Abstract
Elite suppressors are untreated individuals with human immunodeficiency virus type 1 (HIV-1) infection who maintain viral loads <50 copies/mL. Using a single-copy assay, we show that there is no statistically significant difference between the proportions of elite suppressors and patients receiving suppressive highly active antiretroviral therapy who have viral loads of <1 copy/mL.
A rare subset of HIV-1–infected individuals, termed “elite suppressors,” maintain viral loads below the clinical limit of detection (50 copies/mL) and normal CD4+ T cell counts without antiretroviral therapy. Similarly, HIV-infected individuals receiving suppressive HAART maintain viral loads <50 copies/mL, but they do so through the use of antiretroviral therapy [1]. By definition, both infected groups have clinically undetectable viral loads but achieve this level through different biological mechanisms. We have been able to amplify and sequence multiple HIV-1 genes from the plasma of patients receiving HAART [2, 3] and of elite suppressors [4, 5], which suggests that low-level viremia is, in fact, present in both sets of patients. Maldarelli et al. [7] used an ultrasensitive viral load assay with single-copy sensitivity, termed the “single-copy assay” [6], and revealed that individuals with suppression of viremia who receive HAART maintain a steady-state viral load <50 copies/mL (median, 3 copies/mL). Here, in a study with use of archived plasma samples, we used the same assay to measure and compare viral loads in elite suppressors with those in individuals receiving suppressive HAART. To our knowledge, this is the first time such a comparison has been performed, and the results may contribute to the understanding of the mechanisms that lead to viral control in elite suppressors.
Patients and methods
Archived plasma samples from 14 elite suppressors and 15 HAART-treated patients were studied. Informed consent was obtained from each individual before phlebotomy. The elite suppressors were infected for a median duration of 13 years and had a median CD4+ T cell count of 811 cells/μL. They all consistently had viral loads <50 copies/mL, and all HAART-treated patients had viral loads <50 copies/mL for at least 6 months, as determined at the Johns Hopkins Hospital in Baltimore, Maryland (AMPLICOR HIV-1 MONITOR Test, version 1.5; Roche). To prevent selection bias, samples from the first 15 patients who met these criteria were used. Eleven of these patients were receiving a nonnucleoside reverse-transcriptase inhibitor–based regimen, whereas the remaining patients were receiving protease inhibitor–based regimens. A blood sample was obtained from each study participant, with use of acid citrate dextrose as an anticoagulant. Plasma was separated from blood with use of discontinuous density gradient centrifugation. Viral loads were measured from 7 mL of plasma with use of the single-copy assay, a real-time RT-PCR–based assay, as described elsewhere [6]. Virus was concentrated by ultracentrifugation and was lysed by treatment with proteinase K and guanidinium isothiocynate. Viral RNA was precipitated with glycogen and isopropanol. After nucleotide precipitation, the nucleotide-glycogen pellet was washed with 70% ethanol and was air dried. The extracted viral RNA was then resuspended with a Tris-based buffer. Viral RNA was quantified by a 2-step quantitative RT-PCR [6]. Fisher’s exact test was used for statistical analysis of the proportion of undetectable viral loads in the HAART-treated patients and elite suppressors.
Results
Single viral load measurements were performed for 14 elite suppressors and 15 HAART-treated individuals. Of the 14 elite suppressors, 9 (64%) had viral loads that were undetectable (<1 copy/mL) by the single-copy assay. The remaining elite suppressors had various measurable viral loads, as reported elsewhere [8], with a median of 25 copies/mL (figure 1A). In the HAART-treated cohort, 6 (40%) of the 15 individuals had viral loads undetectable by the single-copy assay. The remaining individuals had viral loads ≤4 copies/mL (median, 2 copies/mL). The difference in proportions of patients in each cohort who demonstrated undetectable viral loads was not statistically significant (P >.05); however, the elite suppressors appeared to display a greater range of viral loads than that seen in the HAART-treated individuals.
Figure 1.
