Abstract
Here we report on the severe defects in renal epithelium induced by the transgenic Col2-Cre line used previously for skeletal tissue-specific gene targeting. We demonstrate that conditional ablation of the Kif3a or Pkd1 genes encoding primary cilium/intraflagellar transport-associated proteins using type II collagen-specific Cre transgenic strain results in a severe form of polycystic kidney disease in mice. We detect Col2-Cre recombinase expression in kidney epithelium, which reflects expression of the endogenous Col1α(II) gene in the embryonic renal tubules. We determine the exon 2-containing splice variant of the Col1α(II) gene as a major transcript expressed in kidney. Furthermore, the confocal immunocytochemichal analysis demonstrates deposition of the type II collagen within the mesenchymal-epithelial renal tissue interfaces and its co-localization with the basement membrane marker collagen IV during embryonic kidney morphogenesis.
Keywords: Polycystic kidney, Type II collagen, Kinesin-2, Polycystin, Ciliary proteins
1. Introduction
Ever since type II collagen was discovered as a major component of chick cartilage by Miller and Matukas (Miller and Matukas, 1969), it has been considered as hallmark for chondrocytic differentiation and chondrocyte-specific marker. However, later studies demonstrated that type II collagen was expressed much earlier than the onset of chondrogenesis, which occurs at the time of condensation of the mesenchyme. Moreover, type II collagen has been detected in numerous tissues that never undergo chondrogenic differentiation (Cheah et al., 1991;Thorogood et al., 1986). Nevertheless, several Col2-specific Cre recombinase-expressing transgenic lines have been created and used to facilitate cartilage-specific gene inactivation. In this study, we employed one of the previously described Col2-Cre to inactivate two ciliary proteins, polycystin-1 and kinesin-2. We show that conditional knockout mice develop severe form of polycystic disease, in addition to multiple skeletal defects. Furthermore, we demonstrate that both Col2-Cre and the endogenous type II collagen are expressed in various types of embryonic epithelia, including epithelial cells of the renal tubules. The immunostaining experiments reveal that type II collagen is secreted and deposited at the basal surface of the epithelial cells where it co-localizes with a marker of the basement membrane, type IV collagen.
2. Results
2.1. Col2-Cre-mediated inactivation of kinesin-2 and polycystin-1 results in polycystic kidney disease
Our previous studies demonstrated that kinesin-2 motor is essential during the development of the cranial, axial and appendicular skeletal elements. Genetic ablation of the Kif3a subunit of kinesin-2 in the skeletogenic mesoderm and the neural crest mesenchyme results in abnormal tissue patterning, such as polydactyly, split sternum and midline defects of the craniofacial skeleton. These abnormalities were attributed to both impaired hedgehog signaling as well as a reduced level of Gli3 repressor (Kolpakova-Hart et al., 2007). In the process of studying the role of the ciliary proteins kinesin-2 and polycystin-1 during the endochondral bone growth we employed the Col2-Cre (Col2-Cre10) transgenic mouse strain, which was used in numerous previous studies for conditional gene inactivation in chondrocyte and osteoblast cell lineages (Long et al., 2001;Long et al., 2004;Razzaque et al., 2005). Consistent with the recently published studies (Koyama et al., 2007;Song et al., 2007), Col2-Cre; Kif3a knockout mice developed a severe form of dwarfism and displayed highly disorganized growth plates in the long bones and the cranial base synchondroses (Fig. 1A and data not shown). Because of the retarded overall growth, Col2-Cre; Kif3a knockout mice had to be euthanased upon weaning. Autopsy performed on the mutant mice revealed that in addition to the skeletal abnormalities, all animals developed polycystic kidney disease (Fig.1B).
Fig. 1.

Inactivation of kinesin-2 and polycystin-1 driven by Col2-Cre transgene results in skeletal and renal abnormalities.
A) Col2-Cre;Kif3a mice develop a severe form of dwarfism (left panel) due to premature obliteration of growth plates at the sites of endochondral ossification. H&E stained proximal tibia from P8 wild type (middle panel) and Col2-Cre;Kif3a mice (right panel).
