the origin when response surface was projected onto a two dimensional response-field (eg, the isocontours were curved).
3. Results
3.1 Creaminess and Sweetness by Group
Effects of FP number on creaminess and sweetness have been reported previously [21]; in general, creaminess and sweetness ratings increased with FP number.
3.1.1 Creaminess and Sweetness by Quinine/PROP group
Across all samples and replicates, most sweet and creamy ratings fell below ‘very strong’ for the discordant groups. When ratings exceeded this cutoff (~10% of ratings), they were significantly more likely to come from an individual in the high PROP/low quinine group for both sweetness (χ2(1)=5.16, p=0.023) and creaminess (χ2(1)=7.71, p=0.006). These high PROP/low quinine individuals also reported greater mean creaminess (p=0.01); sweetness showed a similar but non-significant pattern (p=0.14).
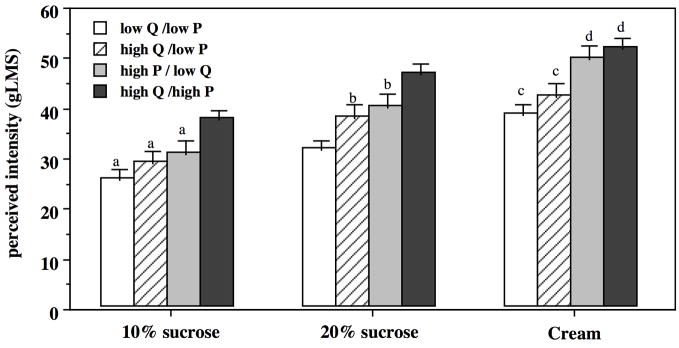
Mixed model ANOVA revealed average sweetness and creaminess differences across all four concordant/discordant groups (Figure 2). For sweetness, the 2-way sucrose concentration by group interaction was significant [F(9,225)=6.44, p<0.0001]. Generally, the two high PROP groups tasted more sweetness than the two low PROP groups, and within a PROP level, the high quinine group tasted greatest sweetness. For creaminess, the 2-way fat level by group interaction was significant [F(6,150)=4.96, p<0.0001]. Again, the two high PROP groups experienced more creaminess than the two low PROP groups, and within a PROP level, the high quinine group experienced greatest creaminess.
Figure 2.
Sweetness and creaminess as measured on the gLMS (17=moderate, 35=strong, 53=very strong) for quinine (Q) and PROP (P) groups; within a stimulus, columns sharing a letter are not different.
3.1.2 Creaminess and Sweetness by Response Surface Models
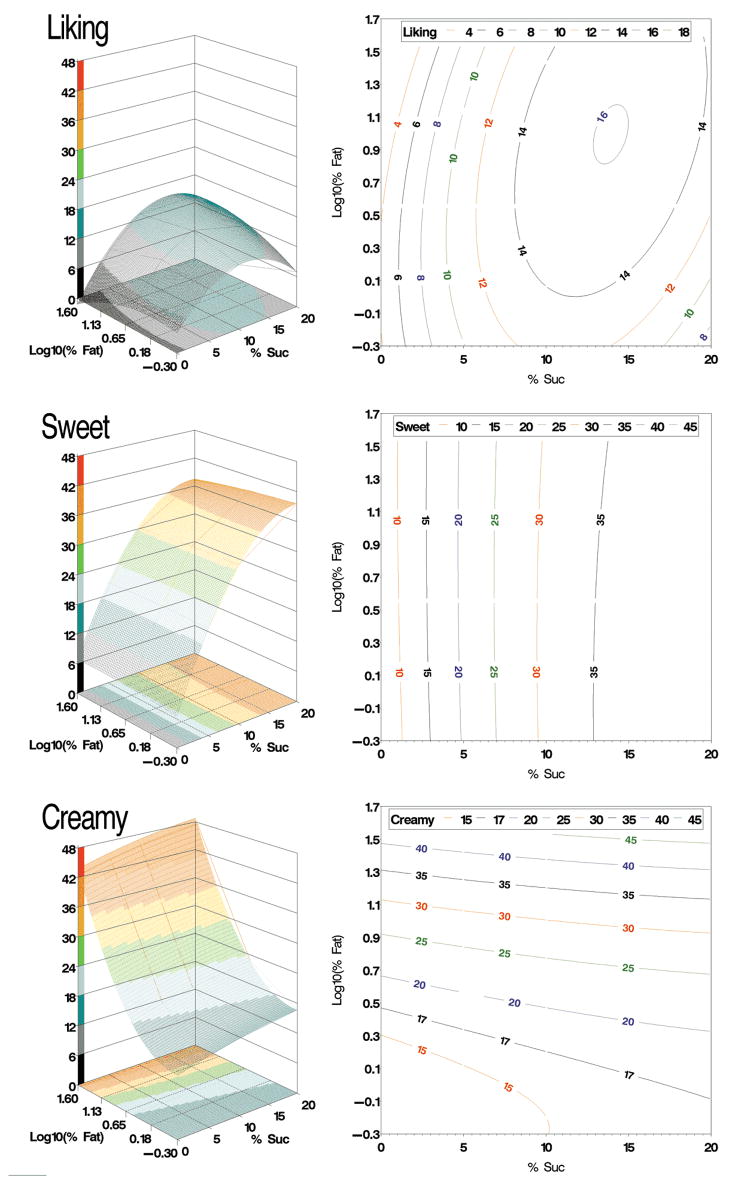
Response surfaces were fit for liking, sweetness and creaminess across the entire sample (Figure 3). For sweetness, the overall response surface was significant (p<0.0001), explaining 46.1% of the variance. Sucrose (p<0.0001) was a significant factor while fat was not (p=0.43). Quadratic terms were needed (p<0.0001) while the cross-product term (p=0.125) was not. There was no synergy, as shown by the straight isocontours on response field (Figure 3, right). Moreover, the isocontours were parallel to the fat axis suggesting that, across the entire sample, fat level did not alter sweetness. Results were similar for all discordant and FP-sex groups in terms of variance explained (ranged from 40.5% to 57.5%), overall fit (all p’s <0.0001), and required factors and terms.
Figure 3.
Response surface models across all subjects in 3-D (left) and 2-D isocontour plots (right) for liking (top; hedonic gLMS), sweetness (middle; gLMS) and creaminess (bottom; gLMS).
For creaminess, the overall response surface model across all individuals explained significant variance (53.9%, p<0.0001). Both fat (p<0.0001) and sucrose (p<0.0001) were significant factors and quadratic terms were required (p<0.0001) while the cross-product (p=0.87) term was not. The isocontour plot (Figure 3, right) revealed straight lines that were angled toward the sucrose axis, suggesting that creaminess increased with sucrose concentration in a simple additive fashion. Results were similar for most subgroup analyses. For all subgroups, the overall fit was significant (all p’s <0.0001), with variance explained ranging from 47.7% to 59.5%; the quadratic term was required while the cross-product term was not. For all subgroups, fat level was a significant factor in creaminess as expected. The sucrose factor was also significant for all except the two discordant groups where sucrose level did not influence creaminess.
