Abstract
Purpose
Studies have investigated time to testosterone (T) recovery in patients who have undergone androgen-deprivation therapy (ADT)—usually by measuring androgens every 3 months—with varying results. This represents the largest study utilizing monthly measurements of both T and dihydroxytestosterone (DHT) to evaluate the kinetics of androgen recovery following limited ADT.
Materials and Methods
Monthly serum androgen levels were analyzed following two 6-month cycles of gonadotropin-releasing hormone agonist (GnRH-A) therapy as part of a randomized placebo-controlled study evaluating the role of thalidomide in delaying time to prostate-specific androgen progression.
Results
By the Kaplan-Meier method, the median time to T normalization in cycle 1 was 15.4 weeks versus 18.3 weeks in cycle 2, with similar recovery times of DHT. Neither on-study PSA, Gleason score, nor thalidomide treatment had a significant impact on time to T normalization. However, in cycle 1, men with low baseline DHT and those who were > 67 years old had significantly longer time to T normalization in a Cox model analysis. Additionally, in cycle 2, patients with prior local radiation therapy had longer time to T normalization, although this was no longer significant in Cox model analysis. Cox model analysis in cycle 2 showed that low baseline DHT and T, low T nadir, and Caucasian race were associated with longer time to T normalization.
Conclusions
The findings of delayed T recovery may be useful for designing and analyzing clinical trials employing limited ADT, and for estimating the duration of treatment-associated side effects in patients undergoing limited ADT.
Keywords: clinical trial, dihydroxytestosterone, intermittent ADT, radiation therapy, testosterone
INTRODUCTION
Androgen-deprivation therapy (ADT) is the cornerstone of initial treatment of androgen-sensitive, metastatic, recurrent, or advanced prostate cancer 1. It is estimated that in the United States alone, 600,000 prostate cancer patients a year are treated with ADT 2. Understanding the kinetics of testosterone (T) recovery after ADT withdrawal is essential to the proper management of patients with prostate cancer, especially the growing number of patients receiving ADT in the neoadjuvant, adjuvant, or intermittent setting. Several studies have investigated the time to T recovery following neoadjuvant, prolonged adjuvant, or intermittent ADT—usually by measuring androgen levels every 3 months—with varying results 3-5. Some data from the trial reported here have been previously published 6. T recovery was analyzed in the initial 80 patients on trial after a single 6-month cycle of gonadotropin-releasing hormone agonist (GnRH-A) therapy. We report here final results on the kinetics of T recovery for the whole cohort of patients after each of 2 cycles of limited GnRH-A therapy.
Studies have shown that local radiation therapy (RT) alone can affect T levels for a limited time 7-9, with potential implications for T reserve in these patients. Temporary ADT (6 to 36 months) is increasingly being used in combination with RT for intermediate- and high-risk prostate cancer patients; intermittent ADT is increasingly being used for patients with biochemical failure following local treatment. Being able to predict when androgens will return to normal in these patients would be valuable for interpreting clinical studies and advising patients of the potential duration of side effects before starting ADT. Furthermore, as pressure mounts to stretch health care budgets, understanding the kinetics of androgen recovery after ADT could help clinicians devise individualized GnRH-A dosing schedules for efficient maintenance of continuous ADT10, 11. To our knowledge, this is the largest study to use monthly measurements of both T and dihydroxytestosterone (DHT) to evaluate the kinetics of androgen recovery following limited ADT.
METHODS
Patients and procedures
The study reported here was a randomized, multicenter, phase III clinical trial of limited intermittent ADT. The study enrolled 159 patients who had undergone previous definitive local therapy and had rising prostate-specific antigen (PSA) with no radiographic evidence of metastatic disease. In cycle 1, patients received 6 months of GnRH-A, which generally consisted of either two 3-month injections of leuprolide (22.5 mg) or goserelin (10.8 mg), followed by thalidomide or placebo until PSA progression, as defined by a PSA concentration of 5 ng/ml or on-study value, whichever occurred first. No anti-androgens were given. Upon PSA progression, patients repeated a 6-month course of GnRH-A, referred to as cycle 2, followed by cross-over to the opposite oral treatment. Prior neoadjuvant or adjuvant hormonal therapy was allowed only if administered > 1 year prior to enrollment. Analysis of changes in measurements of T and DHT were defined endpoints of this trial. T and DHT measurements were obtained upon study enrollment, every 3 months while on GnRH-A, and once a month while on the study drug. Only complete or near complete T or DHT data from patients enrolled on study were included in this analysis. Assays for measuring T and DHT have been previously described 6. Normal range for T was 212 to 742 ng/dl; normal range for DHT was 150 to 980 pg/ml.
