Abstract
Tegaserod relieves overall and multiple individual constipation-predominant IBS (IBS-C) symptoms. However, mechanisms for the relief of abdominal pain/discomfort are not well understood. The effects of tegaserod on rectal sensitivity to distension were measured by the nociceptive flexion RIII reflex, as evidenced by spinal hyperexcitability (i.e. increase or facilitation of the RIII reflex), in IBS-C patients. A randomized, double-blind, placebo-controlled, parallel study was performed in 30 women with IBS-C. The effects of slow ramp rectal distension on the RIII reflex, recorded from the lower limb, were measured before (D1) and after 7 days (D8) of placebo (n = 15) or 6 mg tegaserod bid (n = 15). Pressure–volume and sensation–volume relationships were measured during distension, and patients reported their IBS symptoms daily. On D1, rectal distension facilitated the RIII reflex in both treatment groups. On D8 versus D1 these facilitatory effects were significantly lower (P < 0.001, ANOVA) after tegaserod (mean reduction: −30.3 ± 11.9%) than placebo (mean reduction: −10.1 ± 12.9%). No significant changes in the volume–sensation relationship or differences in compliance were observed with tegaserod or placebo. In conclusion, tegaserod reduces the facilitatory effects of rectal distension on the RIII reflex in women with IBS-C.
Keywords: Adult, Compliance, Constipation, etiology, physiopathology, Double-Blind Method, Female, Humans, Indoles, pharmacology, Irritable Bowel Syndrome, complications, physiopathology, Manometry, Middle Aged, Placebos, Rectum, drug effects, physiopathology, Reflex, drug effects, Serotonin Agonists, pharmacology
Keywords: abdominal pain, constipation-predominant irritable bowel syndrome, IBS-C, RIII reflex, tegaserod
Irritable bowel syndrome (IBS), one of the most common functional gastrointestinal disorders, is characterized by recurrent abdominal pain/discomfort and abnormal bowel function.1 Pain and discomfort may be a consequence of hyperexcitability of nociceptive systems (i.e. central sensitization),2 which could explain the hypersensitivity to rectal distension consistently reported in most IBS patients.3 This new pathophysiological concept highlights the need for standardized and reproducible methods to evaluate visceral sensitivity in humans. We therefore developed a reflexological technique based on the modulation of a somatic nociceptive cutaneomuscular flexion reflex (RIII reflex) during gut distension as a possible tool for objective assessment of visceral sensitivity in humans.4–9
The RIII reflex is a polysynaptic spinal reflex elicited by electrical stimulation of a cutaneous sensory nerve (usually the sural nerve at the ankle) that can be recorded from a flexor muscle on the ipsilateral limb (usually the biceps femoris).10 It is considered to be a reliable index of spinal transmission of nociceptive signals, and indeed, its threshold and amplitude are related closely to those of concomitant painful cutaneous sensations evoked by electrical stimulation.10 In healthy subjects, we demonstrated that gastric or rectal distensions induced a reduction of the RIII reflex response and that the change in the response was correlated with both the volume of the distension and the visceral sensation.4,6,7 Using this methodology we demonstrated that, in about two thirds of IBS patients, slow ramp rectal distension induced an increase (“ie facilitation”)of the RIII reflex response.9 These facilitatory effects, which probably reflect alterations of pain modulatory mechanisms, can therefore be used as an objective and quantifiable index of the hyperexcitability of spinal nociceptive processes in a subgroup of IBS patients. We also showed that this electrophysiological approach is of value for investigating the mechanisms of action of pharmacological agents.5,7,8
The efficacy of tegaserod, a 5-HT4 receptor agonist, in the treatment of IBS with constipation (IBS-C) in women has been confirmed in several large randomized controlled trials.11–16 In addition, we have previously analysed the effects of tegaserod on the modulation of the RIII response induced by rectal distension in healthy women, and showed that it may act directly on nociceptive processes.8
Here we report a double-blind, placebo-controlled study, evaluating the effects of tegaserod on modulation of the RIII reflex induced by rectal distension in women with IBS-C.
MATERIAL AND METHODS
Participants
Following approval from the local Ethics Committee, the study was conducted from 11 July 2003 to 29 October 2004 and included 32 women, aged 18 to 60 years, who provided written informed consent.