3.2 Liking as a function of concentration (physicohedonic functions)
In contrast to the sweet and creamy response surfaces showing monotonic increases with increasing concentration, the liking response surface exhibited an inverted U shape for both sugar and fat; liking first increased and then fell as concentration continued to climb (Figure 3, top left). The overall model was significant (Table 1) with curved isocontours indicating hedonic synergy in fat/sugar mixtures (Figure 3, top right).
Table 1.
Fit Statistics for the Liking Response Surface Models
| Discordant Groups
|
Men
|
Women
|
|||||
|---|---|---|---|---|---|---|---|
| All Subjects
|
High PROP/low quinine
|
High quinine/low PROP
|
Low FP
|
High FP
|
Low FP
|
High FP
|
|
| Overall Fit | p<.0001 | p=.032 | p<.0001 | p<.0001 | p=.0002 | p=.019 | p<.0001 |
| Liking Explained | 3.90% | 3.60% | 8.95% | 4.20% | 5.90% | 3.70% | 4.90% |
| Linear Terms | |||||||
| Sugar | p<.0001 | p=.17 | p<.0001 | p<.0001 | p<.0001 | p=.0156 | p<.0001 |
| Fat | p=.003 | p=.0139 | p=.095 | p=.095 | p<.10 | p=.11 | p=.060 |
| Quadratic Terms | p<.0001 | p=.0428 | p=.011 | p=.018 | p=.0008 | p=.25 | p<.0001 |
| Cross Product | p=.0012 | p=.062 | p=.17 | p=.14 | p=.096 | p=.088 | p=.090 |
| Overall Fit | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 |
| Liking Explained | 7.20% | 13.40% | 14.90% | 11.10% | 12.10% | 20.20% | 7% |
| Linear Terms | |||||||
| Sweetness | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 | p<.0001 |
| Creaminess | p<.0001 | p<.0001 | p=.0054 | p=.0006 | p=.0002 | p<.0001 | p=.078 |
| Quadratic Terms | p=.0008 | p=.0008 | p=.0023 | p<.001 | p<0.12 | p<.0001 | p<.0001 |
| Cross Product | p<.0001 | p<.0001 | p<.0001 | p=.0003 | p=.0004 | p=.19 | p=.61 |
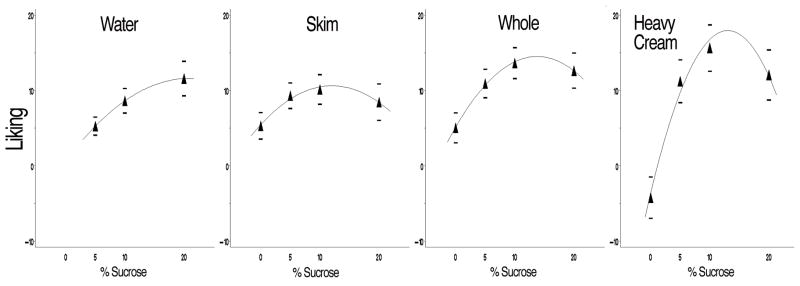
Figure 4 shows liking as quadratic best fit lines with increasing sucrose concentration at each level of fat. Mean liking in the milk initially increased with sucrose concentration before peaking at 10% sucrose. Although peak liking for sweetened heavy cream versus whole milk failed to show differences in means (t78=-0.81, p=0.42), the variability in liking ratings was much greater in the sweetened heavy cream (F-ratio79,79=2.26, p<0.001), suggesting heavy cream could be too creamy for some individuals but not others who enjoy that level of creaminess. Additionally, unsweetened heavy cream was disliked, as the mean rating across all subjects was much lower than for any other stimuli (Figure 4). However, adding sucrose removed this disliking: mean liking for whole milk and heavy cream were different when unsweetened (t77=3.6, p=0.0006) but not at 10% sucrose (see above).
Figure 4.
Quadratic relationship between sucrose level and liking (hedonic gLMS; ±17 moderately like/dislike; ±6 weakly like/dislike; 0=neutral;) across each fat level—skim milk (>0.5%), whole milk (3.5%), heavy cream (36%).
3.2.1 Physicohedonic functions by Quinine/PROP group
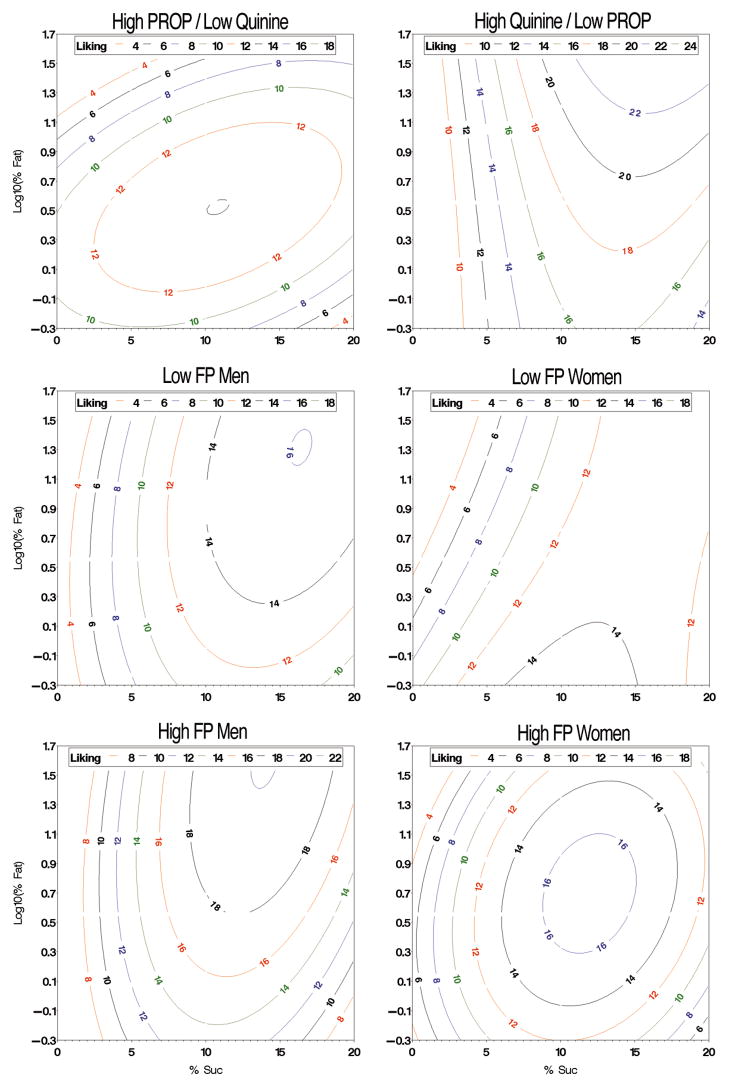
Comparing the two discordant groups revealed large group differences in the concentration of fat and sugar required to achieve maximal liking (Figure 5, top). For the high PROP/low quinine group, peak liking was below ‘moderate’ and near 10% sucrose and 3.3% fat. Liking declined when increasing fat from whole milk to heavy cream at low sucrose levels [Sucrose by Fat F(2,24)=13.2, p <.0002]: the drop was significant for unsweetened stimuli (Fisher’s LSD p <.0001) and 5% sucrose/dairy mixtures (p <.0002) but not 10% mixtures (p =.32). Liking was less influenced by changes in sucrose levels (large oval in the contour plot) and synergy was observed at low concentration (curvature of isocontours toward the origin). For the high quinine/low PROP group, no distinct peak was observed with maximal liking (between ‘moderate’ and ‘strong’) occurring at the highest levels of sucrose and fat. Below 10% sucrose, changes in fat level had minimal impact on liking; above 10% sucrose, increasing fat level heightened liking synergistically.