Statistical analysis
To account for the effects of GnRH-A, time was measured from administration of the final 3-month GnRH-A until the time when normal values of T (212 ng/dl) or DHT (150 pg/ml) were reached, minus 12 weeks. This was done following both cycles of GnRH-A. The probability of normalization of T and DHT as a function of time following both cycle 1 and cycle 2 was determined using the Kaplan-Meier method; statistical significance of the difference between Kaplan-Meier curves was determined using Mantel-Haenszel (log-rank) tests. For those patients who did not reach the normal T or DHT values at the predefined PSA progression, time to normal levels was considered censored at that time. Additional log-rank tests were performed to examine the effects of different variables in cycle 1 and 2, including age, on-study PSA, Gleason score, race, prior definitive therapies, baseline T and DHT levels, T nadir, and thalidomide treatment. Differences between median baseline values were tested using the Wilcoxon rank sum test. A comparison between categorized baseline PSA values prior to cycle 1 and cycle 2 in the same patients was performed using the exact McNemar’s test for paired binomial data. All P values reported were 2-tailed and unadjusted for multiple comparisons, except when initial analysis of Kaplan-Meier curves according to multiple categories indicated that pooling into 2 groups would result in a clear difference in prognosis. In that case, the adjusted P value was determined by multiplying the unadjusted P value by the implicit number of tests performed in order to arrive at that grouping. Factors in the univariate analysis which were deemed important by virtue of their association with T rise in either cycle 1 or 2 (P < 0.10 in log-rank analyses) were further evaluated for their joint association for these particular endpoints using a Cox proportional hazards model.
RESULTS
This study evaluated time to T and DHT normalization after initial treatment with 6 months of GnRH-A (cycle 1), and after patients had received thalidomide or placebo as well as a second 6-month course of GnRH-A (cycle 2). Of the 159 patients enrolled on study, 129 had complete or near complete T data (107 patients had baseline T data; 22 patients had no baseline T measured, but follow-up T data were available). Only these patients were included in this analysis (Table 1). Primary analysis included time to recovery of T to low value (50 to 211 ng/dl) and normal value (> 211 ng/dl). Time to recovery of DHT to > 150 pg/ml was evaluated as a secondary outcome.
Table 1. Clinical characteristics of patients enrolled on study.
| Characteristics | No. of patients (%) |
| Total enrolled on study | 129 |
| Median age | 67 (range: 49-87) |
| Caucasian | 108 (83.7) |
| African American | 21 (16.3) |
| Gleason scores | No. of patients (%) |
| 3 | 1 (0.7) |
| 4 | 3 (2.3) |
| 5 | 10 (7.7) |
| 6 | 26 (20.2) |
| 7 | 53 (41.1) |
| 8 | 22 (17.1) |
| 9 | 14 (10.9) |
| Median 7 (range: 3-9) | |
| Prior definitive therapy | No. of patients (%) |
| Radical prostatectomy alone | 30 (23.3) |
| RT alone | 39 (30.2) |
| Surgery and RT | 60 (46.5) |
| Prior hormonal therapy | No. of patients (%) |
| Yes | 30 (23.3) |
| No | 99 (76.7) |