B) H&E staining of P24 control (left panel) and polycystic kidney (right panel) from Col2-Cre;Kif3a mice. Cy-renal cyst.
C) Early postnatal polycystic kidney phenotype developing in Col2-Cre; Pkd1 mice (P12).
Col2-Cre; Pkd1 mutants displayed a mild reduction in the cortical bone deposition and cranial base defects, which were identical to the abnormalities previously described in Dermo1-Cre; Pkd1 conditional knockout mice (Kolpakova-Hart in preparation). Despite the relatively mild skeletal phenotype, all Col2-Cre; Pkd1 pups died 12 to 14 days after birth. In order to determine the cause of death, the internal organs of the mutant animals were examined. A severe form of polycystic kidney disease was concluded to be a major cause of death of Col2-Cre;Pkd1 conditional knockout animals (Fig. 1C).
2.2. Col2-Cre is expressed in embryonic kidney epithelium
To assess whether the kidney phenotypes observed in Col2-Cre specific kinesin-2 and polycystin-1 conditional knockouts were due to expression of Cre recombinase in kidney tissue, we crossed Col2-Cre mice with the Rosa26R reporter strain, in which β-galactosidase expression was activated by Cre recombinase (Soriano, 1999). In agreement with the previously reported expression pattern, Cre recombinase activity could be detected in the skeletal structures of the developing appendicular, axial and cranial skeleton, such as long bones of limbs, ribs, vertebrae and skull base (Figs. 2 A,B and data not shown). Importantly, Col2-Cre was also found in the epithelial cells of kidney, pancreas, lungs, intestine and ovary (Fig. 2 B–E and data not shown). The histological analysis of the polycystic kidney from the conditional Pkd1 knockout mice revealed Cre activity in the epithelial lining of the renal cysts (Fig. 2F).
Fig. 2.
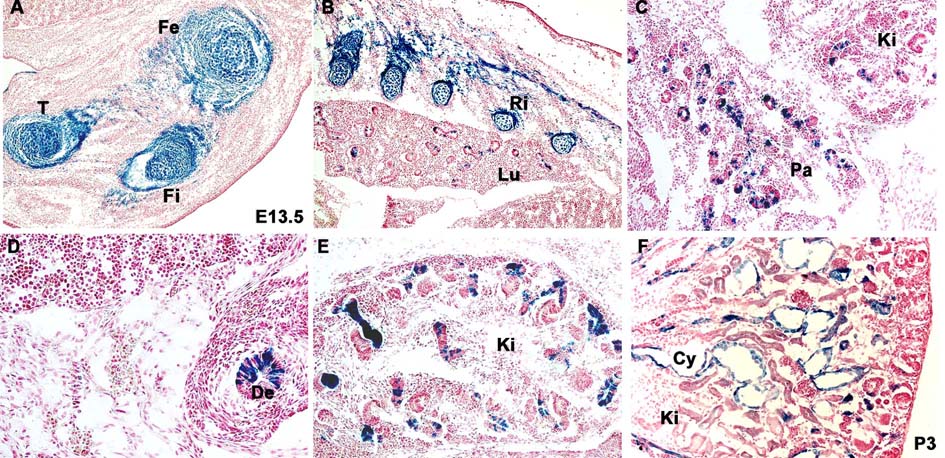
Expression pattern of the Col2-Cre transgene in chondrogenic and nonchondrogenic tissues revealed by activation of the Rosa26 reporter
A–E) β-galactosidase staining of E13.5 Col2-Cre;Rosa26 embryos displays Col2-Cre expression in the skeletogenic mesenchyme of the developing limb (A) and rib bones (B), as well as in the epithelial cells of the branchial (B), pancreatic (C), intestinal (D) and renal (E) tissues.
