Abstract
All retroviruses must circumvent cellular restrictions on the export of unspliced RNAs from the nucleus. While the unspliced RNA export pathways for HIV and Mason-Pfizer monkey virus are well characterized, that of Rous sarcoma virus (RSV) is not. We have previously reported that the RSV direct repeat (DR) elements are involved in the cytoplasmic accumulation of unspliced viral RNA. Here, using fluorescent in situ hybridization (FISH), we demonstrate that unspliced viral RNAs bearing a single point mutation (G8863C) in the DR exhibit a restricted cellular localization in and around the nucleus. In contrast, wild type unspliced viral RNA had a diffuse localization throughout the nucleus and cytoplasm. Since the RSV Gag protein has a transient localization in the nucleus, we examined the effect of Gag over expression on a DR-mediated reporter construct. While Gag did not enhance DR-mediated nuclear export, the dominant negative expression of two cellular export factors, Tap and Dbp5, inhibited expression of the same reporter construct. Further, FISH studies using the dominant negative Dbp5 demonstrated that unspliced wild type RSV RNA was retained within the nucleus. Taken together, these results further implicate the DR in nuclear RNA export through interactions with Tap and Dbp5.
Keywords: RNA export, Rous sarcoma virus, Tap, Dbp5, retrovirus
Introduction
In higher eukaryotic cells, pre-mRNAs are retained within the nucleus until they have been fully spliced to form the mature mRNA found in the cytoplasm (Reed and Hurt, 2002; Erkmann and Kutay, 2004; Vinciguerra and Stutz, 2004). Upon the completion of splicing and consequential mRNP re-arrangements, the RNA is deemed competent for export. Among the factors that associate with the mRNA during splicing is REF/Aly (Stutz et al., 2000; Zhou et al., 2000; Le Hir et al., 2001). This protein is known to interact directly with the nuclear export factor Tap/NXF1 (Stutz et al., 2000). Tap/NXF1 and its co-factor p15/NXT1 facilitate the export of the fully spliced RNA to the cytoplasm (Santos-Rosa et al., 1998; Herold et al., 2001; Jin et al., 2003). Tap and p15 have also been shown to increase the translation of unspliced RNAs in the cytoplasm (Jin et al., 2003). More recently, some hypophosphorylated SR proteins (SRp20, 9G8 and ASF/SF2) have also been shown to bind Tap and promote mRNA export (Huang and Steitz, 2001; Huang et al., 2003, 2004; Lai and Tarn, 2004).
In addition, some RNA helicases associated with the nuclear pore complex (NPC) have also been implicated in nuclear export. In the case of mRNA export, the DEAD box helicase Dbp5 plays a critical role (Snay-Hodge et al., 1998; Hodge et al., 1999; Schmitt et al., 1999; Zhao et al., 2002). While Dbp5 is known to shuttle between the nucleus and cytoplasm (Hodge et al., 1999), it is primarily located in the cytoplasm with a higher concentration around the nuclear rim at steady-state (Snay-Hodge et al., 1998; Schmitt et al., 1999). Dbp5 interacts with CAN/Nup159 and other nuclear shuttling factors (Schmitt et al., 1997), further implicating it in nuclear export. Additionally, Dbp5 mutants which lack an RNA-stimulated ATPase domain cause nuclear accumulation of the RNA (Schmitt et al., 1999). It has also been shown that Dbp5 is responsible for removing Tap from mRNAs during nuclear export in yeast (Lund and Guthrie, 2005).
In contrast to cellular mRNA metabolism, retroviral replication requires unspliced, intron-containing viral RNAs in the cytoplasm. These full-length RNAs are translated in the cytoplasm to produce the viral Gag and Gag-Pol polyproteins (Rabson and Graves, 1997). They are also packaged into new viral particles as genomes. Thus, the accumulation of these unspliced RNAs in the cytoplasm is critical for viral replication. Retroviruses must devise a method to circumvent the host restriction on exporting incompletely spliced RNAs to the cytoplasm.
Complex retroviruses, such as the human immunodeficiency virus (HIV) do so by encoding viral accessory proteins. The HIV Rev protein is a nucleocytoplasmic shuttling protein that binds unspliced and incompletely spliced viral RNAs via the Rev-responsive element (RRE), a cis-acting element located within the env gene (Malim et al., 1989). Upon binding viral RNA, Rev associates with Crm1/exportin-1, a host export factor that typically exports cellular proteins and 5S ribosomal RNA from the nucleus (Fornerod et al., 1997; Neville et al., 1997; Askjaer et al., 1998). Crm1 is known to associate with nucleoporins, including CAN/Nup214, which target the entire RNA/protein complex to the nuclear pore complex for export (Fornerod et al., 1997). Other complex retroviruses, such as human T-cell leukemia virus type 1 (HTLV-1) and feline immunodeficiency virus (FIV), encode similar proteins that contain nuclear export signals and use the Crm1 pathway (Rabson and Graves, 1997). HIV-1 export, requiring Crm1 rather than Tap, also uses a different DEAD box RNA helicase, DDX3, for Rev-RRE-mediated nuclear export (Yedavalli et al., 2004).
Simple retroviruses do not encode known Rev-like export proteins. Instead, they rely on cis-acting RNA sequences within their genome to bind cellular export factors. For the Mason-Pfizer monkey virus (MPMV) and simian retrovirus (SRV), this RNA sequence has been identified as the constitutive transport element (CTE) (Bray et al., 1994; Zolotukhin et al., 1994). This element is comprised of a long stem-loop hairpin (Ernst et al., 1997) that is capable of binding Tap directly (Pasquinelli et al., 1997; Grüter et al., 1998; Braun et al., 1999). It has also been demonstrated that excess CTE expression can inhibit cellular mRNA export (Pasquinelli et al., 1997). Thus, the virus relies on the cellular mRNA export pathway.
Rous sarcoma virus (RSV) is another simple retrovirus that requires a cis-acting element for nuclear export. The direct repeats (DRs) of RSV flank the 3’-most viral gene, src. Since these two DR sequences are over 80% homologous (Ogert et al., 1996), it has been suggested that their functions are redundant. When DR point mutations are present in viral clones bearing a single DR, these clones are replication-defective (Sorge et al., 1983; Simpson et al., 1997, 1998; Ogert and Beemon, 1998; Aschoff et al., 1999). The DRs also play an intergral role in the stability of the unspliced RNA (Ogert et al., 1996; Simpson et al., 1997, 1998).
It has been proposed that the DRs mediate the nuclear export of unspliced viral RNAs (Ogert et al., 1996; Ogert and Beemon, 1998; Simpson et al., 1997, 1998; Yang and Cullen, 1999; Paca et al., 2000), although this notion is not unanimously accepted. It has been suggested that the DRs function only in the cytoplasm, facilitating the viral packaging of genomic RNA (Sorge et al., 1983; Aschoff et al., 1999). However, the DR elements are capable of exporting heterologous RNAs in chicken embryo fibroblasts (CEFs) and HeLa cells (Ogert et al., 1996; Ogert and Beemon, 1998; Simpson et al., 1997; Yang and Cullen, 1999; Paca et al., 2000). Additionally, only one copy of either DR in the context of the virus is required to carry out nuclear export of the RNA (Ogert et al., 1996; Simpson et al., 1998). Leptomycin B (LMB), an inhibitor of Crm1 export, does not affect the ability of the DR to export unspliced RNAs (Paca et al., 2000), revealing the RSV export pathway differs from that of HIV.