| Median on-study PSA | ng/ml |
| 5.1 (range: 0.9-311.8) | |
| Median baseline testosterone | ng/dl |
| Cycle 1 | 311.5 (range: 10-1000) |
| Cycle 2 | 326 (range: 20-1400) |
| Thalidomide | 307.5 (range: 10-1000) |
| Placebo | 338.5 (range: 80-805) |
| Median baseline DHT | pg/ml |
| Cycle 1 | 235 (range: 18.8-1455) |
| Cycle 2 | 142 (range: 50-606) |
Cycle 1
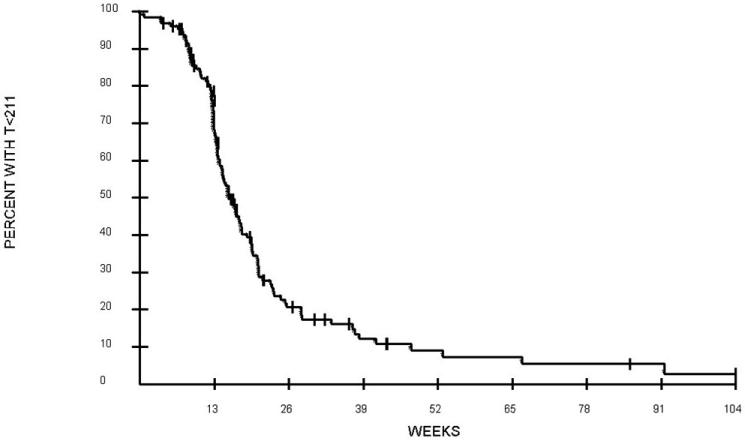
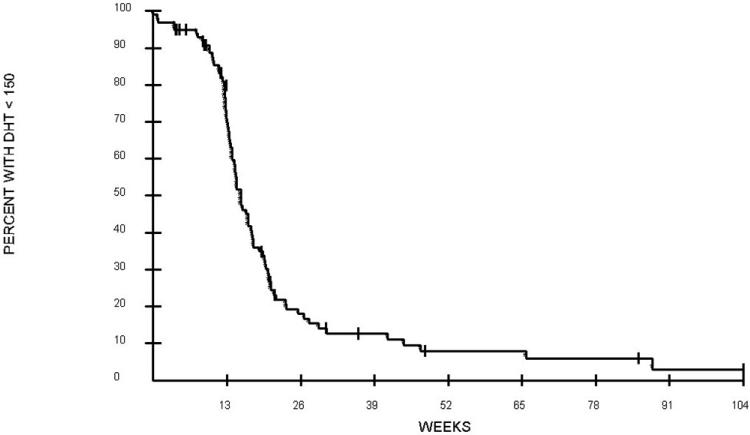
Of 129 evaluable patients, 107 had baseline T measured. Of these patients, 17 had low T levels. During cycle 1, median time to normalization of T was 15.4 weeks (fig. 1, Table 2). The median time to T normalization was 14.4 weeks for patients with normal baseline T levels, but twice as long (31.3 weeks) for patients with low baseline T levels (P = 0.0002). The median time to rise of T to low concentration (50 to 211 ng/dl) was also longer for patients with low baseline T levels (14.5 weeks) compared to patients with normal baseline T levels (10.7 weeks) (P = 0.0048). Baseline DHT was measured in 100 patients; the median time to normalization of DHT was 15.2 weeks (fig. 2, Table 2). Use of thalidomide had no impact on time to serum normalization of T or DHT. The median time to serum T normalization in the thalidomide group was 14.5 weeks versus 16.7 weeks in the placebo group (P = 0.20); the median time to serum DHT normalization in the thalidomide group was 15.2 weeks versus 14.8 weeks in the placebo group (P = 0.31). Further univariate analyses using factors that may affect time to T recovery were performed. These factors including on-study PSA, Gleason scores upon initial diagnosis of prostate cancer, and previous ADT or definitive surgery or RT showed no association with time to normalization of serum androgens. The median age on study was 67 years old. Overall, men ≤ 67 years old had a shorter time to serum T normalization (median 14.7 weeks) than men > 67 years old (17.1 weeks) (P = 0.027 for overall comparison after adjustment for multiple comparisons). Further evaluation among patients with low baseline T showed that age did not affect time to serum androgen recovery (P = 0.68). Among the subset of men with normal baseline T levels, the median time to normalization of T levels in patients > 67 years old was 15.5 weeks versus 13.2 weeks for patients ≤ 67 years old (P = 0.0028). In addition, among patients who were ≤ 67 years old, those with low baseline T had a longer time to normalization of serum T (median 22.4 weeks) than those with normal baseline T (13.2 weeks) (P = 0.0018). During cycle 1, a T nadir of ≤ 20 ng/dl occurred in 94 patients, who experienced a longer time to low T level (50 to 211 ng/dl) recovery, with a median of 12.8 weeks, compared to those who achieved a higher T nadir of > 20 ng/dl with shorter time to low T level recovery of 6.7 weeks (P = 0.0001). The level of T nadir (≤ 20 versus > 20) was not associated with time to T normalization. Further exploratory analyses showed that there was a difference in time to DHT recovery between Caucasians (median 15.4 weeks) and African Americans (12.6 weeks) on-study (P = 0.021). In Cox model analyses, only age and baseline DHT were significantly associated with the time of T rise to normal in cycle 1 (Table 3).