F) β-galactosidase staining of postnatal (P3) polycystic kidney from Col2-Cre;Pkd1;Rosa26 mice shows Col2-Cre-positive mutant cells lining the renal cysts (Cy). De-duodenum, Fe-femur, Fi-fibula, Ki-kidney, Lu-lung, Pa-pancreas, Ri-ribs.
2.3. The Col IIA splice variant of Col1α(II) is expressed in embryonic renal epithelium and the protein is deposited at the base of renal epithelial cells, where it co-localizes with type IV collagen
As the expression pattern of the Col2-Cre transgene utilized in the current study may not faithfully represent the expression of the endogenous Col1α(II), we amplified the endogenous type II collagen-specific mRNA using a combination of primers which recognizes both Col IIA and Col IIB splice variants of the gene. Interestingly, the longer Col IIA transcript appeared to be the predominant form of Col1α(II) mRNA expressed in kidney (Fig. 3A, lanes 3–7), whereas the shorter Col IIB form was the major splice variant detected in skeletal tissue (Fig. 3A, lanes 2 and 8). In order to visualize the expression pattern of the type II collagen in kidney, we also performed in situ hybridization on sections from E13.5 embryos using Col1α(II)-specific probe. Type Col1α(II) mRNA was detected in chondrocytes as well as in a variety of embryonic epithelial tissues, including renal tubule epithelium (Figure 3B–E).
Fig. 3.

Endogenous Col1α(II) is expressed and type II procollagen is secreted by both chondrocytes and epithelial cells during embryonic development in mouse.
A) Distinct expression pattern of type II collagen A (Col IIA) and B (Col IIB) splice variants in skeletal tissue and kidney visualized by RT-PCR. Lanes 1–8: (1) φX174 RF DNA-Hae digest DNA MW marker, (2) femur P1, (3) kidney E13.5, (4) kidney E16.5, (5) kidney E18.5, (6) kidney P1, (7) kidney P11, (8) humerus P1.
B–E) In situ hybridization of Col1α(II)-specific probe demonstrates the gene expression in cartilage (B,C) and the epithelial cells lining the renal tubules (C,D) and duodenum (E). De-duodenum, Ki-kidney, R-ribs, Vr-vertebrae.
F–J) Immunostaining of E13.5 embryos shows type II collagen accumulating around chondrocytes (F) and the basal aspect of the epithelial cells in kidney (G,H) and duodenum (J).
Next, we investigated whether type II collagen protein is synthesized and secreted by embryonic epithelial cells. Immunostaining with monoclonal type II collagen antibody revealed all sites of chondrogenesis both in the trunk and cranium, confirming the specificity of the antibody to this particular collagen, a major extracellular matrix marker of cartilage (Fig. 3 F). In addition, a variety of epithelial tissues, including all components of the digestive tract, bronchial, ovarian, renal epithelia and skin epidermis, were positive for type II collagen (Fig. 3 G–J and data not shown). Most of the protein was found at the base of the epithelial cell layers, the location normally occupied by the basement membrane, a continuous sheet of specialized extracellular matrix material, which separates epithelium from connective tissue. In order to evaluate the topological relationship of type II collagen secreted by epithelial cells and the basement membrane, we performed double immunostaining of sections from E13.5 embryos using antibody specific to type II and a major structural component of the basement membrane, type IV collagen. The domains occupied by these collagens overlapped substantially and mostly in the areas underlying the layers of epithelial and endothelial cells (Fig. 4A–D).
Fig. 4.

Double immunolabelling of type II (green) and type IV (red) collagens in embryonic ovary (A), kidney (B), skin (C) and blood vessels (D) reveals their substantial overlap and suggests that endogenous type II collagen is associated with basement membranes in the embryonic epithelial and endothelial tissues.