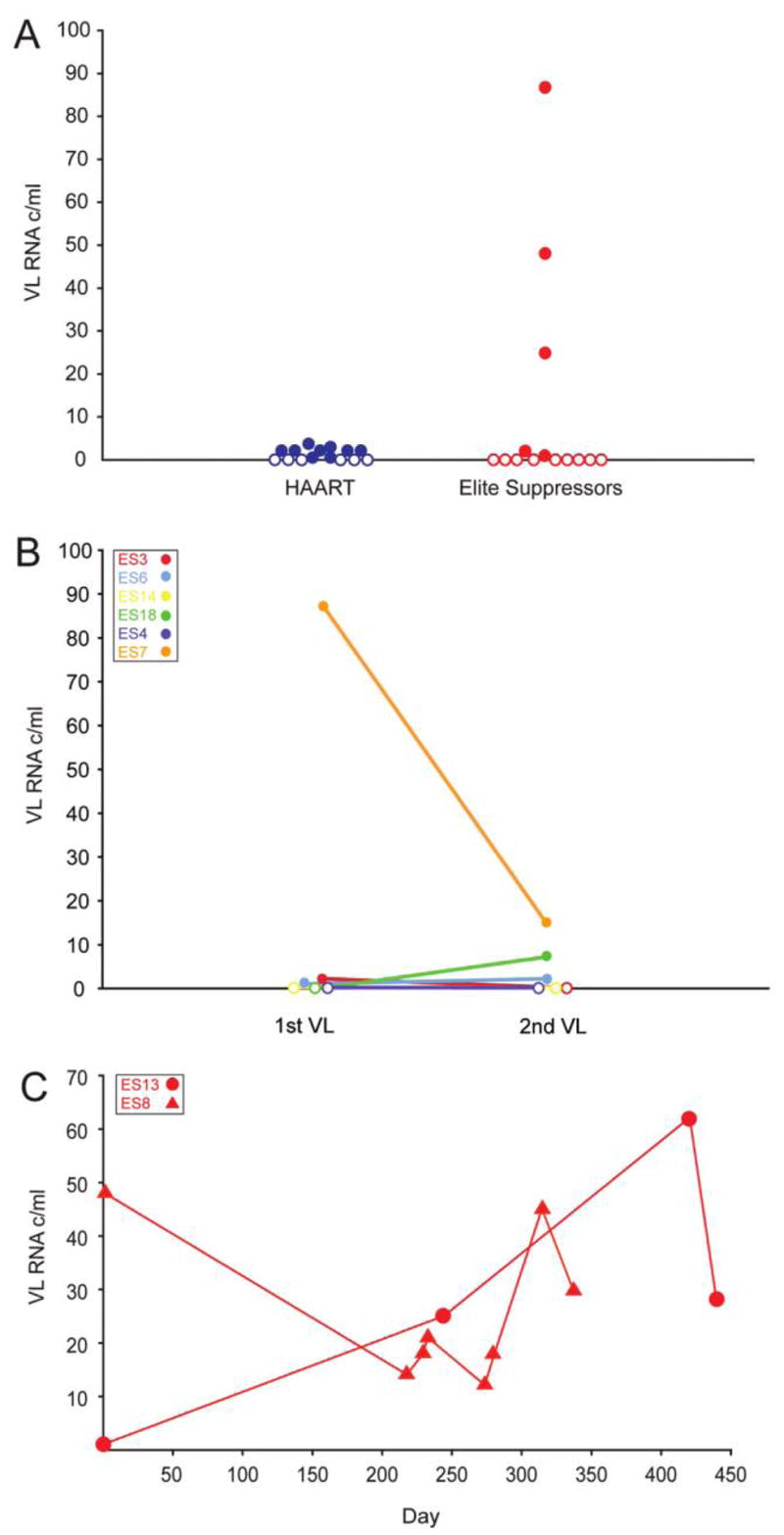
Viral load (VL) measurements. Open symbols represent undetectable viral loads (< 1 copy/mL). A, Single viral load measurements in elite suppressors (red) and HAART-treated individuals (blue). B, Two viral load measurements. Viral loads from 6 elite suppressors were measured in 2 separate blood samples obtained a mean of 8.6 months apart. C, Longitudinal analysis of elite suppressor viral loads in 4 separate blood samples obtained from patient ES13 (circles) and 8 separate blood samples obtained from patient ES8 (triangles). c, Copies.
To further investigate this variation in viral loads, a longitudinal analysis was performed with archived plasma samples. Viral load measurements from 2 separate blood samples obtained a mean of 8.6 months apart were performed for 6 elite suppressors. As shown in figure 1B, some elite suppressors maintained undetectable viral loads during both measurements, whereas others had changes of up to 72 copies/mL. More-intensive sampling was performed for 2 other elite suppressors with detectable viral loads (figure 1C). Patient ES8 had viral loads that fluctuated between 12 and 48 copies/mL during a 337-day period. For patient ES13, viral loads had a range of 1–62 copies/mL over 4 separate measurements during a 440-day period.