FIG. 1. Time to rise in serum T to ≥ 212 ng/dl in 128 evaluable patients during cycle 1. Week 0 begins 12 weeks after last 3-month GnRH-A.
Table 2. Summary of median time to low T and normalization of T and DHT.
| Cycle | Time to T 50-211 ng/dl (wks) | Time to T > 211 ng/dl (wks) | Time to DHT > 150 pg/ml (wks) |
|---|---|---|---|
| 1 | 12.1 | 15.4 | 15.2 |
| 2 | 11.5 | 18.3 | 18.7 |
FIG. 2. Time to rise in serum DHT to ≥ 150 pg/ml in 100 evaluable patients during cycle 1. Week 0 begins 12 weeks after last 3-month GnRH-A.
Table 3. Cox proportional hazards model† for factors associated with T rise in cycle 1 and cycle 2.
| Parameter* | Hazard Ratio (HR) | 95% CI on HR | P value |
|---|---|---|---|
| T > 211 in cycle 1 | |||
| Age (> 67 vs. ≤ 67) | 2.01 | 1.24 - 3.25 | 0.0045 |
| Baseline DHT (< 153 vs. ≥ 153) | 2.34 | 1.32 - 4.15 | 0.0037 |
| T 50-211 in cycle 1 | |||
| T nadir (≤ 20 vs. > 20) | 2.22 | 1.46 - 3.36 | 0.0002 |
| T > 211 in cycle 2 | |||
| Baseline T (low vs. normal) | 4.24 | 1.20 - 15.07 | 0.025 |
| Baseline DHT (< 300 vs. ≥ 300) | 2.94 | 1.41 - 6.12 | 0.0038 |
| Caucasian vs. African American | 4.36 | 1.61 - 11.76 | 0.0037 |
| T nadir (≤ 20 vs. > 20) | 3.59 | 1.81 - 7.11 | 0.0002 |
| T 50-211 in cycle 2 | |||
| T nadir (≤ 20 vs. > 20) | 4.56 | 2.58 - 8.06 | < 0.0001 |
Groups with delayed T rise listed first;
The final model, resulting from backward elimination, is presented. Factors included in the initial models, but which did not meet criteria to remain in the final model were baseline T in cycle 1 (for both models in cycle 1), and prior RT (for both models in cycle 2).
Cycle 2
In cycle 2, median time to serum T normalization was 18.3 weeks—2.9 weeks longer than in cycle 1. The kinetics of the curves was nearly identical, but shifted away from time of GnRH-A administration (data not shown). The overall median time to rise of T to low levels was 11.5 weeks. As in cycle 1, the time to serum T normalization was longer for patients with low baseline T levels at the beginning of cycle 2 (median time not reached) compared to patients with normal T levels (median 18 weeks; P = 0.0022), although this analysis may be limited by the small number of patients (n = 14) with low serum T levels at the beginning of cycle 2. The median time to DHT normalization was 18.7 weeks. Also as in cycle 1, thalidomide did not affect time to serum T normalization, with a median of 18.0 weeks versus 19.2 weeks for placebo (P = 0.70). No association was found between time to serum T normalization and age, on-study PSA, Gleason score, and prior hormonal therapy. One factor which appeared to be potentially associated with a delay in increase of T to low or normal concentration in univariate analyses was a history of RT. In cycle 2, 67 patients had received prior RT and 23 patients had not (Table 4). The median time for an increase of T to low concentration for patients with prior history of RT was 12.1 weeks versus 8.3 weeks for patients with no prior RT (P = 0.0011). The median time for an increase of T to normal concentration was also longer for patients who had undergone RT (20.5 weeks) than for patients who had received no RT (13.2 weeks; P < 0.0001). The median time to normalization of T was 14.6 weeks for patients with a cycle 2 baseline DHT of > 300 ng/dl versus 20.4 weeks for patients with a cycle 2 baseline DHT of 50 to 299 ng/dl (P = 0.0001). Further analysis in cycle 2 showed that a T nadir of ≤ 20 ng/dl occurred in 53 patients, which was associated with longer time to recovery of normal DHT (median 23.2 weeks versus 15.4 weeks; P = 0.036) than for those who had a T nadir of > 20 ng/dl. Longer time to recovery to low T (median 12.3 weeks versus 1.5 weeks; P < 0.0001) and normal T concentrations (median 21.6 weeks versus 15 weeks; P = 0.0001) were also observed for those who achieved a T nadir of ≤ 20 ng/dl compared to those who had a T nadir > 20 ng/dl. Notable differences between Caucasians and African Americans were also observed in cycle 2, with longer time to DHT normalization for Caucasians (median 19.1 weeks) versus African Americans (13 weeks; P = 0.0022), as well as longer time to T normalization for Caucasians (median 19.3 weeks) versus African Americans (13.9 weeks; P = 0.0058). Cox model analysis of the factors affecting T recovery to normal in cycle 2 demonstrated that 4 factors—baseline T, DHT, race, and T nadir—were statistically significantly associated with this outcome when considered jointly (Table 3). History of RT did not remain significant in the Cox analysis after adjusting for these other factors (P = 0.20 when considered jointly with other factors; results not shown).
