Abstract
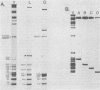
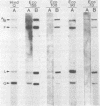
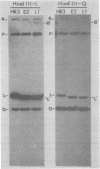
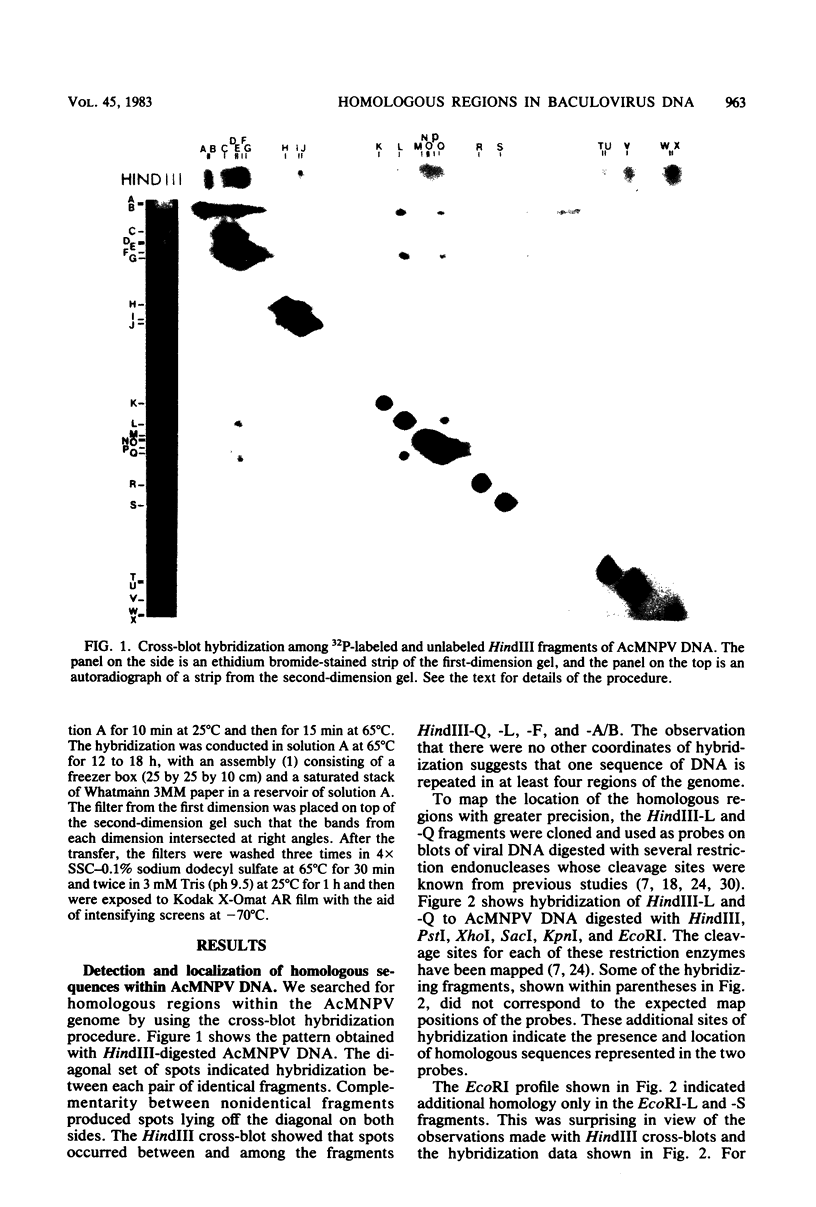
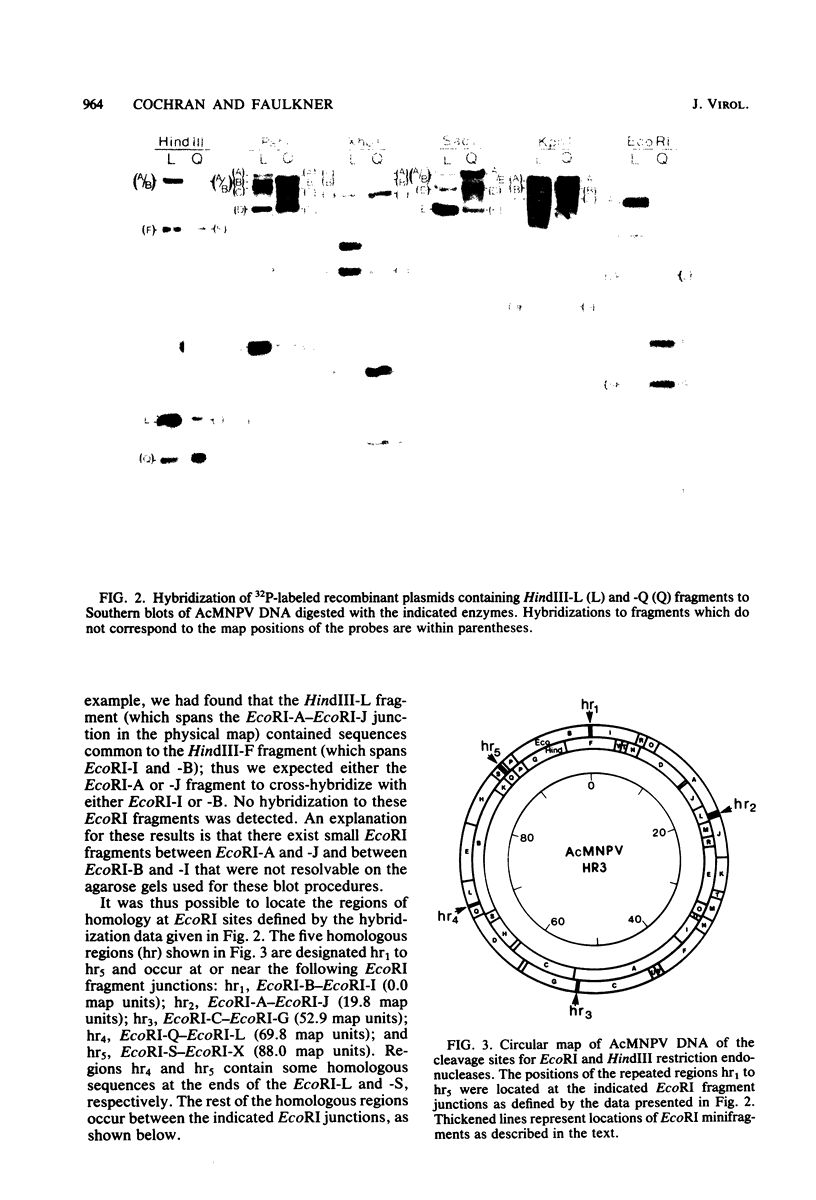
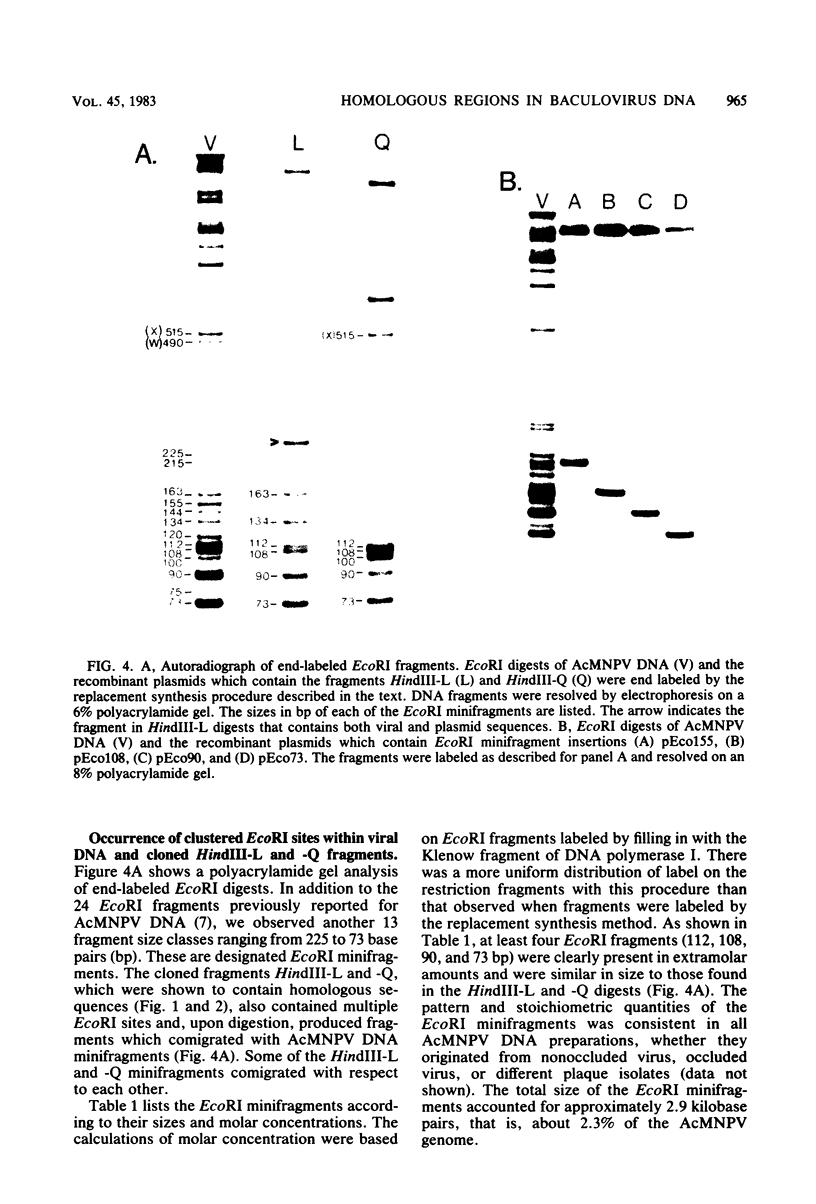
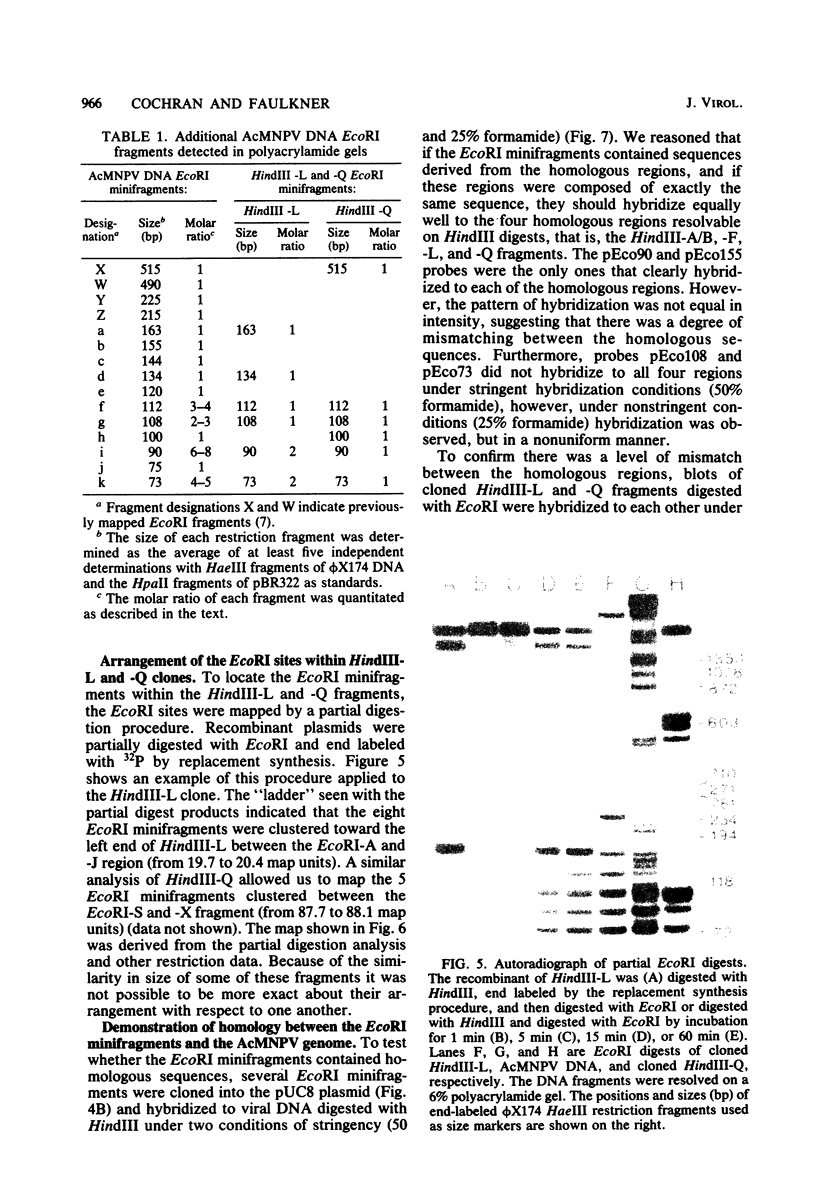
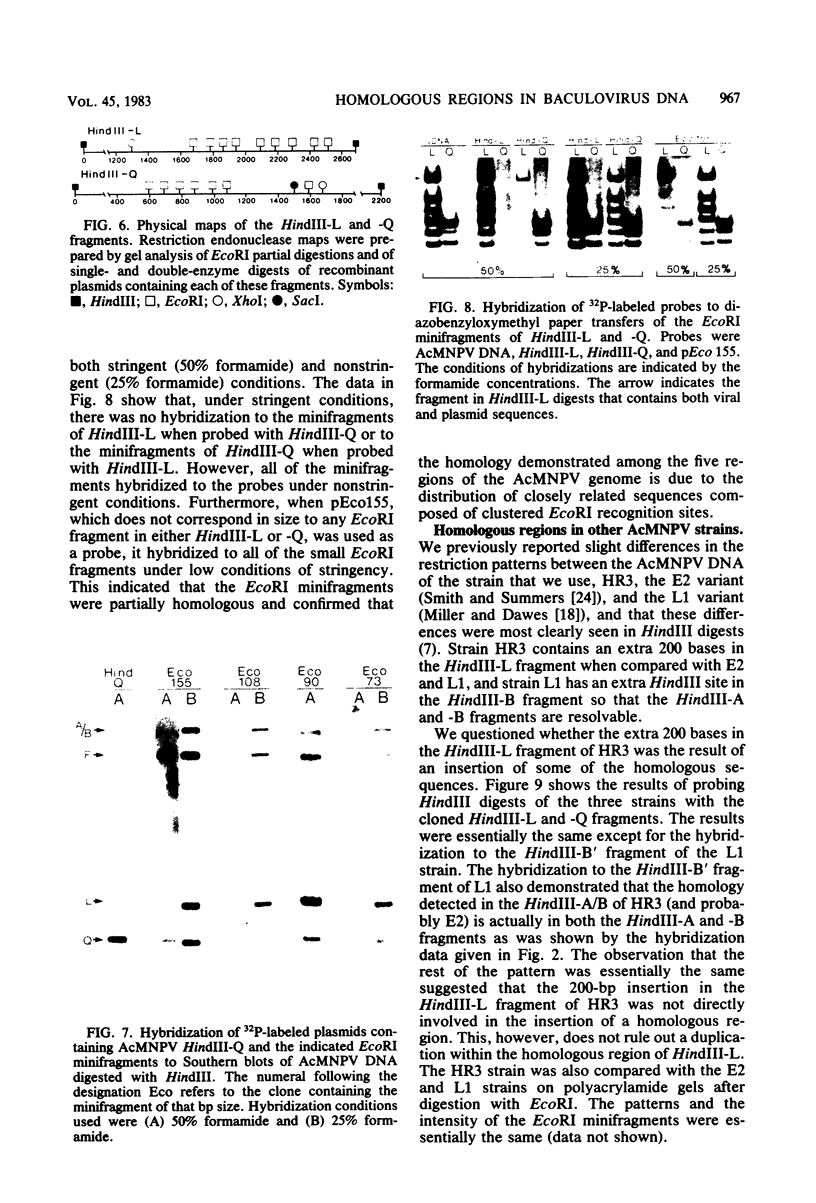
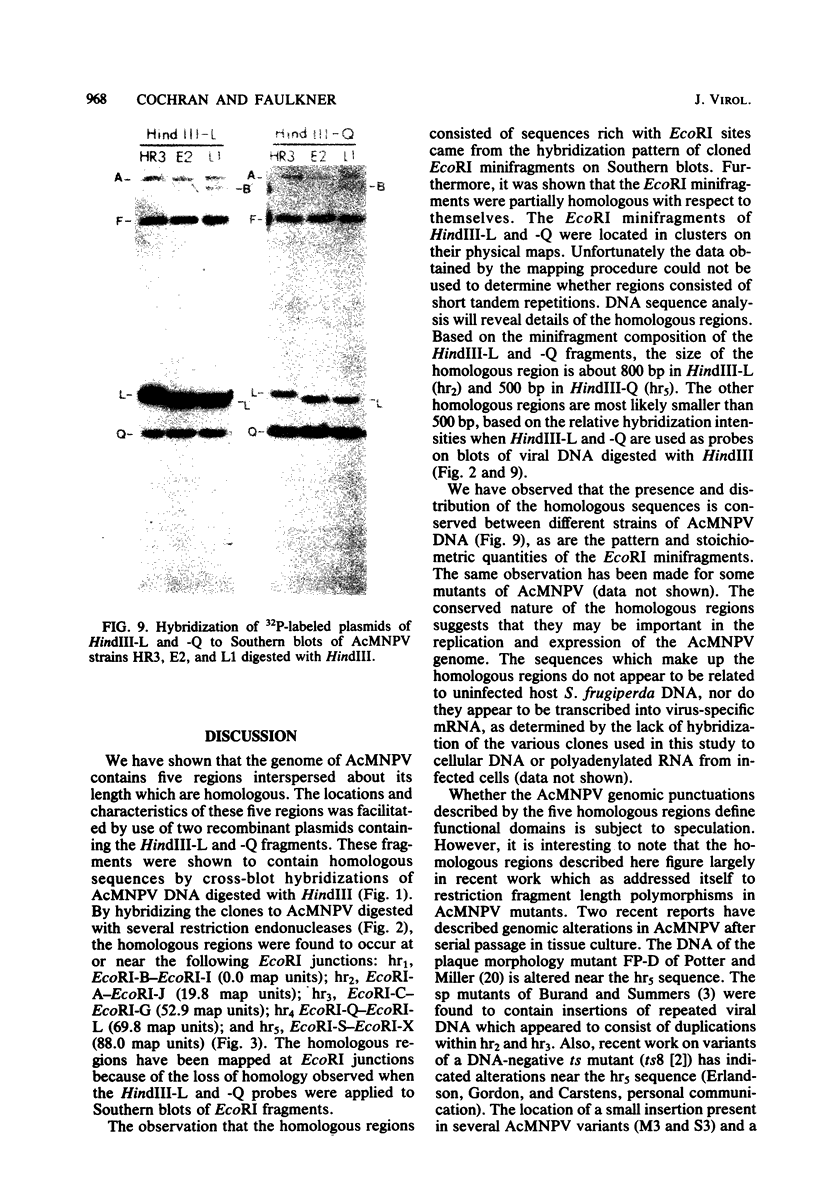
An examination of Autographa californica nuclear polyhedrosis virus DNA revealed the presence of five interspersed regions, rich in EcoRI restriction sites, which shared homologous sequences. These homologous regions (hr), designated hr1 to hr5, occur at or near the following EcoRI fragment junctions: hr1EcoRI-B—EcoRI-I (0.0 map units); hr2, EcoRI-A—EcoRI-J (19.8 map units); hr3, EcoRI-C—EcoRI-G (52.9 map units); hr4, EcoRI-Q—EcoRI-L (69.8 map units); and hr5, EcoRI-S—EcoRI-X (88.0 map units). Four of these regions were identified, by cross-blot hybridization of HindIII-restricted A. californica nuclear polyhedrosis virus DNA, to be within the HindIII-A/B, -F, -L, and -Q fragments. The location of these regions and the identification of a fifth homologous region were confirmed, and their characterization was facilitated, by using two plasmids with HindIII-L or -Q fragment insertions, which contained the homologous regions hr2 and hr5, respectively. The sizes of the homologous regions were about 800 base pairs for hr2, 500 base pairs for hr5, and less than 500 base pairs for hr1, hr3, and hr4. A set of small EcoRI fragments (EcoRI minifragments) which ranged in size from 225 to 73 base pairs were detected in A. californica nuclear polyhedrosis virus DNA and HindIII-L and -Q fragments by polyacrylamide gel analysis. Some of the minifragments in viral DNA were present in extramolar amounts and corresponded in size to some of the minifragments present in HindIII-L and -Q. Clones of some of the EcoRI minifragments were used as probes in hybridizations to digests of viral DNA and of HindIII-L and -Q. The hybridization data, obtained under various levels of stringency, suggested that there was a degree of mismatching between the sequences which were responsible for the homology.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M., Crawford A. M., Faulkner P. Genetic Analysis of a Baculovirus, Autographa californica Nuclear Polyhedrosis Virus I. Isolation of Temperature-Sensitive Mutants and Assortment into Complementation Groups. J Virol. 1979 Jul;31(1):190–198. doi: 10.1128/jvi.31.1.190-198.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabretta B., Robberson D. L., Barrera-Saldaña H. A., Lambrou T. P., Saunders G. F. Genome instability in a region of human DNA enriched in Alu repeat sequences. Nature. 1982 Mar 18;296(5854):219–225. doi: 10.1038/296219a0. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran M. A., Carstens E. B., Eaton B. T., Faulkner P. Molecular Cloning and Physical Mapping of Restriction Endonuclease Fragments of Autographa californica Nuclear Polyhedrosis Virus DNA. J Virol. 1982 Mar;41(3):940–946. doi: 10.1128/jvi.41.3.940-946.