Patients were eligible if they met IBS-C (ROME II) criteria.1 Specifically, they had to experience abdominal pain/discomfort with two of the following characteristics for at least 12 weeks (not necessarily consecutive) of the previous 12 months: 1) relief with defecation; 2) onset associated with a change in stool frequency; 3) onset associated with a change in stool form. Each patient underwent a complete clinical evaluation to exclude organic disease. Women were excluded if they had undergone abdominal surgery other than appendectomy, and only women with a normal total colonoscopy within the previous 5 years were included in the study.
Analgesics, antispasmodics, laxatives and antidiarrhoeal agents were stopped at least 7 days before the start of the experimental protocol. IBS-C criteria were confirmed during a 7-day baseline period, during which patients completed a daily questionnaire about their abdominal pain/discomfort (0 = none to 6 = very severe), the number of bowel movements, and stool consistency (1 = watery to 7 = very hard). At the end of the baseline period, the patients responded ‘Yes’ or ‘No’ to the question ‘Did you have satisfactory relief of your overall symptoms during the last week?’ Only patients who reported at least 4 days with abdominal discomfort or pain score ≥ 3 (i.e. at least moderate pain), mean stool consistency ≥ 4 (i.e. neither loose nor hard) and answered ‘No’ to the question about the overall relief were included in the study.
Nociceptive flexion reflex (RIII reflex)
The RIII reflex was elicited from the lower limb and recorded using an entirely computerized system (PhysioLab System, Notocord, Igny, France) as previously described.4,6–9 Briefly, the subjects were placed in the lateral decubitus position. The sural nerve was electrically stimulated at a frequency of 0.17 Hz (10 stimulations/min) via a pair of surface electrodes placed 2 cm apart on degreased skin over the retromalleolar path of the nerve. Each electrical stimulation consisted of a train of five constant-current pulses of 1-ms duration. Electromyographic responses were recorded from the ipsilateral biceps femoris via a pair of surface electrodes placed 2 cm apart on degreased skin over the muscle. The RIII response was identified as a multiphasic signal appearing between 90 and 180 ms after each stimulation. After amplification, each reflex response was digitized, full-wave rectified, and integrated. The RIII response was thus scored as the integrated surface area of the signal. The RIII reflex threshold in each patient was defined as the average minimal current that elicited the reflex response. The intensity of electrical stimulation of the sural nerve was then adjusted to 20% above the threshold and kept constant during the pre-distension (control), distension and post-distension periods of each experimental sequence (see below).
Rectal distension
An oversized spherical polyvinyl bag (10 cm in diameter; infinite compliance until maximal volume of 600 mL) was mounted on the tip of a double-lumen polyvinyl tube (12 Fr), folded tightly, lubricated and inserted into the rectum. The distal attachment site was 4 cm from the anal verge. The proximal opening of the tube was linked to an electronic barostat (INRA, Toulouse, France) that allowed controlled inflation and deflation of the balloon with air, and continuous monitoring and recording of the volume and pressure inside the balloon. When in place, the balloon was unfolded by slowly injecting air under controlled pressure (< 20 mmHg) and then completely deflated. After a 20-min rest period, the barostat was used to inflate the balloon at a constant rate of 40 mL/min (slow ramp distension).