The Stoltzfus lab (Simpson et al., 1997) has suggested that the DRs serve multiple functions within the cell. Using the Prague A strain of RSV, they report that DR mutations lead to reductions in RNA stability, efficient nuclear export, and cytoplasmic utilization of unspliced viral RNA. They propose that while the DRs are capable of serving these multiple functions, different subelements within the DRs may regulate different tasks. This model is based on the observation that while both the upstream and downstream DRs are capable of directing nuclear RNA export, only the downstream DR can mediate efficient Gag assembly and consequential particle release (Simpson et al., 1998). This difference in functional activity was due to a nucleotide mutation in the upstream DR of Prague A compared to Prague C. Mutation of the nucleotide to match the Prague C sequence restored assembly and release (Simpson et al., 1998). This suggests that the DRs are not only functionally redundant, but that the multiple functions of an individual DR can be uncoupled from one another.
In this study, we have further characterized the role of the RSV DR element. Using fluorescent in situ hybridization (FISH) techniques, we have demonstrated that unspliced viral RNA bearing a point mutation in the DR is retained in or near the nucleus. Given that RSV Gag has a transient localization in the nucleus (Scheifele et al., 2002), we examined the potential for viral Gag to promote nuclear RNA export. Using a nuclear export reporter construct, we showed that Gag does not enhance DR-mediated nuclear export of unspliced RNA, nor does it enhance the export of the same unspliced RNAs containing the RNA packaging signal Ψ. However, dominant negative mutants of two cellular factors known to function in the nuclear export of spliced cellular mRNAs, Tap and Dbp5, reduced DR-mediated nuclear RNA export. In support, FISH experiments using the dominant negative Dbp5 mutant demonstrated that wild type unspliced RNAs were retained in the nucleus. These results further strengthen our previous studies implicating DR involvement in the nuclear export of unspliced viral RNA.
Materials and Methods
Plasmids
The viral clone DR2 is a Prague C derivative that lacks DR1 and the src coding region. The viral mutant G8863C was generated by site-directed mutagenesis usings DR2 as a template. These plasmids have been previously characterized (Ogert et al., 1996; Ogert and Beemon, 1998). They also contain a previously described integrase mutation (D64E) to prevent viral spread after transfection (Kulkosky et al., 1992). The pCMV128, pCMV128DR1 and pCMV-Rev constructs have been previously described (Hope et al., 1990; Paca et al., 2000). pCMV128Ψ was generated by cutting pCMV128 at a unique BamHI site. With the first nucleotide of the viral RNA transcript +1 (RSV Prague C, accession no. V01197), the Ψ sequence (nt 156-315, plus 20 extra nucleotides at both ends) was PCR amplified with primers containing BamHI sites (underlined): sense - 5’ - GTCGTCGGATCCTCGGCCACAGACGGCGTGGCG - 3’; anti-sense - 5’ - GTCGTCGGATCCTCAGTCGTCGGGCTTCCTTCCCG - 3’. The PCR product was BamHI digested and ligated into the linearized pCMV128 vector.
The Tap and dominant negative Tap mutant, TapΔC, were obtained from R. Sandri-Goldin and were previously described (Chen et al., 2002). Expression plasmids for hDbp5 and the dominant negative mutant E243Q/V386N (both of which are fused to GFP) were obtained from E. Izaurralde and were previously described (Schmitt et al., 1999).
Cell culture and transfection
Secondary cultures of CEFs were maintained in Medium 199 (Gibco) supplemented with 2% tryptose phosphate broth (Sigma), 1% calf serum, 1% chick serum, and 1% penicillin/streptomycin (Gibco). QT6 cells were maintained in Medium 199 supplemented with 10% tryptose phosphate broth, 4% calf serum, 1% chick serum, 1% dimethyl sulfoxide and 1% penicillin/streptomycin. All cells were grown at 39°C in the presence of 5% carbon dioxide. Transfections were performed in 6 cm plates using 200 μg/ml DEAE-dextran in serum-free Medium 199 and 1-3 μg of each DNA with cells that were approximately 80% confluent. Cells were incubated for four hours at 39°C, shocked with serum-free Medium 199 containing 10% dimethyl sulfoxide for two minutes at room temperature and then washed twice with serum-free Medium 199.
Fluorescent in situ hybridization
For poly(A)+ mRNA detection, 6 cm plates of transfected CEFs were transferred to Lab-Tek II chamber slides (Nunc) after 24 hours. After another 24 hours, cells were fixed with 3.6% paraformaldehyde. Cells were then washed with 1X phosphate buffered saline (PBS) and permeabilized with 0.5% Triton X-100 (Sigma) in 1X PBS for five minutes at 4°C. Cells were then hybridized at 39°C for 4-24 hours in a hybridization solution (2X SSC, 20% formamide, 0.2% BSA, 1 mg/ml yeast tRNA, 10% dextran sulfate) containing 0.5 ng/μl of a 3’ biotinylated d(T)40 DNA oligonucleotide (Operon Technologies) (14). After hybridization, cells were treated with Texas Red-conjugated streptavidin (Molecular Probes) at 2 μg/ml in 4X SSC for 45 minutes at room temperature (Johnson et al., 1991). Cell nuclei were visualized using 300 nM DAPI (Molecular Probes) in 1X PBS for 4 minutes at room temperature. CEFs were transfected as above with 2 μg of wild type RSV or G8863 (Fig. 1A). Alternatively, CEFs were transfected with 2 μg of wild type RSV and 2 μg of either wild type hDbp5 or E243Q/V386N. To detect unspliced RSV RNA a 22 nt biotinylated DNA oligonucleotide (5’ - TTTGTAAGAGGGACAACATGGC-biotin - 3’, Operon Technologies) complementary to nts 461-482 of the gag gene was used. The final concentration of viral oligonucleotide used was 2.5 ng/μl. Again, the probe was detected using Texas Red-conjugated streptavidin as above. Cells were examined using a Deltavision Deconvolving LM system (Applied Precision, Issaquah, WA) using a Zeiss Axiovert S100-27V scope (Carl Zeiss, Thornwood, NY). Filters of 617 nm, 598 nm, and 497 nm were used to identify the Texas Red, FITC and DAPI signals, respectively. Images were collected with a Photometrics CH300 camera.
Figure 1. A DR point mutation results in the nuclear retention of unspliced RSV RNA.
