Abstract
Nasal colonization with Staphylococcus aureus (SA) increases the risk of surgical site infection (SSI). We first (1) determined the prevalence of asymptomatic nasal colonization with SA, (2) assessed trends in methicillin resistance with time, (3) ascertained risk factors for nasal colonization; and (4) correlated SSI to nasal colonization status and procedure. We performed a cross-sectional analysis of SA nasal colonization among healthy preoperative orthopaedic outpatients between 2003–2005 who were within 2 weeks of surgery. Of 284 patients, 86 (30%) carried SA; of these, 81 (94%) were colonized with methicillin-sensitive and five (6%) with methicillin-resistant SA (MRSA). Total SA colonization increased from 25/78 (32%) in 2003 to 37/97 (38%) in 2005, and colonization with MRSA increased from 0/78 (0%) to four of 97 (4%), respectively. We found no associations between nasal carriage and demographics or procedures. Surgical site infection occurred in nine of 282 (3%), four of which were attributable to SA; these included 0/43 (0%) carriers who received decolonization with 2% mupirocin, two of 43 (4.7%) who declined decolonization, and two of 196 (1.0%) who were noncarriers. Nasal colonization with SA, including MRSA, among preoperative orthopaedic outpatients is increasing and their rates reflect community rates. Knowledge of colonization status may be important in decolonization, choosing perioperative or any subsequent empiric antibiotics.
Introduction
Staphylococcus aureus is among the most common causes of SSI in orthopaedic patients [1]. The association between SA nasal colonization and infection was first reported in 1931 [4]. Since then, it has been well established that development of SSI involving SA is associated with preoperative nasal colonization with the organism [4, 23, 24]. Nasal colonization with SA was the most powerful independent risk factor for SSI after cardiothoracic surgery [14], and in another study of patients undergoing orthopaedic surgery with prosthetic implants, nasal colonization with SA was the most important independent risk factor for development of a SSI. Carriers of SA were nine times more likely to have an SSI develop versus noncarriers (95% confidence interval, 1.7–45.5) [12]. The Centers for Disease Control and Prevention (CDC) describes preoperative nasal colonization with SA as a risk factor for SSI [17].
Methicillin-resistant Staphylococcus aureus (MRSA) in the community is believed to add to the burden of SA colonization, and its prevalence is increasing [13]. Furthermore, Ellis et al. reported a 3.1-fold risk (95% confidence interval, 1.5–6.5) for acquiring MRSA infection in MRSA carriers compared with noncarriers [7]. It is unknown how these data can be applied to a preoperative orthopaedic surgery population and whether MRSA colonization status should play a role in perioperative antibiotic selection and empiric treatment regimens. Staphylococcus aureus is the most important cause of orthopaedic infection at our institution, and we have noted an increasing incidence of MRSA SSIs in patients undergoing orthopaedic surgery. Despite the known association between preoperative colonization with SA and SSI, little is known about the epidemiology of the nasal carriage in an orthopaedic surgery population.
Our primary objective was to (1) determine the prevalence of asymptomatic nasal colonization with SA, including MRSA, among healthy preoperative orthopaedic surgery outpatients at an urban hospital. As secondary objectives, we sought to (2) assess trends in methicillin resistance with time; (3) ascertain risk factors for nasal colonization with SA by demographic data and elective procedure; and (4) correlate the development of SSI to nasal colonization status and procedure.
Materials and Methods
We performed a cross-sectional analysis of SA nasal colonization in preoperative orthopaedic outpatients. Between September 19, 2003, and September 26, 2005, we obtained nasal swabs from the bilateral anterior nares of 284 orthopaedic outpatients scheduled for elective surgery within 2 weeks of their surgery date. Only patients who were unwilling to undergo nasal swabbing were excluded.