Experimental design
Each patient participated in two experimental sessions, separated by 1 week. After verification of inclusion and exclusion criteria, control pre-treatment measurements were taken on the first experimental day (D1) after a 12-hour fast. The experimental session began with the determination of the RIII reflex threshold. Rectal distension was then performed up to the pain threshold (i.e., minimal distension eliciting a pain sensation). The RIII reflex responses were measured continuously for 3 minutes before the distension (i.e., pre-distension control period), during rectal distension, and for 3 minutes following the distension. The sensations elicited by rectal distension were scored as described below. At the end of the first experimental session, the patients were randomized to receive either 6 mg tegaserod twice daily (bid) or placebo for the following 7 days. During these 7 days they were asked to record their symptoms daily (abdominal pain and discomfort, number of bowel movements and stool consistency), in a diary similar to that used for the pre-inclusion period. On Day 8 (D8), at the end of the treatment, the last tablet of tegaserod or placebo was ingested 1 hour before the second experimental session, which was performed as described for D1. During this experimental session the investigators were blinded to the patients’ D1 thresholds and reflex responses
Sensation elicited by rectal distension
Before the experiments, participants were informed of the visceral sensations they might experience. The sensation elicited by the rectal distension was graded from 0 to 6 using a validated verbal questionnaire: 0 = no perception; 1 = initial perception; 2 = sensation of gas; 3 = sensation of stool; 4 = urge to defecate or onset of discomfort; 5 = moderate pain; and 6 = intense or unbearable pain.6,7,9 Subjects reported their sensations at fixed intervals (every 50 mL of distension). Distensions were stopped at the pain threshold (i.e., score 5). If intense or unbearable pain (score 6) was experienced during any level of distension, the experiment was ended immediately.
Data analysis
The primary endpoint was the effect of treatment on the RIII reflex response during rectal distension. Secondary endpoints were the pressure–volume and sensation–volume relationships, and the mean symptom scores and stool consistency scores after treatment.
For each distension on D1 and D8, the RIII responses were averaged at 1-minute intervals in the pre- and post-distension periods and at every 50 mL during the distension. All the reflex responses are expressed as a percentage of the mean value for the control pre-distension period. If the pain threshold was reported before 600 mL, the RIII value recorded at the maximal tolerated volume was reported for subsequent volumes to standardize data (last observation carried forward). Pressure was recorded at fixed intervals (every 50 mL of distension) during slow ramp distension.
Intergroup changes from pre-treatment (D1) to post-treatment (D8) were tested by analysis of variance (ANOVA) or the Wilcoxon rank sum test. ANOVA included treatment and distension volumes as factors. A significance level of 0.05 (two-sided) was used in all cases. Wilcoxon rank sum and Fisher’s exact tests were used to assess the homogeneity of the treatment groups. Adverse events were summarized by body system and by individual adverse events. Severity and relation to study medication were noted. No interim analysis was performed.
RESULTS
Most of the IBS-C patients selected for the study were followed for IBS symptoms by one of the physicians of the gastroenterology department; the others were recruited after advertising in a local paper. The 32 IBS-C patients were screened on D1, of whom two were excluded because they could not tolerate the electrical stimulation. Thus, 30 women with IBS-C (mean age: 42.9 ± 9.5 years) were randomized to receive either placebo (n = 15) or tegaserod (n = 15) and completed the study. Demographic and clinical characteristics were similar in both groups (Table 1).
Table 1.
Baseline demographic and clinical characteristics of the patients
| Variables | Tegaserod (n = 15) | Placebo (n = 15) |
|---|---|---|
| Age (yr) | ||
| Mean (SD) | 44.9 (7.4) | 41.0 (11.1) |
| Range | 29.0–56.0 | 22.0–54.0 |
| Race (%) | ||
| Caucasian | 80.0 | 100.0 |
| Black | 13.3 | 0 |
| Asian | 6.7 | 0 |
| Height (cm) | ||
| Mean (SD) | 161.7 (5.8) | 162.9 (6.3) |
| Range | 154.0–76.0 | 151.0–170.0 |
| Weight (kg) | ||
| Mean (SD) | 60.4 (15.0) | 62.7 (12.1) |
| Range | 41.0–88.0 | 44.0–92.0 |
| Body mass index (kg/m2) | ||
| Mean (SD) | 23.0 (5.3) | 23.6 (4.1) |
| Range | 17.1–33.3 | 17.2–31.8 |
| Abdominal pain (0 = none, 6 = very severe) | ||
| Mean (SD) | 3.06 ± 0.73 | 3.35 ± 0.81 |
| Bowel movements | ||
| Mean (SD) | 0.73 ± 0.47 | 0.87 ± 0.58 |
| Stool consistency (1 = watery, 7 = very hard) | ||
| Mean (SD) | 4.63 ± 1.22 | 5.06 ± 1.16 |
SD = standard deviation.