Figure 5.
Concentration-liking response surfaces for discordant groups (top), and low (middle) and high (bottom) fungiform papillae (FP) groups. Colored lines are liking isocontours (hedonic gLMS; 6 is weak, 17 is moderate and 35 is strong).
The groups differed in changes in liking for whole milk with increasing sucrose in RM ANOVA [Group by Sucrose F(3,78)=3.94, p =.011]. As expected from the RSM plots, liking in the high quinine/low PROP group increased when moving from unsweetened whole milk to 5% (Fisher’s LSD’s p =.064) and 10% (p =.0003) sucrose mixtures before plateauing (0% v 20% p =.0003; 10% v 20% p =.96); in the high PROP/low quinine group, liking was essentially flat (all pairwise p’s >.5).
The response surfaces for the concordant groups (not shown) were generally similar to their respective quinine discordant group. The high/high group (explaining 6.6% of the variance) was very similar to the high quinine/low PROP group. Notably however, these two groups appeared to diverge with regard to high sugar low fat stimuli, which were more liked by the discordant group. The low/low group (explaining 4.5% of liking) was somewhat similar to the high PROP/low quinine group although the oval of liking seen in Figure 5 was rotated such that changes in fat level had limited impact on liking.
3.2.2 Physicohedonic functions by FP-sex groups
The response surface models for FP revealed differences across men and women. In general, men reached optimal liking at higher levels of fat and sucrose, while the women showed a stronger dislike to the highest fat, no sugar stimuli.
For males, low and high FP showed limited differences in the overall shape of the functions (Figure 5, left middle vs. left bottom). Both had optima near ~15% sucrose, yet low FP men peaked at 20% fat vs 40% for high FP men. In both groups, changes in sucrose level appeared to have larger effect on liking than did changes in fat level: increasing sucrose from 0 to 10% rapidly increased liking (the steep gradient in the left side of each plot) whereas changes in fat level had a limited effect. At high fat levels, the high FP men were more responsive to changes in sucrose level as visualized with RSM and subsequently confirmed with RM ANOVA. When moving from 10% to 20% sucrose in heavy cream [Group by Sucrose F(1,41)=2.92, p =.094], liking did not change in the low FP men (Fisher LSD p=.857) while liking dropped significantly in the high FP men (p =.024). RSM also showed high FP men were more responsive to changes in fat level at optimal sucrose levels as moving upward at 15% sucrose required crossing more isocontours (ANOVA confirmation was not possible as ratings were not available). Some curvature was observed in the contour plots, suggesting any potential synergy was limited to high concentration.
Comparing low and high FP females revealed large group differences (Figure 5, middle right vs. bottom right). Low FP females had no distinct peak but showed maximal liking (below ‘moderate’) in skim milk near 12% sucrose. In the high FP females, peak liking reached ‘moderate’ and occurred near 12% sucrose and 5% fat. As was seen with the men, the high FP women (compared to low FP women) were more responsive to changes in sucrose level at high fat levels. In RM ANOVA [Group by Sucrose F(1,34)=3.32, p =.077], liking dropped for high FP women (Fisher LSD p=.061) but not low FP women (Fisher LSD p=.46). Unfortunately, ANOVA could not test differences between the bullseye pattern in the high FP women versus the large plateau in the low FP women, as it fell at liking for 15% sucrose, which was not assessed. Only for the high FP women, the contour plot indicated the existence of synergy given the curvature of isocontours toward the origin.
3.3 Liking as a function of sweetness and creaminess (psychohedonic functions)
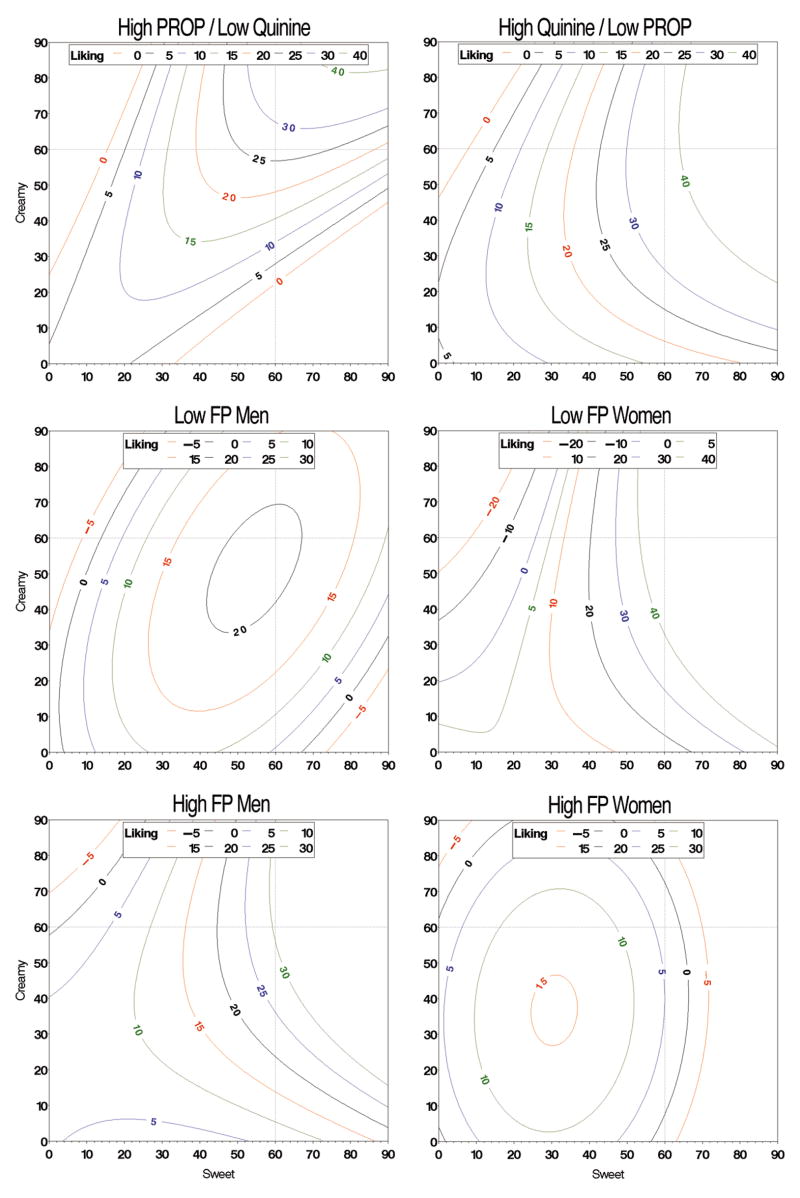
The variance explained by liking as a function of sensation (psychohedonic) was greater than the comparable physicohedonic model (Table 1). Because most ratings occurred below 60, dashed lines are provided to discourage over-interpreting outside the region of interest. Across the entire sample, the overall model was significant (Table 1); sweet and creamy sensations synergized with respect to liking as the isocontours were dramatically curved toward the origin in the contour plot (not shown). Separate RSM models for each phenotypic group are presented below. Most groups liked the sweet and creamy sensations, resulting in more similar models than the liking by concentration models.