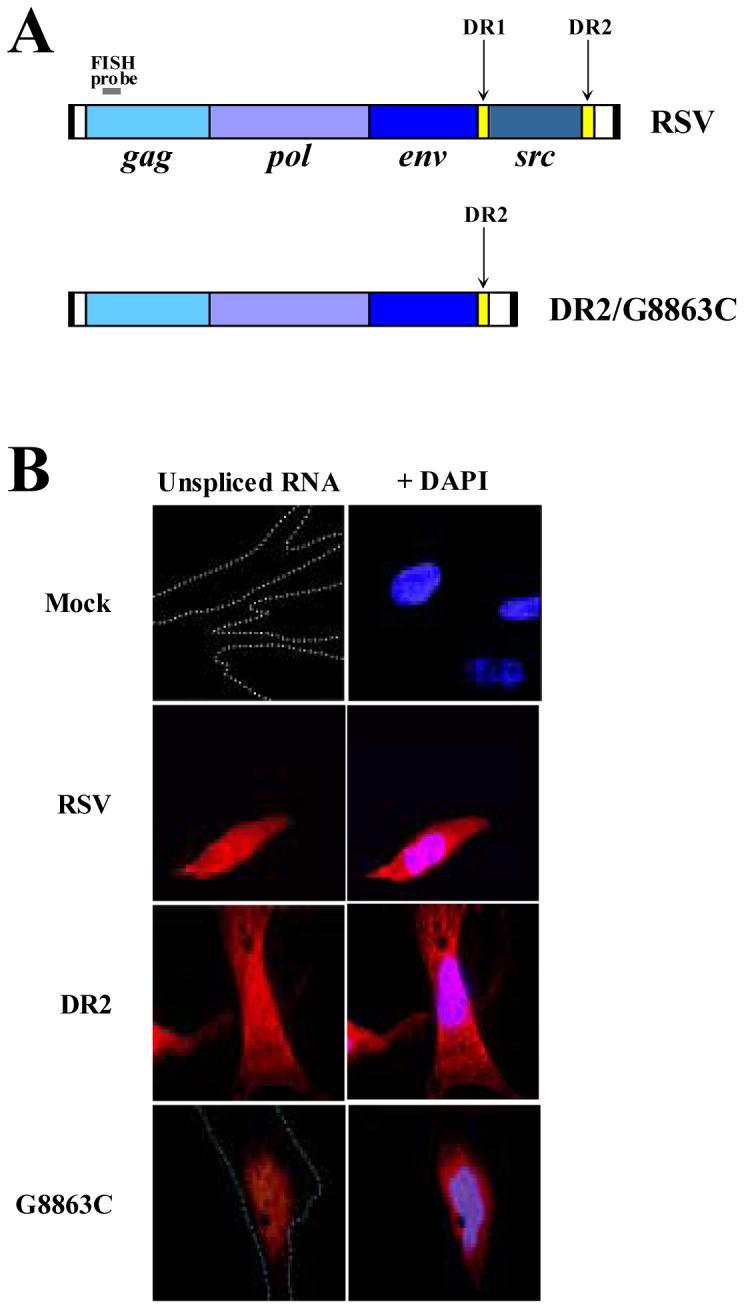
(A) Schematic of the viral clones used. The DR elements flank the src gene of RSV Prague C and are indicated by yellow boxes. In the DR2 and G8863C clones, DR1 and src have been deleted. A small grey bar above the gag gene in both clones indicates the biotinylated DNA oligonucleotide used for FISH analysis, which only binds unspliced viral RNAs. (B) Fluorescent in situ hybridizations of unspliced RSV RNAs in CEFs. Unspliced viral RNA is in red; DNA staining by DAPI is in blue.
CAT assays
The techniques of Gorman et al. (1982) were followed. CEFs were transfected as above in 6 cm plates. After 24 or 48 hours, cells were washed twice with 1X PBS. Cells were incubated for five minutes with 1 ml TEN buffer (40 mM Tris, pH 8.0, 150 mM NaCl and 1 mM EDTA). Cells were scraped, pelleted and resuspended in 100 μl 250 mM Tris, pH 8.0. Cells were frozen in a dry ice-ethanol bath and thawed at 37°C three times. Cell lysates were pelleted and the supernatant saved. Acetyl transferase reactions carried out in 75 μl reactions containing lysate (2-10 μl), 216.67 mM Tris, pH 8.0, 0.5 mM acetyl-CoA, and 0.02 μCi chloramphenicol, D-threo [dichloroacetyl-1,2-14C] (PerkinElmer) for 45 minutes at 37°C. Following incubation, 500 ml ethyl acetate was added and samples vortexed for 30 seconds. The upper phase was removed and placed in a speed vac for 15 minutes. Samples were resuspended in 30 μl ethyl acetate and spotted onto flexible silica gel plates (Whatman). Acetylated and nonacetylated chloramphenicol were separated out by thin-layer chromatography and quantified using InstantImager (Packard) or Typhoon Phosphor Imager (Amersham) instruments.
Results
A point mutation within DR2 blocks nuclear export of unspliced RSV RNA
The DR elements of RSV have been proposed to have several different functions. Several studies have suggested that one function is nuclear export (Ogert et al., 1996; Simpsom et al., 1997; Ogert and Beemon, 1998; Yang and Cullen, 1999). Other labs have suggested the DRs function not in nuclear export but in virion assembly and particle release (Sorge et al., 1983; Aschoff et al., 1999). To help reconcile these differences, we used (FISH) to examine the localization of the unspliced viral RNA within primary chicken embryo fibroblasts (CEFs). Cells were transiently transfected with one of three viral clones: wild type RSV, DR2 or G8863C viral clones (Fig. 1A). DR2 is a clone lacking the src gene and contains only one DR sequence, DR2. G8863C is a replication-defective mutant viral clone of DR2 that inhibits DR-mediated nuclear export (Ogert and Beemon, 1998). Two days later, cells were prepared for FISH analysis using a biotinylated DNA oligonucleotide probe corresponding to a 22 nt region in gag that will only hybridize to full-length viral RNA (Fig. 1A). The wild type RSV showed a uniform distribution of unspliced viral RNA within the nucleus and cytoplasm (Fig. 1B). In striking contrast, the point mutant G8863C demonstrated localization in and around the nucleus. To confirm that the signals seen were the result of RNA:DNA oligonucleotide interactions and not plasmid DNA:DNA oligonucleotide interactions, cells were treated with either RNase A (before probe hybridization) or RNase H (after probe hybridization). Both treatments resulted in the loss of fluorescent signal (data not shown). These results are in agreement with previous studies suggesting the DRs function in nuclear export (Ogert et al., 1996; Simpson et al., 1997; Ogert and Beemon, 1998; Yang and Cullen, 1999).
Gag does not affect the nuclear export of DR-containing or Ψ-containing unspliced reporter RNAs
Recent studies have revealed that RSV Gag proteins spend a short-lived period of time residing within the nucleus of infected QT6 cells (Scheifele et al., 2002). This finding suggests that the Gag protein may be there to select unspliced viral RNAs for genomic packaging. Since a cellular protein has not been found to interact with the DR to promote export, we explored the potential for Gag to mediate this function.
The pCMV128 (Fig. 2) construct has been previously characterized (Hope et al., 1990; Paca et al., 2000). This reporter construct contains a bacterial chloramphenicol acetyltransferase (CAT) gene buried between the HIV splice donor and acceptor sites within env, and contains the Rev-responsive element (RRE). Expression of the CAT gene is only seen when the unspliced RNA is exported to the cytoplasm. pCMV128DR1 contains a single copy of DR1 inserted between the CAT gene and the RRE (Fig. 2). We only studied DR1 in these assays since previous results had shown identical phenotypes for Pr-C DR1 and DR2 constructs (Ogert et al., 1996).
Figure 2. Schematic diagrams of the 128 reporter constructs and the Gag mini-gene.