Effects of tegaserod and placebo on the modulation of RIII reflex by rectal distension
The RIII reflex threshold was not significantly different between the tegaserod (8.0 ± 0.4 mA) and placebo (7.5 ± 0.5 mA) groups on D1, and was not significantly altered by treatment on D8 (8.2 ± 0.6 vs 7.8 ± 0.5 mA, respectively). Sural nerve stimulation at 1.2 times threshold elicited a fairly stable RIII response and evoked a moderate sensation of pain of the pinprick type in every patient; this procedure was well tolerated throughout the experiments.
On D1, the RIII reflex was increased (i.e., facilitation) during rectal distension in both groups, but the magnitude of the facilitation was significantly higher (P < 0.05, ANOVA) in the tegaserod group (134.3 ± 43.9%) than in the placebo group (112.8 ± 50.9%). On D8, compared with D1, the facilitation of the RIII response was significantly more reduced (P < 0.001, ANOVA) by tegaserod treatment (mean reduction = −30.3 ± 11.9%) than by placebo (mean reduction = −10.1 ± 12.9%). The reduction of facilitation was consistent throughout the distension period (Figure 1). An individual example of the effects of tegaserod on the facilitation of the RIII reflex induced by rectal distension is presented in Figure 2.
Figure 1. Overall effects of tegaserod (black circles) and placebo (white circles) on the facilitation of the RIII reflex during rectal distension.
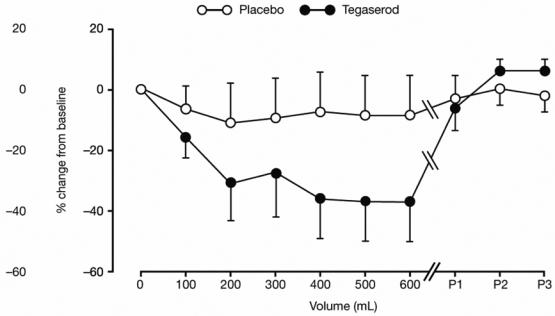
Data (mean ± standard deviation [SD]) are expressed as % change from baseline (D8 minus D1) during rectal distension (0–600 mL). Reduction in the facilitation of the RIII reflex was significantly greater with tegaserod than placebo (P < 0.001, analysis of variance [ANOVA]). Data recorded at each minute of the 3 minutes immediately following distension are also shown (P1–P3).
Figure 2. An example of RIII reflex recordings during slow ramp distension for an individual patient before and after 1 week of treatment with tegaserod.
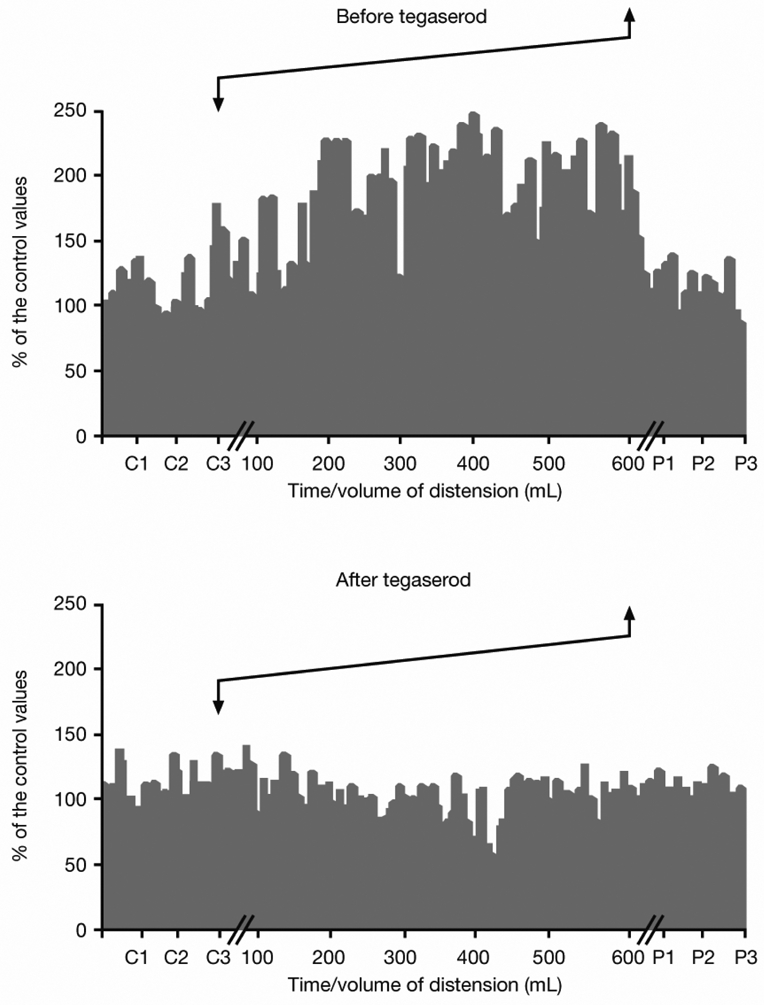
Each bar represents a single reflex response, expressed as a percentage of the control value (i.e., mean value recorded during each minute of the 3-minute control period prior to distension [C1–C3]). The distension is indicated by arrows. Facilitation of the RIII reflex induced by rectal distension was lower on D8 than on D1. P1–P3 refers to each minute of the 3-minute period following distension.
