Abstract
The valgus, osteoarthritic knee is challenging technically and it is unknown whether and how technical and implant variables influence outcomes. We therefore determined the influence of surgical technique of soft tissue balancing and patient and implant factors from 100 unselected cruciate-retaining TKAs for valgus osteoarthritis in patients younger than 75 years of age. From 1987 to 1990, lateral soft tissue balancing was done with an outside-in progression in which the lateral collateral ligament and popliteus were typically released from the femur. From 1991 to 1994, an inside-out technique was use in which the lateral collateral ligament and/or popliteus were typically preserved. The minimum followup was 0.1 year (mean, 8.2 years; range, 0.1–18.2 years). Fourteen of 16 revisions were for wear and/or instability. Popliteus release, lateral collateral ligament release, or greater polyethylene shelf age increased the risk of revision. At 10 postoperative years, survival (end point, revision) was 89% (100 knees), 94% when the shelf age was less than 1 year (n = 73 knees), 97% when the popliteus or lateral collateral ligament was not released (n = 57 knees), and 100% when both conditions were met (n = 39 knees). Cruciate-retaining implants can be successfully used in knees with any degree of valgus osteoarthritis and survival is improved when the surgeon preserves at least one of the structures providing lateral stability in flexion and uses polyethylene with a short shelf life.
Level of Evidence: Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Performing TKA in a knee having valgus osteoarthritis presents many challenges for the treating orthopaedic surgeon. The presence of the typical dysplastic lateral femoral condyle with its inherent excessive distal femoral valgus requires greater medial than lateral resection to restore mechanical alignment, and the diminished anteroposterior dimension can make establishment of optimum femoral component rotation difficult. In addition, typically contracted lateral soft tissues and potentially elongated medial structures make soft tissue balancing difficult.
PCL-substituting implants have traditionally been advocated to eliminate concerns with a potentially abnormal ligament or modifying the joint line, particularly in the presence of severe deformity. No studies, however, prospectively compare clinical outcomes with posterior cruciate ligament (PCL)-retaining and PCL-substituting designs in valgus osteoarthritic knees, so it is not known whether the PCL-substituting implant achieves its goals.
Lateral soft tissue releases required to balance a valgus knee can be performed from the lateral epicondyle (lateral collateral ligament [LCL] and popliteus) followed by release of the deeper capsular structures. Concerns that this could result in lateral instability in flexion resulted in the development of an alternative technique of selective release of the tight lateral structures in extension with the goal of preserving one or more anatomic structures and maintaining lateral stability in flexion [4]. However, there is no consensus as to the relative indications for (or optimum sequence or technique of) the many techniques described for soft tissue balancing in the valgus knee nor is there consensus on the degree of implant constraint that should be used in these cases.
We sought to determine the effect on implant survival and clinical outcome of (1) surgical technique (including preservation of the PCL, LCL, and/or popliteus); (2) implant characteristics (including shelf age of polyethylene, sagittal geometry of the polyethylene, and tibial baseplate material); and (3) patient characteristics (including age, weight, gender, and limb alignment).
Materials and Methods
We retrospectively reviewed prospectively collected data from 86 patients (100 knees) who were younger than 75 years of age and underwent primary, PCL-retaining TKA by the senior author (GAE) for valgus osteoarthritis from 1987 to 1994. Although the patients were selected from others not meeting the study criteria, we included all patients meeting the criteria. For this study, we defined valgus osteoarthritis as a limb aligned in greater than 8° of tibiofemoral valgus in stance and had radiographic narrowing of the lateral compartment and no history of proximal tibial or distal femoral osteotomy. During the study period, the same surgeon used a PCL-substituting variant of the same design in five knees with valgus osteoarthritis. In each case, an intraoperative decision was made to change to a substituting design because the flexion space was considered excessively tight despite PCL recession. The patients consisted of 73 women (14 bilateral knees) and 13 men with a mean age at surgery of 67 years (range, 44–74 years) (Table 1). The minimum followup was 0.1 year (mean, 8.2; range, 0.1–18.2 years). Ninety-two of 100 knees had at least 2 years of clinical followup, two of eight with less than 2 years of followup were revised before 2 years, one of eight was confirmed to be in vivo at 14 years by family interview, one of eight was confirmed to be in vivo at 5 years by examination at another clinic, and the status of four of eight knees was unknown beyond 2 years.
Table 1.