3. Discussion
Since the advent of the conditional gene inactivation method in mice, several independent transgenic Col2-Cre lines have been generated in an attempt to create a chondrocyte-specific Cre deleter strains (Haigh et al., 2000;Long et al., 2001;Ovchinnikov et al., 2000;Sakai et al., 2001;Schipani et al., 2001). These studies demonstrated that Col2-Cre was expressed in the chodrocyte and osteoblast lineages and affected the growth plates, perichondrium, periosteum and the osteoblasts in the bone collar and primary spongiosa. However, differences in the expression pattern of Col2-driven Cre recombinase were also recognized and expression in non-skeletal tissues, such as heart, brain, eye were reported. But to our knowledge, only one non-skeletal phenotype generated by Col2-Cre-driven gene inactivation has been previously described in the literature. In the study, conditional inactivation of one allele of Vegf-a resulted in early embryonic death due to vascular and heart abnormalities (Haigh et al., 2000). The variations in the expression pattern of the individual Col2-Cre strains might be attributed to several factors. First, the regulatory sequences consisted of variable length and configurations of the promoter and enhancer regions from rat, mouse and human Col1α(II) genes were exploited to drive Cre expression. Secondly, the sites where the Col2-Cre expression cassettes were integrated within the genome of these individual strains were most likely distinct and could affect the expression pattern and strength of the transgene. These two factors could result in distinct expression patterns of Cre recombinase, which might not faithfully reproduce the spatial and temporal pattern or/and level of the endogenous type II collagen.
Col2-Cre;Kif3a conditional knockout mice described in this study displayed the skeletal defects described previously (Koyama et al., 2007;Song et al., 2007), such as dwarfism and early postnatal obliteration of cranial base synchondroses. Unexpectedly, the mutant mice also developed early postnatal polycystic kidney disease, similar to that seen in model with kidney-specific inactivation of the Kif3a subunit of kinesin-2 (Lin et al., 2003).
The conditional Col2-Cre;Pkd1 knockout mice were characterized by a mild skeletal phenotype, such as premature closure of the cranial base synchondroses and delayed cortical and intramembranous bone deposition. These features were consistent with another conditional knockout model of Pkd1, where Cre recombinase expression is driven by mesoderm-specific Dermo1-Cre (Kolpakova-Hart in preparation). However, unlike the latter, Col2-Cre;Pkd1 mutant mice invariably died before weaning, as a result of a severe form of polycystic disease.
In adult mammals, type II collagen is mainly found in the skeleton (hyaline cartilage) and in the eye (vitreous humour). As this fibrillar collagen constitutes 60% of the extracellular matrix of chondrocytes, it has been traditionally considered as a cartilage-specific marker. However, several studies described transient embryonic expression of the mouse collagen 1α(II) in non-chondrogenic tissues, such as the epidermis, neural tube, the eye and the heart (Cheah et al., 1991;Linsenmayer et al., 1977;Ng et al., 1993;Smith, Jr. et al., 1976). A few studies detected Col1α(II) mRNA in the embryonic kidney using RT-PCR or Northern blotting. However, the spatial distribution of type II collagen and its transcripts in renal tissue remained unclear. In human fetuses, Col1α(II) mRNA was reported in the kidney mesenchyme, but not in the nephric tubule epithelium (Lui et al., 1995). Nevertheless, a study focusing on early chick development described mesonephric basement membrane, foregut endoderm and surface ectoderm as sites of type II collagen deposition (Kosher and Solursh, 1989). In our study we demonstrate that Col1α(II) is expressed in the epithelial cells of the metanephric derivatives, such as ovary and kidney, and other embryonic epithelia of the ectodermal, endodermal and mesodermal origin.