The difference between the two treatment groups in the mean facilitation effects on D1 was not due to a difference in the magnitude of facilitation itself, but to the unequal distributions of patients presenting facilitation or inhibitory effects. Rectal distension increased the RIII reflex in 20 patients (eight in the placebo group and 12 in the tegaserod group), and decreased the RIII reflex in 10 patients (seven in the placebo group and three in the tegaserod group). Consequently, the data were further analysed by comparing treatment within the subgroups of patients with facilitation and inhibition of the RIII reflex on D1 (Figures 3a and c). The magnitudes of facilitation and inhibition were similar before tegaserod and placebo. On D8 in the subgroup of patients with inhibition, the inhibitory effects were not significantly altered by tegaserod or placebo (Figure 3d). In contrast, in the subgroup of patients with facilitation, facilitation was significantly reduced (P < 0.001) by tegaserod compared with placebo (Figure 3b).
Figure 3. Effects of tegaserod and placebo on the modulation of the RIII reflex during rectal distension according to RIII reflex profile on D1.
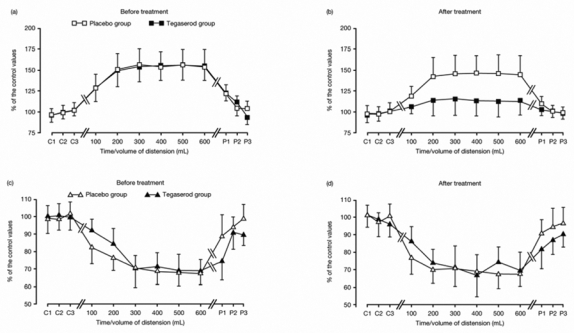
Data (mean ± standard deviation [SD]) are shown for patients with facilitation (squares) before (a) and after treatment (bor for patients with inhibition (triangles) before (c) and after treatment (d),. Tegaserod induced a significant (P < 0.001) reduction of the facilitatory effects (b). In contrast, the inhibitory effects (d) are not altered by tegaserod. No significant changes were observed after placebo.
Pressure–volume and sensation–volume relationships
The pressure–volume relationship was similar in the two groups on D1 and was not significantly affected by the treatment (Figure 4).
Figure 4. Pressure–volume relationships before (a) and after (b) 1 week of treatment with placebo (white) or tegaserod (black).
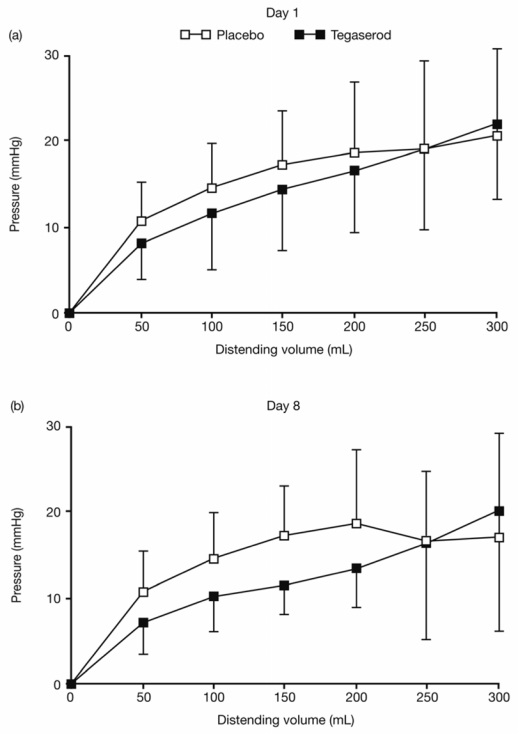
The pressure–volume curves were not significantly altered by treatment.