3.3.1 Psychohedonic functions by Quinine/PROP group
The two discordant groups liked sweet and creamy sensations just above ‘very strong,’ although liking in this region was slightly higher in the high quinine/low PROP group (Figure 6, top). Neither group liked highly creamy unsweet sensations (top left of each plot). The high quinine/low PROP group liked highly sweet, noncreamy sensations whereas the high PROP/low quinine group did not (bottom right of each plot). Keeping creaminess constant at ‘weak’ or ‘moderate’ while increasing sweetness (ie moving from left to right) also showed large group differences – it caused a pronounced increase in liking, but only for the high quinine/low PROP group.
Figure 6.
Same as Figure 5, but for the psychohedonic (sensation-liking) response surfaces.
3.3.2 Psychohedonic functions by FP-sex groups
Although low and high FP males both liked sweet and creamy sensations just above ‘very strong,’ the peak was higher in the high FP men (Figure 6, middle left v. bottom left). At weak or moderate sweetness, increasing creaminess had minimal effect on liking for either group. For men, regardless of FP group membership, increasing sweetness (moving left to right) generally increased liking, particularly at higher levels of creaminess. At lower levels of creaminess, this effect was primarily seen in the low FP males – in high FP males, sweet effects were only observed when creaminess was approaching ‘strong.’
Comparing low and high FP females also revealed differences (Figure 6, middle right v. bottom right). For low FP females, sweet and creamy sensations near ‘very strong’ were liked very much (above ‘strong’ liking), whereas sensations of this magnitude elicited liking near ‘weak’ for the high FP women. In the low FP women, increasing creaminess had little influence on liking; increasing sweetness produced changes in liking. In high FP females, the peak liking never rose above ‘moderate,’ and occurred near ‘strong’ for both sweet and creamy. Changes in sweetness appeared to have a larger effect than changes in creaminess.
4. Discussion
The amount of fat and sugar required to achieve maximal liking varied across groups characterized by two distinct markers of taste phenotype: a) discordance in the bitterness of quinine and PROP and b) the number of fungiform papillae. Within the FP groups, further divergence was observed for men and women. These phenotypical differences in liking were linked to perceptual variation in creaminess and in some cases sweetness – in general, those who perceived more creaminess needed less fat to achieve maximal liking. Controlling for intensity differences by plotting liking as a function of sweet and creamy sensations revealed that the groups were far more similar — all but one subgroup reported peak liking near very sweet and very creamy sensations. This suggests that while taste phenotype strongly influences the concentration of sugar and fat required to achieve peak liking, most exhibit a ‘more is better’ response when an individual’s perceptual experience is considered. The bitter tastants and taste anatomy provided unique groupings to explain variation in sweet/creamy hedonics further supporting the approach to utilize multiple markers of orosensation in explaining dietary behaviors.
The present study continues the work started by Fischer and Griffin [32,37] and continued by our lab to use PROP and quinine to characterize oral sensation [35], Previously, we have shown discordant high quinine/low PROP individuals are more likely to consume sweet foods and obtain more energy from added sugars [2]. Here, we demonstrate that these same individuals have a greater hedonic response to concentrated fat-sugar mixtures. Discordance between PROP and quinine bitterness could reflect taste damage to the chorda tympani nerve (such as with chronic otitis media), which decreases inhibition from other taste nerves to heighten bitterness of quinine (versus PROP) tasted with the whole mouth [73]. Preliminary data support that individuals with reported history of otitis media [9] or diminished quinine bitterness on the tongue tip [74] report greater preference for high-fat sweet foods and are heavier. Alternatively, discordance may result from differential expression of multiple bitter receptors [75]; recent data suggests that multiple genes may be involved in sweet preference [5,6,25]. While it is uncertain whether quinine represents a marker of exposure to taste-related pathologies, a measure of overall taste sensation as Fischer thought [37], or an another genetic variant, the data from the present study support the use of multiple bitter markers and taste anatomy to explain differences in food liking and intake (eg, [2,35]).
4.1 Differences in optimal levels of fat
Drewnowski and coworkers [50] suggested that differential patterns in response surface models for sugar-fat mixtures related to dietary intake and health outcomes. Using the same milk/sugar mixtures described here, they found normal weight women exhibited peak liking at moderate levels of fat and sucrose while the obese required high levels of fat to reach maximal liking. Although our study group lacked sufficient body weight variability to test this relationship directly, we can compare across studies. Drewnowski’s normal weight women had liking patterns most similar to our high PROP/low quinine subjects and high FP women. Our high quinine/low PROP group who exhibited peak liking at the highest amounts of sugar and fat, was similar to obese subjects in the Drewnowski sample. This discordant group required more fat to reach maximal liking than did the high PROP/low quinine group, which is expected, as they also reported significantly less creamy sensations from heavy cream. The Drewnowski sample did not show any intensity differences across groups, but this may reflect limitations of the scale that was used [9].
By comparing the height of the peak rating above the dose-field—the high quinine/low PROP group reported more pleasure out of the most liked samples than the high PROP/low quinine group. This is striking, because longitudinal data demonstrates that increased maximal liking (ie height above the dose-field) for fat/sugar mixtures predicts rate of future weight gain [13]. The argument that taste phenotype is related to adiposity via food liking and intake is buttressed by recent findings from several laboratories. Cross-sectional data demonstrates a negative relationship between PROP bitterness and BMI [76–78], while other studies suggest liking for [22,78,79] or intake of [80] fat may be lower in those for whom PROP is most bitter, although some intake studies do not agree [81,82].