The function(s) of type II collagen in the basement membrane of epithelial cells remains unclear. It was proposed that the protein serves as the extracellular matrix component promoting chondrogenesis because its transient expression was detected on multiple epithelial-mesenchymal tissue interfaces preceding differentiation of the cranial mesenchyme into chondrocytes (Thorogood et al., 1986). Based on that observation, it was suggested that type II collagen had a morphogenetic embryonic function specifying the shape of the vertebrate chondrocranium (Wood et al., 1991). Although intriguing, this hypothesis could not explain the presence of type II collagen at other sites, such as the embryonic cornea, Rathke poach, and epidermis, which are not associated with cartilage formation (Fitch et al., 1989). Clearly, the function of type II collagen in the embryonic kidney has to be different from that suggested for the skeletogenic mesenchyme. One possibility is that the matrix protein plays a role in maintaining the structural strength of the attachment for the adjacent mesenchyme and support for the emerging renal epithelia. Similar to what was reported for several organ primordia within the cephalic region (otic vesicle, the presumptive retinal epithelia) and the trunk (spinal cord neuroepithelium) (Fitch et al., 1989;Thorogood et al., 1986), we found the type II collagen deposition sites under the renal epithelium to overlap with a major basement membrane component, collagen IV. As the renal epithelium is a product of mesenchymal-epithelial transition during embryogenesis, another intriguing possibility is that type II collagen might be involved in maintenance of the tubular epithelial cell identity and prevention of the epithelial-mesenchymal transdifferentiation. The layer containing type II collagen may function as a barrier to immobilize and limit access of macromolecules to renal epithelial and/or mesenchymal cells. Type II procollagen mRNA undergoes alternative splicing producing two forms of polypeptides: procollagen type IIA and type IIB that include or exclude exon 2, respectively. Reportedly, these variants of procollagen II differ in their special and temporal expression pattern in both mouse and human embryonic tissues (Lui et al., 1995;Ryan and Sandell, 1990). Type IIA mRNA was preferentially expressed during embryogenesis and was detected in the prechonrogenic mesenchyme and nonchondrogenic tissues, including ectoderm- and endoderm-derived epithelial cells. In contrast, the type IIB transcript was expressed in differentiating chondrocytes later during the development. We demonstrate that the IIA splice variant is the predominant transcript expressed in renal tissue. Interestingly, the exon 2, which encodes the cysteine-rich domain (CRD) as a part of the N-terminal peptide, is cleaved off after secretion. It was proposed that this cysteine-rich region functions as a growth factor antagonist as it is able to bind TGFβ1, BMP2 and BMP4. Thus in Xenopus embryos, procollagen IIA can functionally substitute for the endogenous CRD-containing extracellular matrix protein and BMP antagonist, Chordin (Larrain et al., 2000). As TGFβ1 is a major mediator of the epithelial-mesenchymal transition (EMT) in general, and in kidney in particular, it is possible that type II collagen deposited under the embryonic kidney basement membrane serves to sequester TGFβ1 secreted by the renal interstitial mesenchyme and to prevent the tubular epithelial cells from de-differentiating.
The expression of type II collagen in the renal tubules and most other epithelia appears to be transient in nature and restricted to embryonic and neonatal stages. The putative embryonic function of the protein in this tissue may be replaced by other mechanisms as the renal tissue architecture is established during later embryonic stages and after birth. We favor the idea that type II collagen may play a role as a basement membrane-associated component serving as mechanical and physiological support for the epithelia during embryonic tissue morphogenesis. The exact spatial relationship between type II collagen and the basement membrane in kidney epithelia is presently uncertain. It may be a component of the lamina fibroreticularis, the transition zone between lamina densa and the surrounding connective tissue. Early immunolocalization studies of the type II collagen in the embryonic otic capsule detected the polypeptide in the reticulate lamina underlying lamina densa of the otocyst (Wood et al., 1991).
Finally, the ability of embryonic epithelial tissues to produce and secrete type II collagen may be related to the evolution of the fibril-forming collagens. Studies in chordates suggest that secretion of a fibrillar collagen with properties similar to the vertebrate type II collagen by endodermal epithelium was the ancestral mode of making pharyngeal cartilages, the gill bars supporting pharyngeal gill slits. The morphological similarity of these cartilage-like structures to hypertrophied basal laminae has also been discussed in several reports (Benito and Pardos, 1997;Rychel et al., 2006;Rychel and Swalla, 2007).