The sensation–volume relationship on D1 was not statistically different in the two treatment groups; the maximal volume of distension tolerated was 363 ± 123 mL in the tegaserod group and 286 ± 131 mL in the placebo group. This was not significantly modified on D8 (change from Day 1 to Day 8 was −70.0 ± 82 mL and −73.3 ± 121 mL in the tegaserod and placebo groups, respectively).
IBS symptoms
Mean IBS symptom scores (abdominal pain/discomfort, frequency of bowel movements, and mean stool consistency) on D1 did not differ significantly between the placebo and tegaserod groups (Table 1). The mean abdominal pain score was significantly lower after treatment with tegaserod than placebo (2.45 ± 1.29 vs 3.06 ± 0.73, P < 0.05). No significant change was observed in the frequency of bowel movements or mean stool consistency scores.
Safety
During the study, three of the 30 patients enrolled reported adverse events; two in the tegaserod group and one in the placebo group. In the tegaserod group, one patient experienced abnormal bowel sounds and another experienced nausea, headache and dry mouth. In the placebo group, one patient reported nausea. No serious adverse event was observed during the study.
DISCUSSION
This placebo-controlled study demonstrates that tegaserod reduces the pathological facilitation of the RIII flexion reflex induced by rectal distension in women with IBS-C. The threshold of the RIII reflex and the elastic properties of the rectum were not altered by tegaserod, suggesting possibly a selective interaction with spinal processing of sensory visceral information. These effects, probably the consequence of a reduction of spinal hyperexcitability, were associated with a significant decrease in abdominal pain/discomfort scores and, therefore, may contribute to the analgesic action of tegaserod.
Phase II and Phase III studies show that tegaserod effectively provides relief from overall and individual IBS symptoms, including a decreased abdominal pain, improved stool consistency, and increased bowel movement frequency with an acceleration of colonic transit time for women with IBS-C.11–16 However, the mechanisms of the analgesic action of tegaserod are not clearly established. In decerebrate cats, tegaserod dose-dependently inhibited the firing of rectal afferents during distension.17 A direct inhibition of mucosal mechanoreceptors may be a likely effect of tegaserod as it is rapidly absorbed and its actions mimic those of endogenous serotonin released from enterochromaffin cells, stimulating intrinsic sensory neurons in the intestinal mucosa.11 The unchanged visceral pain threshold observed here is consistent with this hypothesis. Also, the unchanged RIII reflex threshold in this study suggests that tegaserod does not have general analgesic effects, but may act selectively on sensory visceral processes.
We found that slow rectal distension facilitated the RIII reflex in approximately two thirds of IBS patients. This proportion was similar to that observed in our previous study using the same methodology but with a different cohort of IBS patients.9 Thus, our findings are consistent with IBS being a heterogeneous clinical entity resulting for more than one pathophysiological mechanism.