Discussion
We provide measurements and comparisons of viral loads from elite suppressors and HAART-treated individuals. In elite suppressors, viral loads ranged from undetectable (<1 copy/mL) to 87 copies/mL. Surprisingly, more than one-half of the studied elite suppressors had undetectable viral loads despite recent evidence of viral replication and evolution in these patients [5]. In the HAART-treated individuals, viral loads were found to be ≤4 copies/mL (median, 2 copies/mL). This observation is similar to observations reported elsewhere [7]. In a longitudinal analysis of archived samples, we found that the viral loads in 1 elite suppressor fluctuated between 1 and 87 copies/mL. This fluctuation is unlikely to have been attributable to superinfection, because we analyzed virus from the plasma and resting CD4+ T cells of this patient on multiple occasions and found no evidence of infection with new isolates [5] (authors’ unpublished data). Instead, it is most likely that these observations in elite suppressors may be attributed to individual differences in host immune response.
In elite suppressors, cytotoxic T-lymphocytes are believed to play a significant role in controlling viremia at levels below the clinical limit of detection [9]. It is conceivable that cytotoxic T-lymphocyte–mediated killing of infected cells may not be as efficient in elite suppressors with higher viral loads and greater fluctuations in viral load dynamics as in those individuals with lower viral loads. A greater degree of ongoing replication may occur because the less efficient cytotoxic T-lymphocyte machinery takes time to respond to and control new infection events. On the other hand, patient ES4, from whom we recovered replication-competent HIV isolates [10], had undetectable viral loads at multiple times, which suggests that, in some instances, host factors may be as effective as HAART in controlling replication of virulent HIV-1 isolates.
The human leukocyte antigen B*57 (HLA–B*57) allele has been shown to be highly overrepresented in elite suppressors [11], although a mechanistic role for HLA–B*57 in the control of HIV-1 infection has yet to be defined. In our cohort of 14 elite suppressors, 11 were HLA–B*57 positive, and there did not appear to be a relationship between viral load and HLA–B*57 allele status (data not shown). Patient ES8, from whom we have recovered replication-competent virus [10], presents a particularly interesting situation; the patient is HLA–B*57 positive and has evidence of multiple escape mutations in HLA–B*57–restricted epitopes [5], yet he has maintained viral loads of 12–48 copies/mL for a year without virologic breakthrough. Like the other elite suppressors in our cohort, the patient had low titers of neutralizing antibodies [4]; thus, it is unclear how low-level viremia was being controlled in this case. These data may suggest that, in some patients, suppression of viral loads to 1 copy/mL may not be necessary for long-term control of viral replication.
In summary, this is the first longitudinal study of low-level viremia in elite suppressors and the first study in which viral loads in elite suppressors and HAART-treated individuals were measured and compared through use of a single-copy viral load assay. We did not find a statistically significant difference in the proportions of undetectable viral loads between these 2 cohorts; however, a greater range of viral loads was seen in the elite suppressors. A larger study will be needed to confirm this preliminary finding. Understanding the host factors associated with lower viral loads may provide insight into how elite suppressors control viral replication in the absence of antiretroviral therapy.
Acknowledgments
We thank Dr. Sarah Palmer and Ann Wiegand (National Cancer Institute) for their generous guidance and many helpful discussions in the development of the single-copy assay.
Financial support. National Institutes of Health (K08 AI51191 and R56 AI73185-01A1) and Howard Hughes Medical Institute.
Potential conflict of interest. All authors: no conflicts.
References
- 1.Perelson AS, Essunger P, Cao Y, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–91. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 2.Hermankova M, Ray SC, Ruff C, et al. HIV-1 drug resistance profiles in children and adults with viral load of <50 copies/mL receiving combination therapy. JAMA. 2001;286:196–207. doi: 10.1001/jama.286.2.196. [DOI] [PubMed] [Google Scholar]
- 3.Bailey JR, Sedaghat AR, Kieffer T, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol. 2006;80:6441–57. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey JR, Lassen KG, Yang HC, et al. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J Virol. 2006;80:4758–70. doi: 10.1128/JVI.80.10.4758-4770.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey JR, Williams TM, Siliciano RF, Blankson JN. Maintenance of viral suppression in HIV-1–infected HLA–B*57+ elite suppressors despite CTL escape mutations. J Exp Med. 2006;203:1357–69. doi: 10.1084/jem.20052319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–6. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maldarelli F, Palmer S, King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PloS Pathog. 2007;3:e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufmann DE, Kavanagh DG, Pereyra F, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–54. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 9.Migueles SA, Laborico AC, Shupert WL, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3:1061–8. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 10.Blankson JN, Bailey JR, Thayil S, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. 2007;81:2508–18. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migueles SA, Sabbaghian MS, Shupert WL, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A. 2000;97:2709–14. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]