4.2 Sex differences and FP number
Males liked higher stimulus levels than the women and this was particularly true for fat. Although this could reflect dietary restraint differences, we find this explanation unlikely as our sample was selected to have low dietary restraint, the difference between men and women was minimal, and sample means were comparable to unrestrained eaters (see [59]). More plausible is an oral sensory explanation – although the FP distributions did not differ when split at the median, men may have been skewed toward lower FP numbers when compared in a cumulative fraction plot (69% of the men versus 41% of the women fell below the point of maximal separation). Moreover, a greater number of papillae made men and women more responsive to changes in fat and sucrose level to influence hedonics as well as intensity [21]. In the women, the low and high FP groups had very different response surfaces. The high FP women exhibited optimal liking near 5% fat at 12% sucrose and liking dropped as fat level continued to increase. In contrast, the low FP women had a large plateau near 12% sucrose where changes in fat level had minimal impact on liking. Within men, both the high and low FP groups had peak liking at high levels of fat and sugar. Notably however, in the region above 10% sucrose, the high FP men were more responsive to changes in fat level (Figure 5). Interestingly, Tepper and Nurse found that male PROP nontasters had greater BMIs than did supertasters [79]. Present data suggest that low FP individuals may be less sensitive to changes in fat level and thus may be less able to use oral sensory cues to avoid overconsuming high fat foods and beverage. The influence of oral phenotype on dietary compliance for low-fat diets during weight loss interventions and long-term weight control needs to be tested.
4.3 Additional insight from sensation-liking functions
Bivariate factorial plots revealed mean liking was greatest for the 10% sucrose samples – this is consistent with previous reports based on sip and spit stimulus delivery (eg [83]). However, looking solely at the concentration-liking function ignores the critical contribution of perceived sweetness to sweet liking [25]. Moskowitz [47] made the critical observation that the breakpoint in the hedonic function occurs at a fixed perceptual sweetness, not a fixed concentration. While many authors have noted large variability in patterns of hedonic response across concentration (eg [84–86]), assessing the underlying source of this variability is only possible by accounting for perceptual differences (eg [87]). Presently, all models linking sensation to liking had superior fit compared to the same model linking concentration to liking. While this would be expected theoretically, ANOVA-based approaches preclude direct comparisons; by using the RSM here, we provide an empirical demonstration for sweet/creamy mixtures.
Yeomans and colleagues [27] revisited the relationship between taste phenotype, dietary restraint, sucrose intensity and liking. Those tasting PROP as more bitter were more likely to taste greater sweetness and be sucrose dislikers, consistent with our findings [21,25]. In their analysis, liker and disliker groups persisted after plotting liking as a function of sensation, leading those authors to conclude that liker/disliker classification cannot be explained by differences in sweet intensity related the taste phenotype [27]. Data from the current study agree that intensity is not the sole determinant of liking, but also show that some of the hedonic differences across taste phenotype groups arise from differences in intensity.
Differences in the perceptual/hedonic responses were seen in the discordant PROP/quinine groups. Although both liked very sweet, very creamy sensations, highly sweet non-creamy samples were not liked by the high PROP/low quinine group. Notably, Randall and Sanjur [88] reported that the connection between disliking and non-use was much stronger than the link between liking and consumption. Thus, in light of present data, intake differences previously observed between discordant groups [2] may result in part from the avoidance of highly sweet, low fat foods by high PROP/low quinine individuals.
4.4 Limitations and conclusion
These findings may not generalize to solids as the oral cues of fat content differ between liquid and solid foods and liking for fat is often specific to the food being studied (eg ice cream v. a greasy hamburger) [89]. The semantic labels used here (ie creamy) may not fully capture the range of sensations experienced as other, possibly aversive, fat related sensations like oily or greasy were not measured. Likewise, viscosity changes not captured within the creamy percept could impact liking. Although sip and spit methods like those used here do not have the same predictive power as ad libitum consumption tests, they do predict intake [68,83]. Caution should be taken in generalizing to other populations: the current sample was screened to exclude individuals with high dietary restraint, which can decouple relationships between phenotypic variation, preference and intake, as well as significant history of head trauma, otitis media and other taste pathologies, which can alter taste function and affect liking for high fat foods. Finally, the utility of bitter markers in predicting differences in sensations from, liking for, and intake of sweet or creamy stimuli documented here and elsewhere (eg [78,90–92]) presumably occurs not because these stimuli are themselves bitter, but because bitterness variability captures broader, underlying differences in oral sensation. Likewise, papillae number captures some of the variability in taste and touch fiber density.
Mela [93] notes that hedonic response alone cannot sufficiently explain the variation in food choice, because, the motivation or desire to eat is distinct from the pleasure foods provide. Thus, oral sensation and increased liking for high fat foods does not inherently predestine a specific individual to consume energy dense foods. And even when individuals habitually consume a high-fat diet, they may still resist weight gain [94]. However, across a population, the present finding is consistent with other findings that support a causal chain between phenotypic variation in oral sensation, food preference, intake and health outcomes [10]. O’Rahilly and Farooqi [95] recently suggested that when viewed from a genetic, etiological perspective, “obesity is less a metabolic than a neuro-behavioral disease.” Here, we find that most individuals like highly sweet, highly creamy sensations, but differ in the amount of sugar and fat required to reach optimal liking, suggesting oral sensory phenotype could potentially influence dietary energy density.