Table 4. Clinical characteristics of patients proceeding to cycle 2.
| Parameters | Value |
|---|---|
| Total number of patients | 90 |
| Median age (range, years) | 66 (49 - 97) |
| No. of patients with history of prior RT | 67 |
| No. of patients with history of prior ADT | 20 |
| Median Gleason score | 7 |
| Range | 3 - 9 |
| Median on-study PSA ng/dL | 5.65 |
| Range | 0.9 - 124.2 |
| Median baseline T prior to cycle 1 (range) | 325 (49 - 802) |
| Median baseline T prior to cycle 2 (range) | 326 (20 - 1400) |
All patients initially had androgen-sensitive prostate cancer, as evidenced by a decrease in serum PSA following GnRH-A treatment. In cycle 1 this decreased to ≤ 0.2 ng/dl in 93 of 129 patients (72%) while the remaining patients had a > 90% decrease in PSA, except for one patient who had an 85% decrease in PSA. Of the 90 patients who proceeded to cycle 2, 77 had evaluable baseline PSA data. Of these 77 patients, 44 had undetectable baseline levels of PSA nadirs (≤ 0.2 ng/dl) following both cycles, while 17 had detectable levels following both cycles; 3 had detectable levels after cycle 1 which then became undetectable after cycle 2, while 13 had undetectable levels after cycle 1 which then rose to detectable levels at cycle 2. The tendency to have more patients go from undetectable PSA following cycle 1 to detectable PSA following cycle 2 (n = 13) than go from detectable following cycle 1 to undetectable following cycle 2 (n = 3) was statistically significantly different from an expected 50:50 distribution if there was no influence of a prior cycle on outcome (P = 0.02).
DISCUSSION
ADT is being increasingly utilized to treat different disease states in prostate cancer. Therefore, understanding the kinetics of serum T normalization after limited hormonal therapy in the setting of biochemical recurrence is crucial for the design and interpretation of clinical trials in prostate cancer. Several studies have evaluated the time to serum T normalization after limited hormonal therapy 3-5, 12, leading to the general belief that the effects of T suppression persist long after cessation of GnRH-A therapy. However, the majority of these studies had variable durations of ADT, a limited number of patients, or a large group of patients whose T levels were analyzed only at 3-month intervals, allowing for imprecision in the timing of T recovery.
This study is in agreement with previous studies reporting that time to T recovery is longer in patients at high risk for low T reserve, such as older patients and patients with lower baseline levels of T. The data reported here suggest that patients with prior RT also have longer time to T normalization, although Cox model analysis determined that age, baseline T and DHT, and race jointly appear to be adequate to account for differences in outcome, with less additional impact noted from history of prior RT when these other factors are taken into consideration (P = 0.20 for association with RT after adjusting for the other factors). Some patients did not recover their T (n=27) and DHT (n=18) during cycle 1. Approximately half of these came off trial prior to androgen recovery with the remainder having delayed recovery.
The fact that the time to normalization of T following cycle 2 was affected by baseline T and DHT and nadir T in the Cox model analysis is intuitive and likely directly related to issues of low T reserve. This finding of lower T reserve during cycle 2 has implications for the design of clinical trials. Prolonged T suppression may make the effects of a treatment that has limited activity as a monotherapy, such as thalidomide 13, more readily apparent following the second cycle of GnRH-A therapy. Data from this trial that support this concept will be presented elsewhere. However, the finding of race-associated differences in time to T normalization is intriguing. Previous large studies have shown no differences in serum androgen levels between African Americans and Caucasians 14, 15. There are data showing that sex hormone-binding globulin levels are higher in African Americans than in Caucasians 15, 16, but we are not aware of any study that has analyzed T reserve differences between races.