Summary of study data
| Variable | Value |
|---|---|
| Survival time (years)* | 10 ± 4.4 (0.1–18.2) |
| Radiographic followup (years)* | 8.2 ± 4.5 (0.1–18.2) |
| Patient age at arthroplasty (years)* | 67 ± 6 (44–74) |
| Patient weight (kg)* | 80 ± 15 (53–128) |
| Patient gender | |
| Female | 87 |
| Male | 13 |
| Extension end point of preoperative range of motion (degrees of flexion)* | 8 ± 8 (−3–36) |
| Flexion end point of preoperative range of motion (degrees of flexion)* | 116 ± 13 (85–142) |
| Preoperative tibiofemoral valgus (degrees)* | 16.0 ± 5.0 (6–29.0) |
| Postoperative tibiofemoral valgus (degrees)* | 6.3 ± 2.9 (1–14.0) |
| Tibial component design | |
| Titanium baseplate | 86 |
| Cobalt-chrome baseplate | 11 |
| All polyethylene | 3 |
| Polyethylene articular surface sagittal geometry | |
| Flat | 92 knees |
| Curved | 8 knees |
| Polyethylene shelf age (years)* | 0.9 ± 1.1 (0–6) |
* Values expressed as mean ± standard deviation, with range in parentheses.
All TKAs but one were performed through a medial parapatellar arthrotomy. All implants were of the Anatomic Modular Knee design (DePuy Orthopaedics, Warsaw, IN) with 95 knees having patellar resurfacing. The components were fixed with cement in 42 knees, hybrid fixation was used in 53, and uncemented components were used in five. A titanium tibial baseplate was used in 86 knees and a cobalt-chrome alloy one was used in 11 knees; the remaining three knees were implanted with an all-polyethylene component. The tibial articular sagittal geometry was flat in 92 and curved in eight [10] with 97 of the 100 bearings gamma-irradiation-sterilized in air. The shelf life of the tibial polyethylene averaged 0.9 year (range, 0–6 years). The PCL was completely untouched in 77 knees. In 14 knees, the anterior fibers only were released, a tibial bone block was used in seven, and the ligament was believed to have avulsed from the tibial insertion in two cases. Two knees had a bone staple placed to tighten a lax medial collateral ligament; no releases of the superficial medial collateral ligament were performed.
The surgeon used an “outside-in” progression of lateral soft tissue releases (LCL and popliteus from the lateral epicondyle followed by deeper structures) in 46 knees from 1987 to 1990 and an “inside-out” [4] technique (selective release in extension after bony resections) in 32 knees thereafter (1991 to 1994). Because of potential concerns of controlling the depth of scalpel penetration and to improve visualization of the release, cutting cautery was used to transversely release contracted structures at the level of the meniscal bed. In both groups, the iliotibial band was commonly released (outside-in group: 40 of 46 released; inside-out group: 29 of 32 released), most commonly through transverse release at the joint line or pie-crusting. No LCL, popliteus, or iliotibial band releases were performed in the remaining 22 knees. With the traditional outside-in approach, the popliteus was released in 45 of 46 cases and the LCL was released in 41 knees. In contrast, using the inside-out approach, 14 of 32 knees had a LCL release and 18 of 32 had a popliteus release, but only five of the 32 knees underwent release of both structures. A lateral retinacular release was performed in 33 knees, more commonly in the outside-in technique group (20 of 46 versus eight of 32).
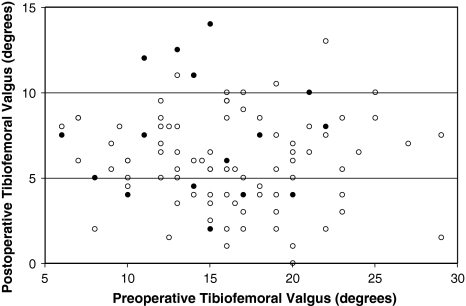
Preoperative and postoperative tibiofemoral valgus was measured from 35- × 43-cm weightbearing anteroposterior radiographs (Fig. 1). Radiographic analysis revealed similar preoperative severity of deformity and degree of correction on the postoperative films in the two groups (Fig. 1).
Fig. 1.
A scatterplot shows the distribution of preoperative and postoperative measurements of valgus deformity with the shaded points representing cases subsequently revised. Neither preoperative nor early postoperative tibiofemoral valgus was associated with revision.
Various surgeons and fellows documented clinical outcome with clinical examination for stability and Knee Society scoring no sooner than 6 months after surgery and at any subsequent followup. All operative reports were reviewed to document the anatomic structures released during soft tissue balancing as well as the sequence of the releases.