The abnormal increase in the RIII reflex during rectal distension may reflect a true hyperexcitability of the spinal viscero-somatic nociceptive processes (i.e., central sensitization), which could contribute to the pathophysiology of chronic abdominal pain in a subgroup of IBS patients. The normal RIII reflex threshold in patients in this study suggests that any such spinal hyperexcitability was not secondary to a direct alteration of the properties of spinal nociceptive neurons, but is the consequence of a failure of segmental and/or heterosegmental (i.e., descending) systems that modulate the spinal transmission of nociceptive signals. Interestingly, studies using other approaches also concluded that IBS symptoms were the result of abnormalities in pain modulation.18 The lower facilitation of the RIII reflex induced by rectal distension after tegaserod treatment may result from action on pain modulatory systems. The role of serotonergic systems in the descending modulation of the spinal transmission of nociceptive signals has been established in animals, but is less well described in humans.19 However, a specific pain modulatory system, Diffuse Noxious Inhibitory Controls (DNIC), initially described in the rat,20 has also been demonstrated in humans and involves serotonergic systems.21–24
An interesting finding with potential clinical significance in the present study is that tegaserod preferentially exhibits effects in patients presenting with a facilitation of the RIII reflex, but not in those presenting with a inhibition of the RIII reflex, during slow ramp rectal distension on D1 before the treatment period. These differential effects are again consistent with IBS being a heterogeneous condition, consisting of different subgroups of patients who respond differently to treatment. Identification of such subgroups may allow therapeutic strategies to be tailored to individual patients and thereby significantly improve treatment outcomes. Classification of patients according to a measurable neurophysiological index might be of particular value because the clinical criteria currently used, such as the ROME II criteria, do not discriminate between subgroups of patients according to pain mechanisms.25 Most Phase II and III clinical studies are performed with subgroups of IBS patients defined by their altered bowel habits (i.e., diarrhoea or constipation). These criteria have no direct relationship with pain mechanisms, and this could explain the relatively small magnitude of the analgesic effects generally reported by Phase II and III trials of IBS treatments. Our findings suggest that analysis of the effects of rectal distension on the RIII reflex may enable identification of subgroups of patients with spinal hyperexcitability that may respond differently to treatment. This interesting hypothesis needs to be tested with other clinically active pharmacological agents.
In conclusion, this study showed that tegaserod had a true objective effect on visceral sensitivity by reducing the facilitatory effects of rectal distension on the RIII reflex in a large subgroup of IBS-C patients.
Acknowledgments
The authors would like to acknowledge the editorial support provided by Mrs Claire Shewbridge of ACUMED® in the preparation of this manuscript. ACUMED’s contribution was funded by Novartis Pharma AG.
This study was supported by an unrestricted grant from Novartis Pharma SA, France.
LIST OF ABBREVIATIONS USED
- ANOVA
analysis of variance
- bid
twice daily
- D1
Day 1 (first experimental day)
- D8
Day 8
- DNIC
Diffuse Noxious Inhibitory Controls
- FDA
Food and Drugs Administration
- IBS
irritable bowel syndrome
- IBS-C
IBS with constipation
- SD
standard deviation
Footnotes
This study was presented at the 20th INT symposium on Neurogastroenterology and Motility (Toulouse, France 2005)and was published in an abstract form in Neurogastroenterol Motil, volume 17, Supplement 2, August 2005, A 158
Amy Wagner is an employee of, and owns stocks and shares in, Novartis Pharma AG, Switzerland. Yolande Loria is an employee of Novartis Pharma SA, France.
References
- 1.Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead W. Rome II: The Functional Gastrointestinal Disorders. 2 McLean, VA: Degnon Associates; 2000. [Google Scholar]