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P. Virus-specific protein synthesis in cells infected by infectious pancreatic necrosis virus. J Virol. 1977 Jan;21(1):242–258. doi: 10.1128/jvi.21.1.242-258.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. A unique form of terminal redundancy in adenovirus DNA molecules. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2391–2395. doi: 10.1073/pnas.69.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrap K. A., Payne C. C. The structural properties and identification of insect viruses. Adv Virus Res. 1979;25:273–355. doi: 10.1016/s0065-3527(08)60572-2. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. C. The DNA contained by nuclear polyhedrosis viruses isolated from four Spodoptera sp. (Lepidoptera, Noctuidae): genome size and homology assessed by DNA reassociation kinetics. Virology. 1977 Jan;76(1):468–471. doi: 10.1016/0042-6822(77)90325-7. [DOI] [PubMed] [Google Scholar]
- Lübbert H., Kruczek I., Tjia S., Doerfler W. The cloned EcoRI fragments of Autographa californica nuclear polyhedrosis virus DNA. Gene. 1981 Dec;16(1-3):343–345. doi: 10.1016/0378-1119(81)90092-5. [DOI] [PubMed] [Google Scholar]
- Miller L. K., Dawes K. P. Physical Map of the DNA Genome of Autographa californica Nuclear Polyhedrosis Virus. J Virol. 1979 Mar;29(3):1044–1055. doi: 10.1128/jvi.29.3.1044-1055.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B. The structure and isomerization of herpes simplex virus genomes. Cell. 1979 Mar;16(3):481–494. doi: 10.1016/0092-8674(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Sato S., Hutchinson C. A., 3rd, Harris J. I. A thermostable sequence-specific endonuclease from Thermus aquaticus. Proc Natl Acad Sci U S A. 1977 Feb;74(2):542–546. doi: 10.1073/pnas.74.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. Restriction Maps of Five Autographa californica MNPV Variants, Trichoplusia ni MNPV, and Galleria mellonella MNPV DNAs with Endonucleases SmaI, KpnI, BamHI, SacI, XhoI, and EcoRI. J Virol. 1979 Jun;30(3):828–838. doi: 10.1128/jvi.30.3.828-838.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stellwag E. J., Dahlberg A. E. Electrophoretic transfer of DNA, RNA and protein onto diazobenzyloxymethyl (DBM) - paper. Nucleic Acids Res. 1980 Jan 25;8(2):299–317. doi: 10.1093/nar/8.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. L., Goodwin R. H., Tompkins G. J., McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro. 1977 Apr;13(4):213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- Vlak J. M., Smith G. E. Orientation of the Genome of Autographa californica Nuclear Polyhedrosis Virus: a Proposal. J Virol. 1982 Mar;41(3):1118–1121. doi: 10.1128/jvi.41.3.1118-1121.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink P. C., Tabata S., Pachl C. The clustered and scrambled arrangement of moderately repetitive elements in Drosophila DNA. Cell. 1979 Dec;18(4):1231–1246. doi: 10.1016/0092-8674(79)90235-6. [DOI] [PubMed] [Google Scholar]
- Wittek R., Moss B. Tandem repeats within the inverted terminal repetition of vaccinia virus DNA. Cell. 1980 Aug;21(1):277–284. doi: 10.1016/0092-8674(80)90135-x. [DOI] [PubMed] [Google Scholar]