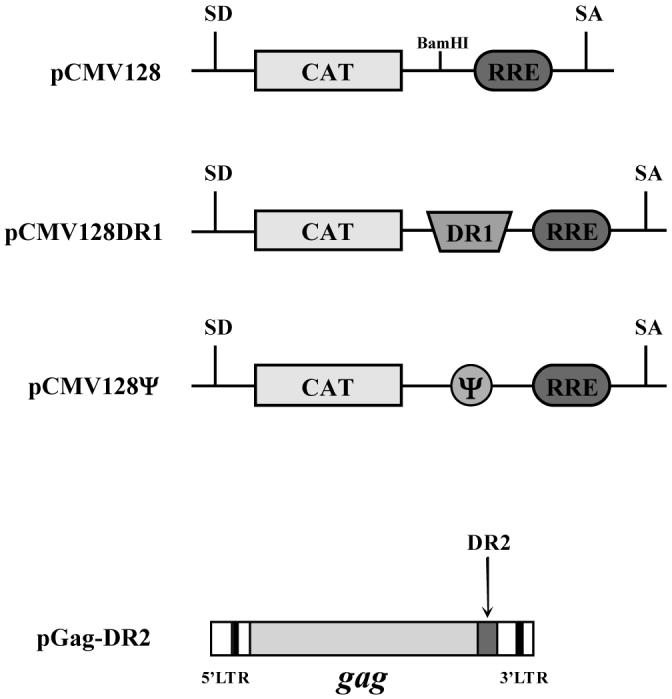
The 128 constructs contain the bacterial chloramphenicol acetyltransferase (CAT) gene cloned into the HIV env gene between viral spliced donor (SD) and splice acceptor (SA) sites. CAT expression is only seen when unspliced RNAs are exported from the nucleus. pCMV128DR1 contains RSV Prague C nts 6897-6989 inserted between the CAT gene and the Rev-responsive element (RRE) at a unique BamHI site. pCMV128Ψ contains the packaging signal, Ψ (136-335), inserted at the same site. pGag-DR2 is an RSV clone that contains only the gag coding region. A copy of DR2 has been retained to promote nuclear export.
To test the potential of Gag to enhance DR-mediated nuclear export, CEFs were transiently transfected with either pCMV128 or pCMV128DR1 and increasing amounts of pGag-DR2, a construct expressing only the gag gene of RSV (Fig. 2). This viral clone was shown to produce full-length Gag and even undergo partial processing by Western blotting (data not shown). Two days post-transfection, cells were assayed for CAT activity (Fig. 3A, upper panel). The samples were normalized to pCMV128 cotransfected with a Rev-expression construct, pCMV-Rev, which served as a positive control for the experiment. The results showed that despite up to a threefold increase in the amount of pGag-DR2 plasmid cotransfected, the relative level of CAT activity was not enhanced over the expression of CAT seen with the pCMV128DR1 construct alone. This suggests that Gag is not involved in DR-mediated nuclear export in CEFs.
Figure 3. Gag does not enhance the nuclear export of DR-containing or Ψ-containing unspliced RNAs.

(A) CEFs (upper panel) or QT6 (lower panel) were transiently transfected with either pCMV128 or pCMV128DR1. pCMV128 was also cotransfected with pCMV-Rev as a positive control, to which all other samples were normalized (lane 2). Increasing amounts of pGag-DR2 were cotransfected with pCMV128DR1 as indicated. Cells were maintained for 48 hours and assayed for CAT activity. The results are from four independently performed transfections. (B) QT6 cells were transiently transfected with pCMV128 or pCMV128Ψ. pCMV128 was also cotransfected with pCMV-Rev as a positive control, to which all other samples were normalized (lane 2). Increasing amounts of gag-DR2 were cotransfected with pCMV128Ψ as indicated. Cells were maintained for 48 hours and assayed for CAT activity. The results are from four independently performed transfections.
The nuclear localization of Gag was observed in QT6 cells (Scheifele et al., 2002), an immortalized quail tumor cell line. To determine if cell type might contribute to there being a difference in nuclear export pathways, we repeated the same experiment in these cells (Fig. 3A, lower panel). Once again, we found that the over-expression of Gag did not enhance the ability of the DR to stimulate nuclear export of unspliced RNA. The slight decrease seen in CAT activity when Gag was cotransfected may possibly be due to competition for cellular transcription factors or export factors. Nonetheless, this leads us to conclude that Gag does not play a role in the DR-mediated export pathway.
Still, Gag may interact with some other region of the unspliced viral RNA to promote nuclear export. One obvious site is the packaging signal Ψ, where Gag is known to bind the viral RNA and multimerize to begin genomic encapsidation (Swanstrom and Wills, 1997). It was recently shown that the murine leukemia virus Ψ signal was capable of promoting nuclear export of intron-containing viral RNAs (Smagulova et al., 2005). To test this possibility, we inserted the RSV Ψ signal into the pCMV128 construct (Fig. 2) and transfected it into QT6 cells with increasing amounts of the pGag-DR2 expression plasmid (Fig. 3B). The results were again normalized to pCMV128 cotransfected with Rev. When transfected alone, the pCMV128Ψ transcripts were not exported. Similarly, export was not induced when the construct was co-expressed with increasing amounts of Gag protein. This suggests that the RSV Ψ signal is also not involved in any potential Gag-directed nuclear RNA export.
The cellular export factor Tap affects DR-mediated mRNA export
Since simple retroviruses such as RSV do not encode accessory proteins to facilitate nuclear export, and viral Gag did not enhance DR-mediated export, we next examined cellular factors that might be recruited by the virus to accomplish this task. The cellular protein Tap (NXF1) is known to function in the nuclear export of spliced cellular mRNAs (Kang and Cullen, 1999; Reed and Hurt, 2002; Vinciguerra and Stutz, 2004). This protein has also been shown to bind directly to the constitutive transport element (CTE) of the Mason-Pfizer monkey virus (MPMV) (Pasquinelli et al., 1997; Braun et al., 1999). Although Tap does not interact directly with the DR elements as detected by gel-shift assays (Paca et al., 2000), it remains possible that Tap associates with unspliced viral transcripts through interactions with an adapter protein that binds the DR directly, somewhat analogous to the role REF/Aly plays in cellular mRNA export (Stutz et al., 2000; Zhou et al., 2000).
To this end, we obtained a previously characterized dominant negative Tap mutant (Chen et al., 2002). Tap interacts with nucleoporins via its C-terminal domain, which is critical for its export activity. The mutant, TapΔC, has a deletion of amino acids 518-619. Deletion of these critical residues allows the protein to bind mRNPs but not export them. Due to the dominant-negative effects of this Tap mutant, cells are only viable for approximately 24 hours after transfection (Chen et al., 2002). CEFs were transiently transfected with either wild type Tap or TapΔC and the pCMV128 constructs (Fig. 2). As a control for export, some samples were also transfected with pCMV-Rev. Cells were assayed for CAT activity after 24 hours (Fig. 4). Samples were again normalized to the CAT activity of pCMV128 cotransfected with pCMV-Rev. As expected, because HIV Rev uses the Tap-independent Crm1 export pathway (Fornerod et al., 1997; Neville et al., 1997; Askjaer et al., 1998), expression of Tap or TapΔC did not have an effect on Rev-dependent CAT expression. Alternatively, the dominant negative Tap mutant did have an inhibitory effect on the expression of pCMV128DR1. In the presence of wild type Tap, pCMV128DR1 had a sixfold increase in CAT activity compared to pCMV128. However, when the mutant was expressed, CAT activity was reduced over 50% when compared to expression with wild type Tap. Although there is still a significant amount of CAT activity seen with TapΔC, we attribute this to residual wild type Tap activity within the cell. Based on these results, in concert with the prior report containing gel-shift assays (Paca et al., 2000), we conclude that although Tap does not bind directly to the DR elements, it does play a functional role in the process of DR-mediated nuclear export.