- 2.Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122:2032–48. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- 3.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 4.Bouhassira D, Chollet R, Coffin B, et al. Inhibition of a somatic nociceptive reflex by gastric distention in humans. Gastroenterology. 1994;107:985–92. doi: 10.1016/0016-5085(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 5.Coffin B, Bouhassira D, Chollet R, et al. Effect of the kappa agonist fedotozine on perception of gastric distension in healthy humans. Aliment Pharmacol Ther. 1996;10:919–25. doi: 10.1046/j.1365-2036.1996.109280000.x. [DOI] [PubMed] [Google Scholar]
- 6.Bouhassira D, Sabate JM, Coffin B, Le Bars D, Willer JC, Jian R. Effects of rectal distensions on nociceptive flexion reflexes in humans. Am J Physiol. 1998;275:G410–17. doi: 10.1152/ajpgi.1998.275.3.G410. [DOI] [PubMed] [Google Scholar]
- 7.Sabate JM, Coffin B, Jian R, Le Bars D, Bouhassira D. Rectal sensitivity assessed by a reflexologic technique: further evidence for two types of mechanoreceptors. Am J Physiol Gastrointest Liver Physiol. 2000;279:G692–9. doi: 10.1152/ajpgi.2000.279.4.G692. [DOI] [PubMed] [Google Scholar]
- 8.Coffin B, Farmachidi JP, Rueegg P, Bastie A, Bouhassira D. Tegaserod, a 5-HT4 receptor partial agonist, decreases sensitivity to rectal distension in healthy subjects. Aliment Pharmacol Ther. 2003;17:577–85. doi: 10.1046/j.1365-2036.2003.01449.x. [DOI] [PubMed] [Google Scholar]
- 9.Coffin B, Bouhassira D, Sabate JM, Barbe L, Jian R. Alteration of the spinal modulation of nociceptive processing in patients with irritable bowel syndrome. Gut. 2004;53:1465–70. doi: 10.1136/gut.2003.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willer JC. Comparative study of perceived pain and nociceptive flexion reflex in man. Pain. 1977;3:69–80. doi: 10.1016/0304-3959(77)90036-7. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M. Review article: tegaserod. Aliment Pharmacol Ther. 2001;15:277–89. doi: 10.1046/j.1365-2036.2001.00925.x. [DOI] [PubMed] [Google Scholar]
- 12.Muller-Lissner SA, Fumagalli I, Bardhan KD, et al. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther. 2001;15:1655–66. doi: 10.1046/j.1365-2036.2001.01094.x. [DOI] [PubMed] [Google Scholar]
- 13.Novick J, Miner P, Krause R, et al. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2002;16:1877–88. doi: 10.1046/j.1365-2036.2002.01372.x. [DOI] [PubMed] [Google Scholar]
- 14.Kellow J, Lee OY, Chang FY, et al. An Asia-Pacific, double blind, placebo controlled, randomised study to evaluate the efficacy, safety, and tolerability of tegaserod in patients with irritable bowel syndrome. Gut. 2003;52:671–6. doi: 10.1136/gut.52.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyhlin H, Bang C, Elsborg L, et al. A double-blind, placebo-controlled, randomized study to evaluate the efficacy, safety and tolerability of tegaserod in patients with irritable bowel syndrome. Scand J Gastroenterol. 2004;39:119–26. doi: 10.1080/00365520310006748. [DOI] [PubMed] [Google Scholar]
- 16.Tack J, Muller-Lissner S, Bytzer P, et al. A randomised controlled trial assessing the efficacy and safety of repeated tegaserod therapy in women with irritable bowel syndrome with constipation (IBS-C) Gut. 2005;54:1707–13. doi: 10.1136/gut.2005.070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schikowski A, Thewissen M, Mathis C, Ross HG, Enck P. Serotonin type-4 receptors modulate the sensitivity of intramural mechanoreceptive afferents of the cat rectum. Neurogastroenterol Motil. 2002;14:221–7. doi: 10.1046/j.1365-2982.2002.00328.x. [DOI] [PubMed] [Google Scholar]
- 18.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 20.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I : Effects on dorsal horn convergent neurones in the rat. Pain. 1979;6:283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- 21.Willer JC, Roby A, Le Bars D. Psychophysical and electrophysiological approaches to the pain- relieving effect of heterotopic nociceptive stimuli. Brain. 1984;107:1095–112. doi: 10.1093/brain/107.4.1095. [DOI] [PubMed] [Google Scholar]
- 22.Chitour D, Dickenson AH, Le Bars D. Pharmacological evidence for the involvement of serotonergic mechanisms in diffuse noxious inhibitory controls (DNIC) Brain Res. 1982;236:329–37. doi: 10.1016/0006-8993(82)90718-1. [DOI] [PubMed] [Google Scholar]
- 23.Kraus E, Besson JM, Le Bars D. Behavioral model for diffuse noxious inhibitory controls (DNIC): potentiation by 5-hydroxytryptophan. Brain Res. 1982;231:461–5. doi: 10.1016/0006-8993(82)90384-5. [DOI] [PubMed] [Google Scholar]
- 24.De Broucker T, Cesaro P, Willer JC, Le Bars D. Diffuse noxious inhibitory controls in man. Involvement of the spinoreticular tract. Brain. 1990;113:1223–34. doi: 10.1093/brain/113.4.1223. [DOI] [PubMed] [Google Scholar]
- 25.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]