Using Cox proportional hazards analysis, we determined which if any of the following 11 factors might compromise arthroplasty survival: patient age at surgery, patient weight at surgery, patient gender, preoperative tibiofemoral valgus, postoperative tibiofemoral valgus, use of a titanium tibial baseplate (yes versus no), shelf age of the polyethylene component, sagittal geometry of the polyethylene component (flat versus curved), release of both the LCL and popliteus tendon (yes versus no), release or pie-crusting of the iliotibial band (yes versus no), and full or partial PCL release (yes versus no). Implant survival time (the years from primary arthroplasty to the date on which the arthroplasty was last known to be in vivo) was the time variable of the analysis. Whether the arthroplasty had been revised as a result of ligamentous instability, polyethylene wear, or osteolysis (no or yes) was the status variable of the analysis. The 11 variables were entered as risk factors and sequentially eliminated until only those that were associated (p < 0.05) with the event remained (Wald backward method of Cox regression). We used SPSS® for Windows® (Version 11.0; SPSS Inc, Chicago, IL) for these analyses. Kaplan-Meier analysis was performed to estimate arthroplasty survival free of revision at fixed intervals including 5, 7.5, and 10 postoperative years.
To assess whether clinical outcome measures could have been influenced by release of the LCL and the popliteus, the 76 knees with at least 5 years of clinical followup were divided into two groups. The first (35 knees) had release of both structures. In the second group (41 knees), the lateral collateral, popliteus, or both structures were preserved. We compared the Knee Society scores of the two groups using Mann-Whitney U-test, Knee Society function scores using a one-way analysis of variance with Tukey’s post hoc test, and Knee Society medial lateral laxity scores using Somer’s d test.
Results
With an end point of any revision (16 such events), Kaplan-Meier survival was 88.7% at 10 postoperative years for all 100 knees (Table 2). Sixteen knees were revised during the study period, 12 for tibial polyethylene wear and/or osteolysis, two for primary ligamentous instability, one for early loosening of a cementless femoral component, and one for late deep infection (yeast).
Table 2.
Results of Kaplan-Meier survival analysis performed with an end point of any revision and with the survival time of each arthroplasty as the time variable
| Postoperative survival interval | All cases (%) | Tibial polyethylene shelf age less than 1 year (%) | Popliteus or lateral collateral ligament not released (%) | Tibial polyethylene shelf age less than 1 year and popliteus or lateral collateral ligament not released (%) |
|---|---|---|---|---|
| Number of knees/revisions | 100/16 | 73/9 | 54/3 | 39/2 |
| 5 years | 95.8 ± 2.1 | 100 | 100 | 100 |
| 7.5 years | 94.6 ± 2.4 | 100 | 100 | 100 |
| 10 years | 88.7 ± 3.6 | 93.7 ± 3.5 | 97.1 ± 2.9 | 100 |
The survival data are presented as the cumulative survival percentage ± standard error.
Surgical technique influenced both implant survival and clinical outcome because both better survival and higher clinical outcome scores were associated with preservation of the LCL and preservation of the popliteus tendon with the best survival when both structures were preserved. Thirteen of 46 knees that had release of both the popliteus and LCL were revised (12 for wear, osteolysis, or instability). Only three of 54 knees that had preservation of either or both structures were revised (two for wear, osteolysis, or instability). Release of both the PCL and popliteus was one of the two factors that made revision resulting from wear, osteolysis, or instability more likely (Table 3). Release of both the LCL and popliteus increased the likelihood of revision by 19.9 times (95% confidence interval, 2.4–162.5 times; p < 0.01). The most recent Knee Society score examinations on the 76 knees with 5 or more years of followup suggested the structures released also influenced clinical outcome. Knee Society knee scores were higher (p < 0.01) in the 41 knees in which the LCL or popliteus was preserved (89 ± 13) than in the 35 knees in which both were released (76 ± 24) (Table 4). Knees that had releases of both the LCL and popliteus had lower Knee Society mediolateral laxity scores (more laxity) at their most recent examination than those that had release of neither or one of the two structures (p = 0.03; Somer’s d value = 0.23) (Table 4). The remaining surgical factors (PCL recession or release, iliotibial band release, postoperative tibiofemoral valgus) did not influence the risk of revision resulting from wear, osteolysis, or instability.
Table 3.