Also in accordance with prior studies, we found that while all patients remained sensitive to ADT, responsiveness during cycle 2 was blunted. In paired analyses, based on PSA levels only when available at both time points in the same subjects, although 61 of 77 patients had concordant levels (detectable or not) following both cycle 1 and cycle 2, there was a tendency to have more patients with detectable levels (n = 13) following the second cycle who had undetectable levels during cycle 1 than the obverse (n = 3), which was statistically significantly different from an expected 50:50 distribution if there had been no influence of a prior cycle on outcome (P = 0.02). These findings likely reflect the emergence over time of castrate-resistant populations of prostate cancer cells.
While this study obtained multiple, frequent (monthly) measurements of T and DHT following a uniform GnRH-A administration of 6 months of therapy, the study nevertheless had several limitations. The vast majority of patients received leuprolide injections, and thus no comparisons can be made between different available GnRH agonists. Furthermore, relatively few patients (< 25%) had prior ADT, and those who did reported various time points since that prior ADT. Thus, the baseline T reserve for that subgroup of patients may not be uniform. A recent study reported that patients with a BMI >30 kg/m2, although they had lower levels of T prior to ADT, had higher T nadirs than patients with a BMI <25 kg/m2.17 This may effect the time to T normalization, however we did not analyze this in our study. Finally, the large majority of patients were Caucasians, thus limiting generalization of data to other races.
CONCLUSIONS
Following 6 months (24 weeks) of GnRH-A therapy in patients with androgen-sensitive prostate cancer, T levels returned to normal after an additional 15.4 weeks during cycle 1 and after 18.3 weeks during cycle 2; DHT levels returned to normal after an additional 15.2 weeks during cycle 1 and after 18.7 weeks during cycle 2. Not surprisingly, patients affected by factors associated with lower T reserves had longer time to androgen normalization. These data have significant implications for the design and analysis of clinical trials utilizing limited ADT. Furthermore, intermittent ADT is being increasingly employed in the large population of patients with biochemical failure, and appears to have similar clinical outcomes to continuous ADT in this patient population18-20 with less cost and possibly, fewer side effects. Being able to more accurately predict the duration of a patient’s risk of side effects related to iatrogenically reduced levels of T could result in better-informed consent from patients receiving intermittent ADT. Finally, being able to give GnRH-A less frequently, while carefully monitoring T levels, is appealing at a time of rising health care costs.
ACKNOWLEDGMENTS
The authors thank the research nurses, nursing staff, and fellows for providing patient care at the NCI and all participating centers; Maya Goldfarb for data management assistance; the NCI Cancer Therapy Evaluation Program (CTEP) for sponsoring the trial; and Bonnie L. Casey for expert editorial assistance. Most importantly, we thank all of the prostate cancer patients who graciously participated in this study, which was supported by the Intramural Research Program of the NIH, NCI, Center for Cancer Research.
Key for definitions of abbreviations
- ADT
Androgen deprivation therapy
- GnRH-A
Gonadotropin-releasing hormone agonist
- PSA
Prostate specific antigen
- DHT
Dihydroxytestosterone
- T
Testosterone
- RT
Radiation therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None declared.