Acknowledgments
This manuscript was completed in partial fulfillment of the requirements for a PhD at the University of Connecticut by JEH. The project was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2003-35200-12943 and NIH Institutes of Deafness and Communication Disorders grant number DC00283. JEH received additional support from the Pangborn Sensory Science Scholarship Fund for 2006; the award is underwritten by GlaxoSmithKline Consumer Healthcare Division, which has no other interest in this work. The authors thank Megan Philips and Julie Peterson for collecting these data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pilgram FJ, Kamen JM. Predictors of Human Food Consumption. Science. 1963;13:501–2. doi: 10.1126/science.139.3554.501. [DOI] [PubMed] [Google Scholar]
- 2.Duffy VB, Peterson J, Dinehart M, Bartoshuk LM. Genetic and environmental variation in taste: Associations with sweet intensity, preference and intake. Top Clin Nutr. 2003;18:209–220. [Google Scholar]
- 3.Holt SHA, Cobiac L, Beaumont-Smith NE, Easton K, Best DJ. Dietary habits and the perception and liking of sweetness among Australian and Malaysian students: A cross-cultural study. Food Qual Prefer. 2000;11(4):299–312. [Google Scholar]
- 4.Pangborn RM, Giovanni ME. Dietary intake of sweet foods and of dairy fats and resultant gustatory responses to sugar in lemonade and to fat in milk. Appetite. 1984;5(4):317–27. doi: 10.1016/s0195-6663(84)80004-5. [DOI] [PubMed] [Google Scholar]
- 5.Mennella JA, Pepino MY, Reed DR. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115(2):e216–22. doi: 10.1542/peds.2004-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keskitalo K, Knaapila A, Kallela M, Palotie A, Wessman M, Sammalisto S, Peltonen L, Tuorila H, Perola M. Sweet taste preferences are partly genetically determined: identification of a trait locus on chromosome 16. Am J Clin Nutr. 2007;86(1):55–63. doi: 10.1093/ajcn/86.1.55. [DOI] [PubMed] [Google Scholar]
- 7.Mattes RD, Mela DJ. Relationships between and among selected measures of sweet-taste preference and dietary intake. Chem Senses. 1986;11(4):523–39. [Google Scholar]
- 8.Liem DG, de Graaf C. Sweet and sour preferences in young children and adults: role of repeated exposure. Physiol Behav. 2004;83(3):421–429. doi: 10.1016/j.physbeh.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HD, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006;361(1471):1137–48. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffy VB. Variation in oral sensation: implications for diet and health. Curr Opin Gastroenterol. 2007;23(2):171–7. doi: 10.1097/MOG.0b013e3280147d50. [DOI] [PubMed] [Google Scholar]
- 11.Mela DJ, Sacchetti DA. Sensory preferences for fats: relationships with diet and body composition. Am J Clin Nutr. 1991;53(4):908–15. doi: 10.1093/ajcn/53.4.908. [DOI] [PubMed] [Google Scholar]
- 12.Duffy VB, Lanier SA, Hutchins HL, Pescatello LS, Johnson MK, Bartoshuk LM. Food Preference Questionnaire as a Screening Tool for Assessing Dietary Risk of Cardiovascular Disease within Health Risk Appraisals. J Am Diet Assoc. 2007;107(2):237–45. doi: 10.1016/j.jada.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Salbe AD, DelParigi A, Pratley RE, Drewnowski A, Tataranni PA. Taste preferences and body weight changes in an obesity-prone population. Am J Clin Nutr. 2004;79(3):372–8. doi: 10.1093/ajcn/79.3.372. [DOI] [PubMed] [Google Scholar]
- 14.Duffy VB, Bartoshuk LM. Food acceptance and genetic variation in taste. J Am Diet Assoc. 2000;100(6):647–55. doi: 10.1016/S0002-8223(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 15.Duffy VB. Associations between oral sensation, dietary behaviors and risk of cardiovascular disease (CVD) Appetite. 2004;43(1):5–9. doi: 10.1016/j.appet.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Monneuse MO, Bellisle F, Louis-Sylvestre J. Impact of sex and age on sensory evaluation of sugar and fat in dairy products. Physiol Behav. 1991;50(6):1111–7. doi: 10.1016/0031-9384(91)90569-a. [DOI] [PubMed] [Google Scholar]
- 17.Fischer R, Griffin F, Kaplan AR. Taste Thresholds, Cigarette Smoking, and Food Dislikes. Med Exp Int J Exp Med. 1963;210:151–67. doi: 10.1159/000135346. [DOI] [PubMed] [Google Scholar]
- 18.Duffy VB, Bartoshuk LM, Lucchina LA, Snyder DJ, Tym A. Supertasters of PROP (6-n-propylthiouracil) rate the highest creaminess to high-fat milk products. Chem Senses. 1996;21:598. [Google Scholar]
- 19.Tepper BJ, Nurse RJ. Fat perception is related to PROP taster status. Physiol Behav. 1997;61(6):949–54. doi: 10.1016/s0031-9384(96)00608-7. [DOI] [PubMed] [Google Scholar]
- 20.Prescott J, Bartoshuk LM, Prutkin J. 6-n-Propylthiouracil Tasting and the Perception of Nontaste Oral Sensations. In: Prescott J, Tepper BJ, editors. Genetic Variation in Taste Sensitivity. New York: Marcel Dekker; 2004. pp. 89–104. [Google Scholar]
- 21.Hayes JE, Duffy VB. Revisiting sugar-fat mixtures: sweetness and creaminess vary with phenotypic markers of oral sensation. Chem Senses. 2007;32(3):225–36. doi: 10.1093/chemse/bjl050. [DOI] [PubMed] [Google Scholar]
- 22.Keller KL, Steinmann L, Nurse RJ, Tepper BJ. Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite. 2002;38(1):3–12. doi: 10.1006/appe.2001.0441. [DOI] [PubMed] [Google Scholar]
- 23.Keller KL, Tepper BJ. Inherited taste sensitivity to 6-n-propylthiouracil in diet and body weight in children. Obesity Research. 2004;12(6):904–912. doi: 10.1038/oby.2004.110. [DOI] [PubMed] [Google Scholar]
- 24.Prescott J, Soo J, Campbell H, Roberts C. Responses of PROP taster groups to variations in sensory qualities within foods and beverages. Physiol Behav. 2004;82(2–3):459–69. doi: 10.1016/j.physbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Duffy VB, Hayes JE, Dinehart ME. Genetic Differences in Sweet Taste Perception. In: Spillane WJ, editor. Optimising the sweet taste in foods. Cambridge (UK): Woodhead Publishing; 2006. [Google Scholar]
- 26.Looy H, Weingarten HP. Facial expressions and genetic sensitivity to 6-n-propylthiouracil predict hedonic response to sweet. Physiol Behav. 1992;52(1):75–82. doi: 10.1016/0031-9384(92)90435-5. [DOI] [PubMed] [Google Scholar]
- 27.Yeomans MR, Tepper BJ, Rietzschel J, Prescott J. Human hedonic responses to sweetness: role of taste genetics and anatomy. Physiol Behav. 2007;91(2–3):264–73. doi: 10.1016/j.physbeh.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Miller IJ, Jr, Reedy FE., Jr Variations in human taste bud density and taste intensity perception. Physiol Behav. 1990;47(6):1213–9. doi: 10.1016/0031-9384(90)90374-d. [DOI] [PubMed] [Google Scholar]
- 29.Hayes JE, Bartoshuk LM, Kidd JK, Duffy VB. Supertasting and PROP Bitterness Depends on More Than the TAS2R38 Gene. Chem Senses. 2008 doi: 10.1093/chemse/bjm084. Advance Access published on January 21, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Corlis R, Splaver G, Wisecup P, Fischer R. Myers-Briggs type personality scales and their relation to taste acuity. Nature. 1967;216(5110):91–2. doi: 10.1038/216091a0. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan AR, Glanville EV, Fischer R. Taste Thresholds for Bitterness and Cigarette Smoking. Nature. 1964;202:1366. doi: 10.1038/2021366a0. [DOI] [PubMed] [Google Scholar]