Results of regression analysis testing for associations between 11 factors and revision events
| Factor | Linked with revision event? |
|---|---|
| Tibial polyethylene shelf age | Yes (p < 0.01) |
| Release of lateral collateral ligament and popliteus | Yes (p < 0.01) |
| Patient age | No |
| Patient weight | No |
| Patient gender | No |
| Preoperative tibiofemoral valgus angle | No |
| Postoperative tibiofemoral valgus angle | No |
| Use of titanium baseplate | No |
| Use of sagittally flat insert | No |
| Release/pie-crusting of iliotibial band | No |
| Recession/release of posterior cruciate ligament | No |
Cox proportional hazards regression analysis was used to identify if the 11 listed factors were associated (p < 0.05) with an event of revision as a result of tibial polyethylene wear, osteolysis, or instability (the time variable of the analysis for each of the 100 knees was the number of years that had elapsed from the date of surgery to the date that the implants were last known to be in situ).
Table 4.
Most recent followup examination of 76 knees examined at 5 or more postoperative years
| Group | Patient age (years)* | Length of followup (years)* | Mediolateral laxity at 30° of flexion per knee society criteria | Anteroposterior drawer at 90° of flexion per knee society criteria | Knee society knee score (points)* | Knee society function score (points)* |
|---|---|---|---|---|---|---|
| Popliteus and lateral collateral ligament released (35 knees) | 75 ± 7 | 11 ± 4 | < 6°: 18 | < 5 mm: 28 | 76 ± 24 | 58 ± 28 |
| 6°–9°: 12 | 5–10 mm: 6 | |||||
| 10°–14°: 4 | > 10 mm: 1 | |||||
| > 14°: 1 | ||||||
| Either popliteus or lateral collateral ligament or both preserved (41 knees) | 78 ± 4 | 10 ± 3 | < 6°: 30 | < 5 mm: 35 | 89 ± 13 | 62 ± 29 |
| 6°–9°: 9 | 5–10 mm: 5 | |||||
| 10°–14°: 2 | > 10 mm: 1 | |||||
| > 14°: 0 |
* Values are expressed as mean ± standard deviation.
One implant characteristic influenced outcome. With any 1-year increase in shelf age, the risk of revision increased by 3.1 times (95% confidence interval, 1.7–5.9 times; p < 0.01). Other implant factors (titanium baseplate, sagittal articular insert curvature) did not influence risk of revision. In the 73 knees with polyethylene shelf age less than 1 year, survivorship was 93.7% compared to the overall survival of 88.7%. This increased further to 97% in the 54 knees in which the LCL or popliteus was preserved. If one of these two structures was retained and the shelf life of the polyethylene was less than 1 year (39 knees), survivorship was 100%.
Age, weight, gender, preoperative alignment did not influence risk of revision.
There were no peroneal nerve complications.
Discussion
The valgus, osteoarthritic knee presents a major reconstructive challenge both in the technical aspects of the surgery itself and in the reduction in survivorship resulting from ligament imbalance in these knees [2]. The complexities of addressing the deformity, osseous deficiencies, and soft tissue imbalances have been well documented with many differing surgical techniques described [1, 3–9, 11]. There is no consensus as to the relative indications for (or optimum sequence or technique of) the many techniques described for soft tissue balancing in the valgus knee. There is also no consensus on the degree of implant constraint that should be used in these cases. Many authors have advocated the use of PCL-substituting implant designs, avoiding concerns with PCL balancing and dealing with a potentially abnormal native ligament [1, 3, 4, 7, 9]. In fact, some authors have advocated the use of primary constrained components both with [3] and without [1] stem extensions. Conversely, others have published results that support the use of PCL-retaining designs with various soft tissue balancing techniques [5–8].
We note several limitations to this study. First, the numbers are likely inadequate to detect all potentially important patient or implant variables. Second, the predominance of female patients of relatively low body weight (typical of valgus osteoarthritis) makes valid comparisons of implant, technique, or implant factors to existing literature (with marked predominance of varus osteoarthritis) subjective at best. The cases represent two sequential cohorts of patients not randomized to one technique or the other for soft tissue balancing. Lastly, the two techniques can result in the same anatomic end point with any potential benefit of the “inside-out” technique lost if both popliteus and the LCL require release for adequate soft tissue balance.