Figure 4. A dominant negative mutant of Tap adversely affects the DR-mediated export of mRNA.
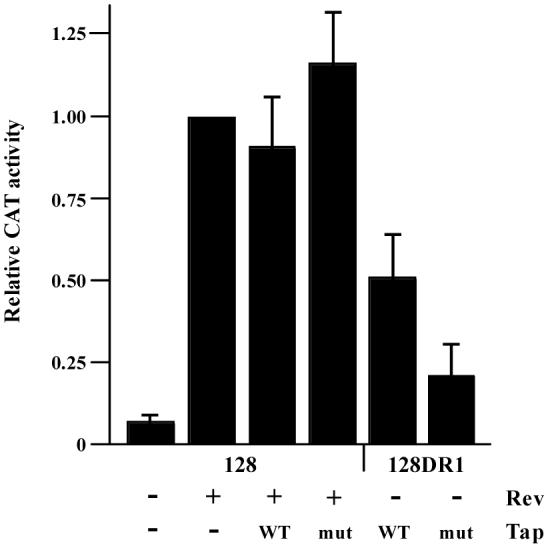
CEFs were transiently transfected with pCMV128 or pCMV128DR1. Cotransfected plasmids are indicated below the bar graph. Cells were maintained for 24 hours and assayed for CAT activity. All samples are normalized to pCMV128 + pCMV-Rev (lane 2). The results are from four independently performed transfections. WT, wild type Tap; mut, dominant negative mutant TapΔC.
The cellular RNA helicase Dbp5 affects DR-mediated mRNA export
Dbp5 is a DEAD-box RNA helicase involved in the nuclear export of poly(A)+ mRNA in yeast and vertebrate cells (Snay-Hodge et al., 1998; Hodge et al., 1999; Schmitt et al., 1999; Zhao et al., 2002). Since Tap is involved in unspliced RSV RNA export, we asked if Dbp5 was also involved. Using the same pCMV128 constructs, CEFs were transfected with either wild type human Dbp5 (hDbp5) or a dominant negative Dbp5 mutant, E243Q/V386N (Schmitt et al., 1999). The first mutation is in the highly conserved DEAD-box domain, which leads to the loss of ATP hydrolysis. The second mutation severely reduces the protein’s ability to bind RNA (Schmitt et al., 1999).
Cells were transiently transfected with the pCMV128 constructs and the Dbp5 clones. After 48 hours, cells were assayed for CAT activity (Fig. 5). CAT expression was increased with Rev cotransfection and this activity was not affected by the over-expression of wild type or mutant Dbp5, suggesting that Dbp5 is not involved in RRE-mediated nuclear RNA export. This result is consistent with the recent report identifying DDX3 as the nuclear pore complex protein used by HIV (Yedavalli et al., 2004).
Figure 5. A dominant negative mutant of Dbp5 adversely affects the DR-mediated export of mRNA.
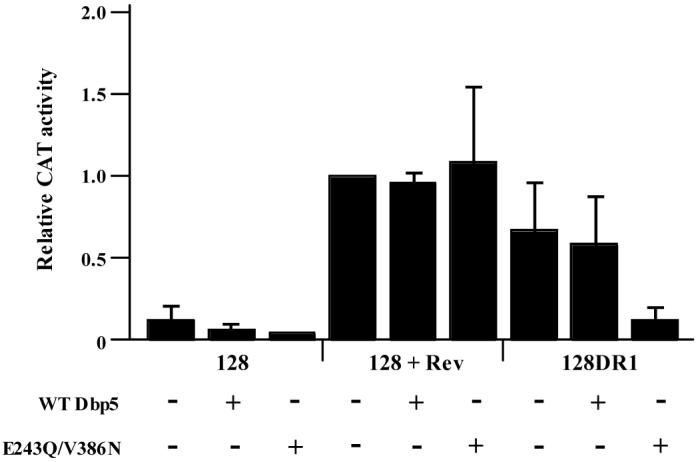
CEFs were transiently transfected with pCMV128, pCMV128 + pCMV-Rev or pCMV128DR1. Additionally, either wild type Dbp5 (WT Dbp5) or the dominant negative mutant (E243Q/V386N) was cotransfected as indicated. Cells were maintained for 48 hours and assayed for CAT activity. All samples are normalized to pCMV128 + pCMV-Rev without a Dbp5 over-expression clone (lane 4). These results are the average of six independent experiments.
Once again, however, the dominant negative mutant had a strong effect on the export of DR-regulated transcripts. With no Dbp5 or with wild type Dbp5 over-expression, the pCMV128DR1 construct supported nuclear export (CAT activity was ∼70% that of pCMV128 + Rev) (Fig. 5). However, when the mutant Dbp5 protein was expressed, CAT activity dropped to approximately 15% of pCMV128 + Rev levels, similar to the activity of the pCMV128 construct alone (Fig. 5). In addition to Tap, this result allows us to infer that the helicase Dbp5 is directly involved in the DR-mediated nuclear export of RNA.
Dbp5 is involved in the nuclear export of unspliced RSV RNA
Dbp5 is an RNA helicase mainly localized throughout the cytoplasm and on the cytoplasmic side of nuclear pore complexes (Snay-Hodge et al., 1998; Schmitt et al., 1999; Zhao et al., 2002). Since the CAT reporter gene system is based on the translation of an mRNA, it is possible that the protein is involved in unwinding the RNA for translation rather than export. To this end, we once again used FISH to examine the localization of unspliced RSV RNA in the presence of dominant negative Dbp5.
First, we examined the effect of human Dbp5 over-expression on cellular mRNAs in CEFs. The human Dbp5 protein sequence is 94%, 58% and 46% homologous to murine, fly and yeast Dbp5s, respectively (Schmitt et al., 1999). It has been shown to be involved in nuclear mRNA export based on Xenopus oocyte injection experiments. However, it is not essential for mRNA export in Drosophila (Schmitt et al., 1999).
CEFs were transiently transfected with no DNA, wild type Dbp5 or the dominant negative Dbp5. Cells were prepared for FISH two days later using a biotinylated oligo d(T) probe to identify the cellular poly(A)+ RNA (Fig. 6A). As expected, mock-transfected cells demonstrated a uniform distribution of mRNA throughout the cell. Additionally, the cells transfected with wild type Dbp5 (Fig. 6A, second row) demonstrated the same distribution of mRNA within the cell. Alternatively, cells transfected with the mutant Dbp5 showed a marked nuclear retention of mRNA within the cell (Fig. 6A, third and fourth rows). Because the Dbp5 clones are fused to GFP, we were able to discriminate between transfected and non-transfected cells. These results demonstrate that Dbp5 is involved in the nuclear export of cellular mRNAs in primary CEFs.
Figure 6. Dbp5 is involved in the nuclear export of cellular poly(A)+ mRNA and unspliced RSV RNA in CEFs.