- 32.Fischer R, Griffin F, England S, Garn SM. Taste thresholds and food dislikes. Nature. 1961;191:1328. doi: 10.1038/1911328a0. [DOI] [PubMed] [Google Scholar]
- 33.Glanville EV, Kaplan AR. Food Preference and Sensitivity of Taste for Bitter Compounds. Nature. 1965;205:851–853. [Google Scholar]
- 34.Falciglia GA, Norton PA. Evidence for a genetic influence on preference for some foods. J Am Diet Assoc. 1994;94(2):154–8. doi: 10.1016/0002-8223(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 35.Dinehart ME, Hayes JE, Bartoshuk LM, Lanier SL, Duffy VB. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol Behav. 2006;87(2):304–13. doi: 10.1016/j.physbeh.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Hayes JE, Chapo AK, Bartoshuk LM, Duffy VB. Orosensory and genetic taste (GT) markers predict alcohol intake across age cohorts. Chem Senses. 2005;30(3):A6. [Google Scholar]
- 37.Fischer R, Griffin F. Quinine Dimorphism: A Cardinal Determinant of Taste Sensitivity. Nature. 1963;200:343–7. doi: 10.1038/200343a0. [DOI] [PubMed] [Google Scholar]
- 38.Prutkin J, Fisher EM, Etter L, Fast K, Gardner E, Lucchina LA, Snyder DJ, Tie K, Weiffenbach J, Bartoshuk LM. Genetic variation and inferences about perceived taste intensity in mice and men. Physiol Behav. 2000;69(1–2):161–73. doi: 10.1016/s0031-9384(00)00199-2. [DOI] [PubMed] [Google Scholar]
- 39.Dinehart ME. Master’s Thesis. Storrs: University of Connecticut; 2005. The bitter and the sweet : oral sensory markers of vegetable and sugar intake. [Google Scholar]
- 40.Lawless H. Taste of Creatine and Creatinine. Chem Sens Flav. 1979;4(3):249–258. [Google Scholar]
- 41.Hansen JL, Reed DR, Wright MJ, Martin NG, Breslin PA. Heritability and genetic covariation of sensitivity to PROP, SOA, quinine HCl, and caffeine. Chem Senses. 2006;31(5):403–13. doi: 10.1093/chemse/bjj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartoshuk LM, Duffy VB, Reed D, Williams A. Supertasting, earaches and head injury: genetics and pathology alter our taste worlds. Neurosci Biobehav Rev. 1996;20(1):79–87. doi: 10.1016/0149-7634(95)00042-d. [DOI] [PubMed] [Google Scholar]
- 43.Sainz E, Cavenagh MM, Gutierrez J, Battey JF, Northup JK, Sullivan SL. Functional characterization of human bitter taste receptors. Biochem J. 2007;403(3):537–43. doi: 10.1042/BJ20061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coombs CH, Avrunin GS. Single-peaked functions and the theory of preference. Psychol Rev. 1977 [Google Scholar]
- 45.Beebe-Center JG. The Psychology of Pleasantness and Unpleasantness. New York: D. Van Nostrand Company, Inc; 1932. [Google Scholar]
- 46.Pfaffmann C. Wundt’s Schema of Sensory Affect in the Light of Research on Gustatory Preference. Psychol Res. 1980;42:165–174. doi: 10.1007/BF00308700. [DOI] [PubMed] [Google Scholar]
- 47.Moskowitz HR, Kluter RA, Westerling J, Jacobs HL. Sugar sweetness and pleasantness: evidence for different psychological laws. Science. 1974;184(136):583–5. doi: 10.1126/science.184.4136.583. [DOI] [PubMed] [Google Scholar]
- 48.Khuri AI, Cornell JA. Response surfaces : designs and analyses. xii. New York: M. Dekker; 1987. p. 405. [Google Scholar]
- 49.Drewnowski A, Greenwood MR. Cream and sugar: human preferences for high-fat foods. Physiol Behav. 1983;30(4):629–33. doi: 10.1016/0031-9384(83)90232-9. [DOI] [PubMed] [Google Scholar]
- 50.Drewnowski A, Brunzell JD, Sande K, Iverius PH, Greenwood MR. Sweet tooth reconsidered: taste responsiveness in human obesity. Physiol Behav. 1985;35(4):617–22. doi: 10.1016/0031-9384(85)90150-7. [DOI] [PubMed] [Google Scholar]
- 51.Sunday SR, Halmi KA. Taste perceptions and hedonics in eating disorders. Physiol Behav. 1990;48(5):587–94. doi: 10.1016/0031-9384(90)90196-b. [DOI] [PubMed] [Google Scholar]
- 52.Warwick ZS, Schiffman SS. Sensory evaluations of fat-sucrose and fat-salt mixtures: relationship to age and weight status. Physiol Behav. 1990;48(5):633–6. doi: 10.1016/0031-9384(90)90202-f. [DOI] [PubMed] [Google Scholar]
- 53.Drewnowski A, Henderson SA, Barratt-Fornell A. Genetic sensitivity to 6-n-propylthiouracil and sensory responses to sugar and fat mixtures. Physiol Behav. 1998;63(5):771–7. doi: 10.1016/s0031-9384(97)00540-4. [DOI] [PubMed] [Google Scholar]
- 54.Duffy VB, Peterson J, Bartoshuk LM. Associations between taste genetics, oral sensations and alcohol intake. Physiol Behav. 2004;82(2–3):435–445. doi: 10.1016/j.physbeh.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 55.Laessle RG, Tuschl RJ, Kotthaus BC, Pirke KM. A comparison of the validity of three scales for the assessment of dietary restraint. J Abnorm Psychol. 1989;98(4):504–7. doi: 10.1037//0021-843x.98.4.504. [DOI] [PubMed] [Google Scholar]
- 56.Herman CP, Polivy J. Anxiety, restraint, and eating behavior. J Abnorm Psychol. 1975;84(6):66–72. [PubMed] [Google Scholar]
- 57.Herman C, Polivy J. Restrained eating. In: Stunkard A, editor. Obesity. Philadelphia, PA: W.B. Saunders; 1980. pp. 208–225. [Google Scholar]
- 58.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 59.Gorman B, Allison D. Measures of Restrained Eating. In: Allison D, editor. Handbook of assessment methods for eating behaviors and weight-related problems. London: Sage Publications; 1996. pp. 149–183. [Google Scholar]
- 60.Bartoshuk LM, Duffy VB, Miller IJ. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol Behav. 1994;56(6):1165–71. doi: 10.1016/0031-9384(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 61.Helson H. Adaptation-level theory; an experimental and systematic approach to behavior. xvii. New York: Harper & Row; 1964. p. 732. [Google Scholar]
- 62.Lawless HT, Horne J, Speirs W. Contrast and Range Effects for Category, Magnitude and Labeled Magnitude Scales in Judgments of Sweetness Intensity. Chem Senses. 2000;25:85–92. doi: 10.1093/chemse/25.1.85. [DOI] [PubMed] [Google Scholar]
- 63.Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, Snyder DJ. Labeled scales (eg, category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Qual Pref. 2003;14(2):125–138. [Google Scholar]
- 64.Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004;82(1):109–14. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 65.Green BG, Shaffer GS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 1993;18(6):683–702. [Google Scholar]
- 66.Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 1996;21(3):323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- 67.Bartoshuk LM, Fast K, Snyder DJ. Differences in Our Sensory Worlds: Invalid Comparisons With Labeled Scales. Current Directions in Psychological Science. 2005;14(3):122–125. [Google Scholar]
- 68.Lanier SA, Hayes JE, Duffy VB. Sweet and bitter tastes of alcoholic beverages mediate alcohol intake in of-age undergraduates. Physiol Behav. 2005;83(5):821–831. doi: 10.1016/j.physbeh.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Moser EB. Repeated Measures Modeling With PROC MIXED. Proceedings of the 29th SAS Users Group International Conference; 2004. [Google Scholar]
- 70.Dallal GE. Multi-Factor Analysis of Variance [internet] [cited 17 Oct 2006];2001 Available at: http://www.tufts.edu/~gdallal/anova2.htm.