We used only two basic techniques in this series. The first, the outside-in approach (used from 1987 to 1990), involved initial release of structures from the lateral epicondyle (LCL, popliteus) followed by the deeper static stabilizing structures. This, by definition, has the potential to result in lateral instability in flexion. To address this concern, we used an alternative approach (from 1991 to 1994) developed by Ranawat, the results of which were subsequently published by Elkus et al. [4]. This “inside-out” approach allowed the potential for preserving lateral stability in flexion but was described in combination with a PCL-substituting implant and without a comparison group. We sought to answer if adopting this surgical technique using a PCL-retaining design did in fact result in preservation of lateral stabilizing structures.
The data suggest preservation of structures providing lateral flexion stability was possible and much more frequent with the inside-out technique adopted in 1991. More importantly, preservation of lateral stabilizing structures improved survivorship and clinical outcome. Conversely, division of these structures increased the risk of revision and was associated with lower Knee Society scores and clinical stability.
The only implant factor influencing survivorship was polyethylene shelf life. This is surprising because it increased the risk of revision despite the fact that 73% of the inserts had a shelf life of less than 1 year and 87% less than 2 years.
The senior surgeon used a single PCL-retaining design in all but five valgus knees during the study period with the decision to change to the PCL-substituting version of the same implant made based on a tight flexion space, not varus/valgus concerns. This is supported by our findings that in the 100 knees preoperative valgus deformity did not influence outcome (Table 1).
Our 88.7% 10-year implant survival rate approaches the 85% result reported by Elkus et al. [4] at 15 years and comparable to others with shorter followup and unspecified survivorship. To our knowledge, Knee Society scores have been infrequently reported specifically in TKA for valgus arthritis [3, 6] with comparable overall results but no relationship to technique or patient or implant variables evaluated.
We conclude cruciate-retaining implants can be used in a wide range of patients with valgus osteoarthritis and that survival is improved when the LCL and/or the popliteus tendon are preserved and when polyethylene with a short shelf age is used.
Footnotes
The institution of one or more of the authors (MBC, WGH, GAE) has received funding from the Inova Health System. One author (GAE) received royalties from and one author (JPM) is a consultant for DePuy Orthopaedics (Warsaw, IN).
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Anderson JA, Baldini A, MacDonald JH, Pellici PM, Sculco TP. Primary constrained condylar knee arthroplasty without stem extensions for the valgus knee. Clin Orthop Relat Res. 2006;442:199–203. [DOI] [PubMed]
- 2.Berend ME, Ritter MA, Medling JB, Faris PM, Keating EM, Redelman R, Faris GW, Davis KE. Tibial component failure mechanisms in total knee arthroplasty. Clin Orthop Relat Res. 2004;428:26–34. [DOI] [PubMed]
- 3.Easely ME, Insall JN, Scuderi GR, Bullek DD. Primary constrained condylar knee arthroplasty for the arthritic valgus knee. Clin Orthop Relat Res. 2000;380:58–64. [DOI] [PubMed]
- 4.Elkus M, Ranawat CS, Rasquinha VJ, Babhulkar S, Rossi R, Ranawat A. Total knee arthroplasty for severe valgus deformity. J Bone Joint Surg Am. 2004;86:2671–2676. [DOI] [PubMed]
- 5.Engh GA. The difficult knee severe varus and valgus. Clin Orthop Relat Res. 2003;416:588–563. [DOI] [PubMed]
- 6.Krackow KA, Jones MM, Teeny SM, Hungerford DS. Primary total knee arthroplasty in patients with fixed valgus deformity. Clin Orthop Relat Res. 1991;273:9–18. [PubMed]
- 7.Lombardi AV Jr, Dodds KL, Berend KR, Mallory TH, Adams JB. An algorithmic approach to total knee arthroplasty in the valgus knee. J Bone Joint Surg Am. 2004;86(Suppl 2):62–71. [DOI] [PubMed]
- 8.Politi J, Scott R. Balancing severe valgus deformity in total knee arthroplasty using a lateral cruciform retinacular release. J Arthroplasty. 2004;19:553–557. [DOI] [PubMed]
- 9.Stern SH, Moeckel BH, Insall JN. Total knee arthroplasty in valgus knees. Clin Orthop Relat Res. 1991;273:5–8. [PubMed]
- 10.Uvehammer J, Regner L, Karrholm J. Flat vs concave tibial joint surface in total knee arthroplasty: randomized evaluation of 39 cases using radiostereometry. Acta Orthop Scand. 2001;72:257–265. [DOI] [PubMed]
- 11.Whiteside LA. Soft tissue balancing the knee. J Arthroplasty. 2002;17:23–27. [DOI] [PubMed]