Regardless of the biological function of type II collagen in non-cartilagenous tissues, its broad and relatively strong expression in embryonic epithelia suggests that the use of Col2-Cre transgenic strains would not restrict gene inactivation to the skeletal tissues. Based on our data, the outcome would largely depend on the embryonic function(s) of the gene of interest and the generated phenotypes should, therefore, be interpreted with caution.
4. Materials and methods
4.1. Mouse strains
Generation of Pkd1 and Kif3a floxed mice were described previously (Marszalek et al., 2000;Starremans et al., 2008). Mice harboring floxed Kif3a alleles in C57BL/6 background were a generous gift from Larry Goldstein and were genotyped as previously described. Col2-Cre10 deleter strain was kindly provided by Dr. Fanxin Long. The presence or absence of the Cre transgene was determined using PCR with primers specific to the Cre-recombinase coding sequence: Cre5′ (TGC TCT GTC CGT TTG CCG) and Cre3′ (ACT GTG TCC AGA CCA GGC). Genomic tail DNA was amplified by PCR; 35 cycles of denaturation at 94°C for 30 seconds, annealing at 58°C for 60 seconds, and elongation for 90 seconds at 72°C in reaction buffer containing 2.5 mM MgCl2, 1 × PCR buffer (Roche Applied Science, Indianapolis, IN) and 0.2 µM for each primer.
In all timed pregnancies, the day of the vaginal plug appearance was considered as E0.5. For harvesting E13.5 embryos, pregnant females were euthanized by CO2 intoxication and the uterus was dissected out.
4.2 Total RNA isolation and RT-PCR
Total RNA was isolated from new born limbs skeletal elements and kidney at various embryonic or postnatal stages using Tri Reagent™ (Sigma, St. Louis, MO) reagent. Subsequent cDNA synthesis was performed using the iScript ™Select cDNA Synthesis Kit (BioRad Laboratories, Hercules, CA). For RT-PCR, the OLJ8/OLJ10/OLJ12 primers and PCR conditions were used as previously described (Ng et al., 1993).
4.3 Immunohistochemistry, non-radioactive in situ hybridization and β-gal staining
For immunohistochemistry, E13.5 embryos were fixed in 4% buffered paraformaldehyde overnight at 4°C, incubated with 20% sucrose, embedded in Tissue-Tek OCT compound (Sakura Finetek USA Inc.) and sectioned at 7 µm. Cryosections were permeabilized with 0.1%Triton X-100 and incubated overnight with mouse anti-collagen type II (dilution 1:100, NeoMarkers) and rabbit anti-collagen type IV (dilution 1:200, Chemicon). To improve antibody penetration, sections were treated with pepsin (2 mg/ml in Tris-HCl, pH 2.0) for 10 minutes at 37°C. For immunofluorescence detection, sections were incubated with goat anti-mouse and goat anti-rabbit secondary antibodies conjugated with Alexa 546 or Alexa 488 (dilution 1:200, Molecular Probes). Immunofluorescence microscopy images were obtained using Nikon TE/2000 laser scanning microscope.
For nonradioactive in situ hybridization, E13.5 embryos were fixed in 4% buffered paraformaldehyde overnight at 4°C and subsequently dehydrated in graded alcohol series and embedded in paraffin (Paraplast Plus, McCormick Sci.). Seven-micrometer-thick sections were dewaxed, rehydrated, rinsed in PBS (pH 7.4) and postfixed with 4% PFA-PBS (pH 7.4) for 10 min. Sections were rinsed in PBS (pH 7.4), treated with 10 µg of proteinase K/ml for 30 min at 37°C, acetylated with 0.25% acetic anhydride for 10 min, washed three times in PBS (pH 7.4), and dehydrated in an ascending ethanol series. Air-dried sections were hybridized with digoxigenin-UTP-labeled antisense riboprobe for mouse collagen type II overnight at 52°C. After hybridization, sections were washed three times for 30 min each at 55°C in 50% formamide, 2× sodium citrate-chloride buffer (SSC [1× SSC is 0.015 M sodium citrate and 0.15 M NaCl]), and twice in 1× SSC for 15 min at room temperature. The sections were then incubated with an alkaline phosphatase-coupled digoxigenin-specific antibody (Roche) diluted 1:500 in PBS (pH 7.4) containing 2% sheep serum and 0.1% Triton X-100 for 2 h at room temperature. After rinsing in PBS (pH 7.4), color detection was performed according to the recommendation of the manufacturers.