(A) FISH analysis was done using a biotinylated oligo-d(T) probe to detect all cellular poly(A)+ mRNA. Cells were transfected with either wild type or dominant negative mutant (E243Q/V386N) Dbp5 and assayed for RNA localization two days later. Poly(A)+ mRNA is shown in red; DNA staining by DAPI is in blue. (B) Cells were transfected with wild type RSV and either the wild type or dominant negative Dbp5 mutant. Cells were assayed for unspliced viral RNA localization by FISH two days later using the DNA oligonucleotide described in Fig. 1. Unspliced viral RNA is in red; DNA staining by DAPI is in blue.
We next transfected CEFs with wild type RSV clones along with either the wild type or mutant Dbp5 over-expression constructs. Cells were prepared for FISH analysis two days later (Fig. 6B) using the same oligonucleotide probe described previously (Fig. 1). In cells transfected with wild type Dbp5, the unspliced RSV RNA was uniformly located throughout the cell, similar to the result obtained without Dbp5 over-expression (compare Fig. 6B top row to Fig. 1B, second and third rows). However, when the dominant negative mutant was expressed, strong nuclear retention of unspliced viral RNA was seen (Fig. 6B, bottom row). This result demonstrates that Dbp5 is involved in the nuclear export of unspliced RSV RNA. In combination with the prior results using the 128 constructs (Fig. 5), we conclude that this involvement is accomplished through interactions (either direct or indirect) with the DR elements.
Discussion
The focus of this study was to further investigate the role of the RSV DR elements. Although the DRs appear to be involved in several facets of replication, we examined the potential role the DRs play in the nuclear export of unspliced RNA. Using FISH studies, we demonstrated that a point mutation in DR results in a nuclear/perinuclear localization of unspliced RSV RNA. We have ruled out the viral Gag as being involved in DR-mediated nuclear export. We have identified two cellular proteins, Tap and Dbp5, which affect the expression of a reporter gene construct containing a copy of the DR. Furthermore, FISH studies demonstrated that when a dominant negative form of Dbp5 was co-expressed with wild type RSV, the RNA was once again restricted to a nuclear localization.
Although we do not present evidence for direct interactions between Tap, Dbp5 and the DR, we have formulated a model involving the two proteins as they assist in the export of the viral RNA (Fig. 7). After transcription, the DRs interact with a yet unknown cellular adapter, “X.” This protein is then able to recruit Tap and, presumably, its co-factor p15. This viral mRNP complex is deemed export-competent and is moved to the NPC. During the export process, Dbp5 acts as the functional helicase that permits the cytoplasmic localization of the viral RNA. This export process is similar to that of cellular mRNA, and differs only in the fact that cellular mRNAs are spliced. It is also similar to the MPMV unspliced RNA export pathway, which is mediated by the CTE (Bray et al., 1994; Ernst et al., 1997; Pasquinelli et al., 1997; Grüter et al., 1998). However, in contrast to the CTE, the direct interaction of Tap with the viral RNA has not been demonstrated (Paca et al., 2000).
Figure 7. Overview of cellular mRNA and unspliced retroviral RNA export.
Cellular mRNA export is mediated by the addition of REF/Aly during mRNA splicing, which recruits Tap and p15 to promote nuclear export. The RNA helicase Dbp5 is also involved in this export pathway. HIV employs the RRE, which binds the viral Rev protein, which in turn binds Crm1. This export pathway is Tap-independent, and instead uses DDX3 as the RNA helicase associated with export. MPMV utilizes the CTE to export unspliced viral RNAs. This structured element binds Tap directly and accesses the cellular mRNA pathway, presumably including p15 (Jin et al., 2003) and the helicase Dbp5. RSV uses the DR elements to recruit a yet unidentified adapter protein “X,” which in turn binds Tap and presumably p15. This complex is then targeted for nuclear export via Dbp5.
It is clear that the next step in further characterizing the nuclear export of this viral RNA is to identify the protein adapter (“X” in Fig. 7). Gag was unable to enhance DR-mediated export of unspliced reporter RNAs. It has been shown with the pCMV128 constructs that the DR can promote the nuclear export of unspliced RNA in the absence of Gag (Ogert et al., 1996; Ogert and Beemon, 1998; Simpson et al., 1998; Yang and Cullen, 1999; Paca et al., 2000). Transfection of the pCMV128DR1 construct and subsequent treatment with LMB, an inhibitor of the Crm1 export pathway, did not impair DR-mediated CAT activity (Paca et al., 2000). Conversely, LMB treatments were shown to trap Gag in the nucleus of transfected cells (Scheifele et al., 2002). Additionally, the idea of Gag inducing unspliced viral RNA export does not explain how the first unspliced messages escape the nucleus to initiate Gag synthesis. Taken together, these results suggest that Gag and DR-mediated RNA export are not linked. Alternatively, we explored the possibility that Gag interacts with the packaging signal Ψ to induce unspliced RNA export. While this result was also negative, it is important to note that these results do not rule out the potential for nuclear Gag:RNA interactions (and subsequent export) to exist, they merely rule out two candidate regions where this interaction could occur.
Other candidate adaptor proteins must be considered given the recent expansion of proteins that are now being reported as capable of promoting nuclear export. One obvious candidate would be the REF/Aly protein, which is the cellular adapter for the bulk of cellular mRNAs (Fig. 7) (Stutz et al., 2000; Zhou et al., 2000). This protein is recruited to mRNAs by the RNA helicase UAP56/HEL (Gatfield et al., 2001). However, in metazoans, REF/Aly recruitment is thought to occur during splicing (Erkmann and Kutay, 2004; Vinciguerra and Stutz, 2004).
A more likely set of candidates are the SR proteins. Recent studies have demonstrated that SR proteins, in the hypophosphorylated state, bind mRNA, promote nuclear export (Huang and Steitz, 2001), and are capable of binding Tap (Huang et al., 2003, 2004; Lai and Tarn, 2004). Only some shuttling SR proteins, such as ASF/SF2, 9G8 and SRp20 have this ability, while non-shuttling SR proteins, such as SC35, do not (Huang and Steitz, 2001; Lai and Tarn, 2004). In further support of these non-REF/Aly adapters binding Tap to promote export, a similar finding has been made in yeast. In Saccharomyces, the essential protein Npl3p has SR-like characteristics. This protein shuttles between the nucleus and cytoplasm, and interacts with Mex67p, the yeast Tap homolog (Gilbert and Guthrie, 2004).
A report by Kang and Cullen (1999) claims that the quail Tap protein is variant and somewhat non-functional, as evidenced by CTE-mediated nuclear export in Qcl-3 only functioning once wild type, exogenous Tap was provided in trans. They report that this quail Tap variant binds the CTE but is inadequate to support CTE export. However, DR-containing RNAs were efficiently exported in these cells (Yang and Cullen, 1999). This suggests that while the same protein is involved in nuclear export, a different domain of the protein may be required for promoting export via other mRNA adapter proteins.
A primary objective in this study was to rectify the differences between various studies regarding the potential functions of the DR. Currently, it is believed that the DR functions in nuclear RNA export (Ogert et al., 1996; Ogert and Beemon, 1998; Simpson et al., 1998, Yang and Cullen, 1999; Paca et al., 2000), stability of unspliced viral RNA (Ogert et al., 1996; Ogert and Beemon, 1998; Simpson et al., 1998), Gag assembly (Simpson et al., 1997, 1998) and genomic viral packaging (Sorge et al., 1983; Aschoff et al., 1999). While we cannot address all of these issues in one study, we have provided some resolution to the conflict concerning the ability of the DR to support the nuclear export of unspliced viral RNAs.