- 71.Drewnowski A, Moskowitz HR. Sensory characteristics of foods: new evaluation techniques. Am J Clin Nutr. 1985;42(5 Suppl):924–31. doi: 10.1093/ajcn/42.5.924. [DOI] [PubMed] [Google Scholar]
- 72.Freund RJ, Littell RC. SAS System for regression. viii. Cary, N.C: SAS Institute; 2000. p. 236. [Google Scholar]
- 73.Bartoshuk LM, Snyder DJ, Grushka M, Berger AM, Duffy VB, Kveton JF. Taste damage: previously unsuspected consequences. Chem Senses. 2005;30(Suppl 1):i218–i219. doi: 10.1093/chemse/bjh192. [DOI] [PubMed] [Google Scholar]
- 74.Hayes JE. Statistical modeling of links between taste markers, fat/sweet sensation and preference, and central adiposity. Storrs, CT: University of Connecticut; 2007. [Google Scholar]
- 75.Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 2002;277(1):1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- 76.Duffy VB, Fast K, Cohen Z, Chodos E, Bartoshuk LM. Genetic taste status associates with fat food acceptance and body mass index in adults. Chem Senses. 1999;24:545–546. (abstract) [Google Scholar]
- 77.Tepper BJ, Ullrich NV. Influence of genetic taste sensitivity to 6-n-propylthiouracil (PROP), dietary restraint and disinhibition on body mass index in middle-aged women. Physiol Behav. 2002;75(3):305–12. doi: 10.1016/s0031-9384(01)00664-3. [DOI] [PubMed] [Google Scholar]
- 78.Duffy VB, Lucchina LA, Bartoshuk LM. Genetic Variation in Taste: Potential Biomarker for Cardiovascular Disease Risk? In: Prescott J, Tepper BJ, editors. Genetic Variation in Taste Sensitivity. New York: Marcel Dekker; 2004. pp. 195–227. [Google Scholar]
- 79.Tepper BJ, Nurse RJ. PROP taster status is related to fat perception and preference. Ann N Y Acad Sci. 1998;855:802–4. doi: 10.1111/j.1749-6632.1998.tb10662.x. [DOI] [PubMed] [Google Scholar]
- 80.Savage J, Davidson H. Genetic sensitivity to PROP and its relationship with energy intake and short term satiety. Eur J Clin Nutr. 2000;54(Supplement 4):S15–S16. [Google Scholar]
- 81.Kamphuis MM, Westerterp-Plantenga MS. PROP sensitivity affects macronutrient selection. Physiol Behav. 2003;79(2):167–72. doi: 10.1016/s0031-9384(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 82.Yackinous CA, Guinard JX. Relation between PROP (6-n-propylthiouracil) taster status, taste anatomy and dietary intake measures for young men and women. Appetite. 2002;38(3):201–9. doi: 10.1006/appe.2001.0481. [DOI] [PubMed] [Google Scholar]
- 83.Zandstra EH, de Graaf C, van Trijp HCM, van Staveren WA. Laboratory hedonic ratings as predictors of consumption. Food Qual Prefer. 1999;10(4–5):411–418. [Google Scholar]
- 84.Pangborn RM. Individual Variation in affective responses to taste stimuli. Psychon Sci. 1970;21(2):125–126. [Google Scholar]
- 85.Lundgren B, Jonsson B, Pangborn RM, Sontag AM, Barylko-Pikielna N, Pietrzak E, Garruti RS, Chaib MA, Yoshida M. Taste discrimination vs hedonic response to sucrose in coffee beverage. An interlaboratory study. Chem Senses Flav. 1978;3(3):249–265. [Google Scholar]
- 86.Thompson DA, Moskowitz HR, Campbell RG. Taste and olfaction in human obesity. Physiol Behav. 1977;19(2):335–7. doi: 10.1016/0031-9384(77)90348-1. [DOI] [PubMed] [Google Scholar]
- 87.de Graaf C, van Staveren W, Burema J. Psychophysical and psychohedonic functions of four common food flavours in elderly subjects. Chem Senses. 1996;21(3):293–302. doi: 10.1093/chemse/21.3.293. [DOI] [PubMed] [Google Scholar]
- 88.Randall E, Sanjur D. Food preferences – their conceptualization and relationship to consumption. Ecol Food Nutr. 1981;11:151–161. [Google Scholar]
- 89.Drewnowski A, Shrager EE, Lipsky C, Stellar E, Greenwood MR. Sugar and fat: sensory and hedonic evaluation of liquid and solid foods. Physiol Behav. 1989;45(1):177–83. doi: 10.1016/0031-9384(89)90182-0. [DOI] [PubMed] [Google Scholar]
- 90.Tepper BJ. 6-n-Propylthiouracil as a Genetic Taster Marker for Fat Intake, Obesity, and Chronic Disease Risk. In: Prescott J, Tepper BJ, editors. Genetic Variation in Taste Sensitivity. New York: Marcel Dekker; 2004. pp. 155–178. [Google Scholar]
- 91.Kirkmeyer SV, Tepper BJ. Consumer reactions to creaminess and genetic sensitivity to 6-n-propylthiouracil: A multidimensional study. Food Qual Prefer. 2005;16(6):545–556. [Google Scholar]
- 92.de Wijk RA, Dijksterhuis G, Vereijken P, Prinz JF, Weenen H. PROP sensitivity relfects sensory discrimination between custard desserts. Food Qual Pref. 2007;18(4):597–604. [Google Scholar]
- 93.Mela DJ. Determinants of food choice: relationships with obesity and weight control. Obes Res. 2001;9(Suppl 4):249S–255S. doi: 10.1038/oby.2001.127. [DOI] [PubMed] [Google Scholar]
- 94.Blundell JE, Cooling J. Routes to obesity: phenotypes, food choices and activity. Br J Nutr. 2000;83(Suppl 1):S33–8. doi: 10.1017/s0007114500000933. [DOI] [PubMed] [Google Scholar]
- 95.O’Rahilly S, Farooqi IS. Genetics of obesity. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1095–105. doi: 10.1098/rstb.2006.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]