REFERENCES
- 1.Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 2.Michaelson MD, Kaufman DS, Lee H, McGovern FJ, Kantoff PW, Fallon MA, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickles T, Agranovich A, Berthelet E, Duncan GG, Keyes M, Kwan W, et al. Testosterone recovery following prolonged adjuvant androgen ablation for prostate carcinoma. Cancer. 2002;94:362. doi: 10.1002/cncr.10219. [DOI] [PubMed] [Google Scholar]
- 4.Nejat RJ, Rashid HH, Bagiella E, Katz AE, Benson MC. A prospective analysis of time to normalization of serum testosterone after withdrawal of androgen deprivation therapy. J Urol. 2000;164:1891. [PubMed] [Google Scholar]
- 5.Hall MC, Fritzsch RJ, Sagalowsky AI, Ahrens A, Petty B, Roehrborn CG. Prospective determination of the hormonal response after cessation of luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Urology. 1999;53:898. doi: 10.1016/s0090-4295(99)00061-8. [DOI] [PubMed] [Google Scholar]
- 6.Gulley JL, Figg WD, Steinberg SM, Carter J, Sartor O, Higano CS, et al. A prospective analysis of the time to normalization of serum androgens following 6 months of androgen deprivation therapy in patients on a randomized phase III clinical trial using limited hormonal therapy. J Urol. 2005;173:1567. doi: 10.1097/01.ju.0000154780.72631.85. [DOI] [PubMed] [Google Scholar]
- 7.Pickles T, Graham P. What happens to testosterone after prostate radiation monotherapy and does it matter? J Urol. 2002;167:2448. [PubMed] [Google Scholar]
- 8.Zagars GK, Pollack A. Serum testosterone levels after external beam radiation for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 1997;39:85. doi: 10.1016/s0360-3016(97)00311-8. [DOI] [PubMed] [Google Scholar]
- 9.Daniell HW, Clark JC, Pereira SE, Niazi ZA, Ferguson DW, Dunn SR, et al. Hypogonadism following prostate-bed radiation therapy for prostate carcinoma. Cancer. 2001;91:1889. doi: 10.1002/1097-0142(20010515)91:10<1889::aid-cncr1211>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Wagmiller J, Griggs J, Dick A, Sahasrabudhe D. Individualized strategy for dosing luteinizing hormone-releasing hormone agonists for androgen-independent prostate cancer: identification of outcomes and costs. J Oncol Pract. 2006;2:57. doi: 10.1200/jop.2006.2.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oefelein MG. Health related quality of life using serum testosterone as the trigger to re-dose long acting depot luteinizing hormone-releasing hormone agonists in patients with prostate cancer. J Urol. 2003;169:251. doi: 10.1016/S0022-5347(05)64079-7. [DOI] [PubMed] [Google Scholar]
- 12.Oefelein MG. Time to normalization of serum testosterone after 3-month luteinizing hormone-releasing hormone agonist administered in the neoadjuvant setting: implications for dosing schedule and neoadjuvant study consideration. J Urol. 1998;160:1685. [PubMed] [Google Scholar]
- 13.Figg WD, Dahut W, Duray P, Hamilton M, Tompkins A, Steinberg SM, et al. A randomized phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen-independent prostate cancer. Clin Cancer Res. 2001;7:1888. [PubMed] [Google Scholar]
- 14.Litman HJ, Bhasin S, Link CL, Araujo AB, McKinlay JB. Serum androgen levels in black, Hispanic, and white men. J Clin Endocrinol Metab. 2006;91:4326. doi: 10.1210/jc.2006-0037. [DOI] [PubMed] [Google Scholar]
- 15.Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab. 2007;92:2519. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 16.Mohler JL, Gaston KE, Moore DT, Schell MJ, Cohen BL, Weaver C, et al. Racial differences in prostate androgen levels in men with clinically localized prostate cancer. J Urol. 2004;171:2277. doi: 10.1097/01.ju.0000127739.88383.79. [DOI] [PubMed] [Google Scholar]
- 17.Smith MR. Obesity and sex steroids during gonadotropin-releasing hormone agonist treatment for prostate cancer. Clin Cancer Res. 2007;13:241. doi: 10.1158/1078-0432.CCR-06-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calais Da Silva FM, Calais Da Silva F, Bono A, Brausi M, Whelan P, Queimadelos A, et al. Phase III intermittent MAB vs continuous MAB. J Clin Oncol (Meeting Abstracts) 2006;24:4513. [Google Scholar]
- 19.de Leval J, Boca P, Yousef E, Nicolas H, Jeukenne M, Seidel L, et al. Intermittent versus continuous total androgen blockade in the treatment of patients with advanced hormone-naive prostate cancer: results of a prospective randomized multicenter trial. Clin Prostate Cancer. 2002;1:163. doi: 10.3816/cgc.2002.n.018. [DOI] [PubMed] [Google Scholar]
- 20.Miller K, Steiner U, Lingnau A, Keilholz U, Witzsch U, Haider A, et al. Randomised prospective study of intermittent versus continuous androgen suppression in advanced prostate cancer. J Clin Oncol (Meeting Abstracts) 2007;25:5015. [Google Scholar]