Based on our FISH studies, we noted that the unspliced viral RNA is located primarily within the nucleus. However, a fraction of the viral RNA seems to be cytoplasmic with a perinuclear location (Fig. 1B, bottom row). Previous reports used nuclear/cytoplasmic fractionations (Sorge et al., 1983; Ogert et al., 1996; Simpson et al., 1997, 1998; Ogert and Beemon, 1998; Aschoff et al., 1999), which can easily contain contaminating materials. This might explain why some reports see cytoplasmic accumulations (Sorge et al., 1983; Aschoff et al., 1999) and others do not (Ogert et al., 1996; Ogert and Beemon, 1998). Reported defects in packaging can also be addressed by this result. If the RNA is trapped at this perinuclear location, possibly because of incorrect interactions with Dbp5 at the cytoplasmic side of the NPC, the availability of unspliced RSV RNAs for packaging would be restricted.
There are other examples of RNA export elements being involved in genomic RNA packaging. It has long been known that there are various blocks to HIV replication in murine cells, including blocks to viral assembly. A study from the Malim group recently illustrated that transferring the CTE of MPMV restored HIV Gag processing and budding in 3T3 cells (Swanson et al., 2004). While this restoration was only achieved when multiple copies of the CTE were present, it does support the notion that a nuclear export element can have downstream effects on viral replication.
We conclude that the DR is directly involved in the nuclear export of unspliced RSV RNA. We suggest that the DR utilizes a cellular mRNA pathway since Tap and Dbp5 are both involved in this export process. In the future, we wish to further characterize this pathway by identifying the cellular factor that bridges the interactions between Tap and the viral RNA, protein “X.” We cannot, however, rule out other potential roles that the DR may play in viral RNA packaging and Gag assembly.
Acknowledgements
This work was supported by NIH grant R01 CA48746 to K.L.B. J.J.L. was supported in part by NIH predoctoral training grant T32GM07231. We thank Eliza Izarraulde for the Dbp5 clones, Rozanne Sandri-Goldin for the Tap clones, Tom Hope for the 128 construct, and Anna Marie Skalka for the RSV integrase mutant. We thank members of the DR Consortium for helpful discussions and Jason Weil for review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aschoff JM, Foster D, Coffin JM. Point mutations in the avian sarcoma/leukosis virus 3’ untranslated region result in a packaging defect. J. Virol. 1999;73(9):7421–7429. doi: 10.1128/jvi.73.9.7421-7429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askjaer P, Jensen TH, Nilsson J, Englmeier L, Kjems J. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J. Biol. Chem. 1998;273(50):33414–33422. doi: 10.1074/jbc.273.50.33414. [DOI] [PubMed] [Google Scholar]
- Bray M, Prasad S, Dubay JW, Hunter E, Jeang K-T, Rekosh D, Hammarskjöld M-L. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc. Natl. Acad. Sci. USA. 1994;91(4):1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun IC, Rohrbach E, Schmitt C, Izaurralde E. TAP binds to the constitutive transport element (CTE) through a novel RNA-binding motif that is sufficient to promote CTE-dependent RNA export from the nucleus. EMBO J. 1999;18(7):1953–65. doi: 10.1093/emboj/18.7.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IH, Sciabica KS, Sandri-Goldin RM. ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway. J. Virol. 2002;76(24):12877–12889. doi: 10.1128/JVI.76.24.12877-12889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkmann JA, Kutay U. Nuclear export of mRNA: from the site of transcription to the cytoplasm. Exp. Cell Res. 2004;296(1):12–20. doi: 10.1016/j.yexcr.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Ernst RK, Bray M, Rekosh D, Hammarskjöld M-L. A structured retroviral RNA element that mediated nucleocytoplasmic export of intron-containing RNA. J. Virol. 1997;17(1):135–144. doi: 10.1128/mcb.17.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornerod M, Ohno M, Yoshida M, Mattaj IW. Crm1 is an export receptor for the leucine-rich nuclear export signals. Cell. 1997;90(6):1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Fornerod M, van Deursen J, van Baal S, Reynolds A, Davis D, Murti KG, Fransen J, Grosveld G. The human homolog of yeast Crm1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component, Nup88. EMBO J. 1997;16(4):807–816. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatfield D, Le Hir H, Schmitt C, Braun IC, Köcher T, Wilm M, Izaurralde E. The DexH/D box protein HEL/UAP56 is essential for mRNA nuclear export in Drosophila. Curr. Biol. 2001;11(21):1716–1721. doi: 10.1016/s0960-9822(01)00532-2. [DOI] [PubMed] [Google Scholar]
- Gilbert W, Guthrie C. The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol. Cell. 2004;13(2):201–212. doi: 10.1016/s1097-2765(04)00030-9. [DOI] [PubMed] [Google Scholar]
- Gorman CM, Moffat LF, Howard BH. Recombinant genomes which express chloramphenicol acetyltransferase in mammaliam cells. Mol. Cell. Biol. 1982;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber BK, Izauralde E. Tap, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell. 1998;1(5):649–659. doi: 10.1016/s1097-2765(00)80065-9. [DOI] [PubMed] [Google Scholar]
- Herold A, Klymenko T, Izaurralde E. NXF1/p15 heterodimers are essential for mRNA nuclear export in Drosophila. RNA. 2001;7(12):1768–1780. [PMC free article] [PubMed] [Google Scholar]
- Hodge CA, Colot HV, Stafford P, Cole CN. Rat8p/Dbp5p is a shuttling transport factor that interacts with Rat7p/Nup159p and Gle1p and suppresses the mRNA export defect of xpo1-1 cells. EMBO J. 1999;18(20):5778–5788. doi: 10.1093/emboj/18.20.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope TJ, Huang X, McDonald D, Parslow TG. Steriod receptor fusion of the human immunodeficiency virus type 1 Rev transactivator: Mapping cryptic functions of the arginine-rich motif. Proc. Natl. Acad. Sci. 1990;87(19):7787–7791. doi: 10.1073/pnas.87.19.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Gattoni R, Stevenin J, Steitz JA. SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol. Cell. 2003;11(3):837–843. doi: 10.1016/s1097-2765(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Huang Y, Steitz JA. Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol. Cell. 2001;7(4):899–905. doi: 10.1016/s1097-2765(01)00233-7. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yario TA, Steitz JA. A molecular link between SR protein dephosphorylation and mRNA export. Proc. Natl. Acad. Sci. 2004;101(26):9666–9670. doi: 10.1073/pnas.0403533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Guzik BW, Bor YC, Rekosh D, Hammarskjöld M-L. Tap and NXT promote translation of unspliced mRNA. Genes Dev. 2003;17(24):3075–86. doi: 10.1101/gad.1155703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CV, Singer RH, Lawrence JB. Fluorescent detection of nuclear RNA and DNA: implications for genomic organization. Methods Cell Biol. 1991;35:73–99. [PubMed] [Google Scholar]
- Lai M-C, Tarn W-Y. Hypophosphorylated ASF/SF2 binds Tap and is present in messenger ribonucleoproteins. J. Biol. Chem. 2004;279(30):31745–31749. doi: 10.1074/jbc.C400173200. [DOI] [PubMed] [Google Scholar]