For β-galactosidase staining, frozen sections were washed fixed in 0.2% glutaraldehyde in PBS, washed in 10mM MgCl2 in PBS and treated in detergent (0.005% NP40 in PBS) for 10 min on ice. β-galactosidase activity assay was performed by incubating the sections in the solution containing 5mM K3Fe(CN)6, 5mM K4Fe(CN)6·3H2O, 1mM MgCl2, 0.01% sodium deoxycholate, 0.009% NP40, 1mg/ml X-gal for 2 to 4 hours at 37°C. Colour reaction was stopped by post-fixation in 4% paraformaldehyde in PBS for 10 min at RT. Sections were counterstained with Nuclear Fast Red (Vector Laboratories, Burlingame, CA), dehydrated in alcohol and cleared with xylene.
Acknowledgements
We are grateful to Fanxin Long, Beate Lanske and Yukiko Maeda for generating and providing Col2-Cre transgenic mice. We would like to thank Dr. Jennifer Waters and Lara Petrak at the Nikon Imaging Center at Harvard Medical School for professional advice and assistance with confocal microscopy. This work was supported by grants R01 AR036819 and R21 AR053143 (to B.R.O.) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benito J, Pardos F. Hemichordata. In: Harrison FW, Ruppert EC, editors. Hemichordata, Chaetognatha and Invertabrate Chordates. New York: Wiley-Liss; 1997. pp. 15–101. [Google Scholar]
- Cheah KS, Lau ET, Au PK, Tam PP. Expression of the mouse alpha 1(II) collagen gene is not restricted to cartilage during development. Development. 1991a;111:945–953. doi: 10.1242/dev.111.4.945. [DOI] [PubMed] [Google Scholar]
- Fitch JM, Mentzer A, Mayne R, Linsenmayer TF. Independent deposition of collagen types II and IX at epithelial-mesenchymal interfaces. Development. 1989;105:85–95. doi: 10.1242/dev.105.1.85. [DOI] [PubMed] [Google Scholar]
- Haigh JJ, Gerber HP, Ferrara N, Wagner EF. Conditional inactivation of VEGF-A in areas of collagen2a1 expression results in embryonic lethality in the heterozygous state. Development. 2000;127:1445–1453. doi: 10.1242/dev.127.7.1445. [DOI] [PubMed] [Google Scholar]
- Kolpakova-Hart E, Jinnin M, Hou B, Fukai N, Olsen BR. Kinesin-2 controls development and patterning of the vertebrate skeleton by Hedgehog- and Gli3-dependent mechanisms. Dev. Biol. 2007;309:273–284. doi: 10.1016/j.ydbio.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosher RA, Solursh M. Widespread distribution of type II collagen during embryonic chick development. Dev. Biol. 1989;131:558–566. doi: 10.1016/s0012-1606(89)80026-0. [DOI] [PubMed] [Google Scholar]
- Koyama E, Young B, Nagayama M, Shibukawa Y, Enomoto-Iwamoto M, Iwamoto M, Maeda Y, Lanske B, Song B, Serra R, Pacifici M. Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development. 2007;134:2159–2169. doi: 10.1242/dev.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrain J, Bachiller D, Lu B, Agius E, Piccolo S, De Robertis EM. BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development. 2000;127:821–830. doi: 10.1242/dev.127.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmayer TF, Smith GN, Jr, Hay ED. Synthesis of two collagen types by embryonic chick corneal epithelium in vitro. Proc. Natl. Acad. Sci. U. S. A. 1977;74:39–43. doi: 10.1073/pnas.74.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F, Chung UI, Ohba S, McMahon J, Kronenberg HM, McMahon AP. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. 2004;131:1309–1318. doi: 10.1242/dev.01006. [DOI] [PubMed] [Google Scholar]
- Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- Lui VC, Ng LJ, Nicholls J, Tam PP, Cheah KS. Tissue-specific and differential expression of alternatively spliced alpha 1(II) collagen mRNAs in early human embryos. Dev. Dyn. 1995;203:198–211. doi: 10.1002/aja.1002030208. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Matukas VJ. Chick cartilage collagen: a new type of alpha 1 chain not present in bone or skin of the species. Proc. Natl. Acad. Sci. U. S. A. 1969;64:1264–1268. doi: 10.1073/pnas.64.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LJ, Tam PP, Cheah KS. Preferential expression of alternatively spliced mRNAs encoding type II procollagen with a cysteine-rich amino-propeptide in differentiating cartilage and non-chondrogenic tissues during early mouse development. Dev. Biol. 1993;159:403–417. doi: 10.1006/dbio.1993.1251. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26:145–146. [PubMed] [Google Scholar]
- Razzaque MS, Soegiarto DW, Chang D, Long F, Lanske B. Conditional deletion of Indian hedgehog from collagen type 2alpha1-expressing cells results in abnormal endochondral bone formation. J. Pathol. 2005;207:453–461. doi: 10.1002/path.1870. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Sandell LJ. Differential expression of a cysteine-rich domain in the amino-terminal propeptide of type II (cartilage) procollagen by alternative splicing of mRNA. J. Biol. Chem. 1990;265:10334–10339. [PubMed] [Google Scholar]
- Rychel AL, Smith SE, Shimamoto HT, Swalla BJ. Evolution and development of the chordates: collagen and pharyngeal cartilage. Mol. Biol. Evol. 2006;23:541–549. doi: 10.1093/molbev/msj055. [DOI] [PubMed] [Google Scholar]
- Rychel AL, Swalla BJ. Development and evolution of chordate cartilage. J. Exp. Zoolog. B Mol. Dev. Evol. 2007;308:325–335. doi: 10.1002/jez.b.21157. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hiripi L, Glumoff V, Brandau O, Eerola R, Vuorio E, Bosze Z, Fassler R, Aszodi A. Stage-and tissue-specific expression of a Col2a1-Cre fusion gene in transgenic mice. Matrix Biol. 2001;19:761–767. doi: 10.1016/s0945-053x(00)00122-0. [DOI] [PubMed] [Google Scholar]
- Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GN, Jr, Linsenmayer TF, Newsome DA. Synthesis of type II collagen in vitro by embryonic chick neural retina tissue. Proc. Natl. Acad. Sci. U. S. A. 1976;73:4420–4423. doi: 10.1073/pnas.73.12.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Haycraft CJ, Seo HS, Yoder BK, Serra R. Development of the post-natal growth plate requires intraflagellar transport proteins. Dev. Biol. 2007;305:202–216. doi: 10.1016/j.ydbio.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Starremans PG, Li X, Finnerty PE, Guo L, Takakura A, Neilson EG, Zhou J. A mouse model for polycystic kidney disease through a somatic in-frame deletion in the 5' end of Pkd1. Kidney Int. 2008 doi: 10.1038/ki.2008.111. In press. [DOI] [PubMed] [Google Scholar]
- Thorogood P, Bee J, von der MK. Transient expression of collagen type II at epitheliomesenchymal interfaces during morphogenesis of the cartilaginous neurocranium. Dev. Biol. 1986;116:497–509. doi: 10.1016/0012-1606(86)90150-8. [DOI] [PubMed] [Google Scholar]
- Wood A, Ashhurst DE, Corbett A, Thorogood P. The transient expression of type II collagen at tissue interfaces during mammalian craniofacial development. Development. 1991;111:955–968. doi: 10.1242/dev.111.4.955. [DOI] [PubMed] [Google Scholar]