- Le Hir H, Gatfield D, Izaurralde E, Moore MJ. The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J. 2001;17(20):4987–97. doi: 10.1093/emboj/20.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund MK, Guthrie C. The DEAD-box protein Dbp5p is required to dissociate Mex67p from exported mRNPs at the nuclear rim. Mol. Cell. 2005;20(4):645–651. doi: 10.1016/j.molcel.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Kang Y, Cullen BR. The human Tap protein is a nuclear mRNA export factor that contains novel RNA-binding and nucleocytoplasmic transport sequences. Genes Dev. 1999;13(9):1126–1139. doi: 10.1101/gad.13.9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkosky J, Jones KS, Katz RA, Mack JPG, Skalka AM. Residues critical for retroviral integrative recombination in a region that is highly conserved among retroviral/retrotransposon integrases and bacterial insertion sequence transposases. Mol. Cell. Biol. 1992;12(5):2331–2338. doi: 10.1128/mcb.12.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH, Hauber J, Le SY, Maizel JV, Cullen BR. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Neville M, Stutz F, Lee L, Davis LI, Rosbash M. The importin-beta family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export. Curr. Biol. 1997;7(10):767–75. doi: 10.1016/s0960-9822(06)00335-6. [DOI] [PubMed] [Google Scholar]
- Ogert RA, Lee LH, Beemon KL. Avian retroviral RNA element promotes unspliced RNA accumulation in the cytoplasm. J. Virol. 1996;70(6):3834–3843. doi: 10.1128/jvi.70.6.3834-3843.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogert RA, Beemon KL. Mutational analysis of the Rous sarcoma virus DR posttranscriptional control element. J. Virol. 1998;72(4):3407–3411. doi: 10.1128/jvi.72.4.3407-3411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paca RE, Ogert RA, Hibbert CS, Izaurralde E, Beemon KL. Rous sarcoma virus DR posttranscriptional elements use a novel RNA export pathway. J. Virol. 2000;74(20):9507–9514. doi: 10.1128/jvi.74.20.9507-9514.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquinelli AE, Ernst RK, Lund E, Grimm C, Zapp ML, Rekosh D, Hammarskjöld M-L, Dahlberg JE. The constitutive transport element (CTE) of Mason-Pfizer monkey virus (MPMV) accesses a cellular mRNA export pathway. EMBO J. 1997;16(24):7500–7510. doi: 10.1093/emboj/16.24.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson AB, Graves BJ. Synthesis and processing of viral RNA. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor Laboratory Press; Woodbury, NY: 1997. pp. 205–262. [PubMed] [Google Scholar]
- Reed R, Hurt E. A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell. 2002;108(4):523–531. doi: 10.1016/s0092-8674(02)00627-x. [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H, Moreno H, Simos G, Segref A, Fahrenkrog B, Pante N, Hurt E. Nuclear mRNA export requires complex formation between Mex67p and Mtr2p at the nuclear pores. Mol. Cell. Biol. 1998;18(11):6826–6838. doi: 10.1128/mcb.18.11.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifele LZ, Garbitt RA, Rhodes JD, Parent LJ. Nuclear entry and CRM-1 dependent nuclear export of the Rous sarcoma virus Gag polyproteins. Proc. Natl. Acad. Sci. USA. 2002;99(6):3944–3949. doi: 10.1073/pnas.062652199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C, von Kobbe C, Bachi A, Pante N, Rodrigues JP, Boscheron C, Rigaut G, Wilm M, Seraphin B, Carmo-Fonseca M, Izaurralde E. Dbp5, a DEAD-box protein required for mRNA export, is recruited to the cytoplasmic fibrils of nuclear pores complex via a conserved interaction with CAN/Nup159p. EMBO J. 1999;18(15):4332–4347. doi: 10.1093/emboj/18.15.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SB, Zhang L, Craven RC, Stoltzfus CM. Rous sarcoma virus direct repeat cis elements exert effects at several points in the viral life cycle. J. Virol. 1997;71(12):9150–9156. doi: 10.1128/jvi.71.12.9150-9156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SB, Guo W, Winistorfer SC, Craven RC, Stoltzfus CM. The upstream direct repeat sequence of Prague A Rous sarcoma virus is deficient in mediating efficient Gag assembly and particle release. Viology. 1998;247(1):86–96. doi: 10.1006/viro.1998.9233. [DOI] [PubMed] [Google Scholar]
- Smagulova F, Maurel S, Morichaud Z, Devaux C, Mougel M, Houzet L. The highly structured encapsidation signal of MuLV RNA is involved in the nuclear export of its unspliced RNA. J. Mol. Biol. 2005;354(5):1118–1128. doi: 10.1016/j.jmb.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Snay-Hodge CA, Colot HV, Goldstein AL, Cole CN. Dbp5p/Rat8p is a yeast nuclear pore-associated DEAD-box protein essential for RNA export. EMBO J. 1998;17(9):2663–2676. doi: 10.1093/emboj/17.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge J, Ricci W, Hughes SH. cis-Acting RNA packaging locus in the 115-nucleotide direct repeat of Rous sarcoma virus. J. Virol. 1983;48(3):667–675. doi: 10.1128/jvi.48.3.667-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz F, Bachi A, Doerks T, Braun IC, Seraphin B, Wilm M, Bork P, Izaurralde E. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with Tap/Mex67p and participates in mRNA nuclear export. RNA. 2000;6(4):638–650. doi: 10.1017/s1355838200000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CM, Puffer BA, Ahmad KM, Doms RW, Malim MH. Retroviral mRNA nuclear export elements regulate protein function and virion assembly. EMBO J. 2004;23(13):2632–2640. doi: 10.1038/sj.emboj.7600270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R, Wills JW. Synthesis, assembly and processing of viral proteins. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor Laboratory Press; Woodbury, NY: 1997. pp. 263–334. [PubMed] [Google Scholar]
- Vinciguerra P, Stutz F. mRNA export: an assembly line from genes to nuclear export. Curr. Opin. Cell Biol. 2004;16(3):285–92. doi: 10.1016/j.ceb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Yang J, Cullen BR. Structural and functional analysis of the avian leukemia virus constitutive transport element. RNA. 1999;5(12):1645–1655. doi: 10.1017/s1355838299991616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedavalli VSRK, Neuveut C, Chi Y, Kleiman L, Jeang K-T. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell. 2004;119(3):381–392. doi: 10.1016/j.cell.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Zhao J, Jin S-B, Björkroth B, Wieslander L, Daneholt B. The mRNA export factor Dbp5 is associated with Balbani ring mRNP from gene to cytoplasm. EMBO J. 2002;21(5):1177–1187. doi: 10.1093/emboj/21.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Luo MJ, Straesser K, Katahira J, Hurt E, Reed R. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature. 2000;407(6802):401–405. doi: 10.1038/35030160. [DOI] [PubMed] [Google Scholar]
- Zolotukhin AS, Valentin A, Pavlakis GN, Felber BK. Continuous propagation of RRE(-) and Rev(-)RRE(-) human immunodeficiency virus type 1 molecular clones containing a cis-acting element of simian retrovirus type 1 in human peripheral blood lymphocytes. J. Virol. 1994;68(12):7944–7952. doi: 10.1128/jvi.68.12.7944-7952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]