Staphylococcus aureus was cultured and resistance to methicillin was characterized using standard methods. The nasal swab was plated on mannitol salt agar (BD, Franklin Lakes, NJ) and incubated overnight at 35°C to 37°C in a non-CO2 incubator. After overnight incubation, plates were interpreted. Any yellow colonies were subcultured on a blood agar plate. Gram-positive cocci in clusters from the blood agar plate were subjected to catalase and coagulase tests after 24 hours incubation. Isolates that were catalase and coagulase-positive were inoculated onto selective agar containing 6 μg/mL of oxacillin. Isolates that showed no growth after 24 hours on the oxacillin plate were reported as methicillin-sensitive SA (MSSA) and isolates that showed growth after 24 hours were reported as MRSA [3, 18]. We did not assess for mupirocin susceptibilities.
There were 118 (41.5%) males and 166 (58.5%) females screened, with a mean age of 50.7 years (range, 18–85 years). Based on ethnicity, there were 104 (36.6%) whites, 54 (19.0%) blacks, 117 (41.2%) Hispanics, and nine (3.2%) other. By surgical procedure, there were 94 (33.1%) arthroplasties, 63 (22.2%) arthroscopies, 57 (20.1%) podiatric procedures, 12 (4.2%) fracture fixations, two (0.7%) hand surgeries, 23 (8.1%) hardware removals, and 33 (11.6%) other surgical procedures.
The prevalence of SA nasal colonization was defined as the number of patients who had SA grow from the culture of their preoperative nasal swab divided by the total number of patients screened. In addition, we divided the study period by calendar year and calculated period-specific prevalence to assess changes in methicillin resistance with time. We used a two-tailed Fisher’s exact test to determine statistical significance (p < 0.05).
To assess risk factors for nasal colonization with SA, we reviewed medical records for demographic and surgical procedure data. Demographic variables collected included age, gender, and ethnicity. The surgical procedures were categorized as arthroplasty, arthroscopy, podiatry procedures, fracture fixation procedures, hand surgery, hardware removal, and other surgical procedures. The other surgical procedures group was comprised of patients having biopsies, bursectomies, and soft tissue procedures. We did not assess for prior antibiotic use. We conducted univariate and comparative statistical analyses to determine whether any of these variables could be risk factors for nasal colonization with SA using independent samples t-tests, Fisher’s exact tests, and chi square analyses. We performed binary logistic regression analyses to estimate odds ratios and corresponding 95% confidence intervals for SA carriage for each potential risk factor. We performed statistical analyses using SPSS software (SPSS 11.5, Chicago, IL). Statistical significance was set at p < 0.05.
Patients in whom SA was detected were offered nasal decolonization with no less than six doses of 2% mupirocin calcium ointment before their surgery. No other decolonization methods were used. Prophylactic antibiotic regimens included cefazolin (or if cephalosporin allergic, clindamycin) infused within 60 minutes before incision and continued up to 24 hours after wound closure. Cutaneous antisepsis was achieved using chlorhexidine. Patients were followed for 30 days after their operative procedure to assess development of infection. All postoperative SSIs, including superficial, deep incisional, and organ/space infections, were noted and classified according to CDC definitions [10]. Two of the 284 patients could not be located for followup.
Results
Among 284 healthy preoperative orthopaedic surgery outpatients presenting within 2 weeks of their surgical procedure, 86 patients (30.3%) asymptomatically carried SA in their anterior nares. Of the 86 carriers, 81 (94%) were colonized with MSSA and five (6%) were colonized with MRSA. Thus, of the 284 patients screened during the 2-year period, MRSA was colonized in 1.8% of patients.
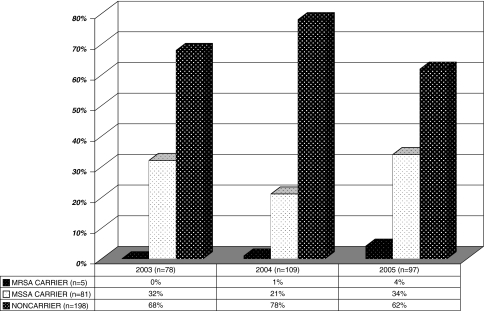
We observed an increase (p = 0.04) in prevalence of total SA colonization with time (Fig. 1). In 2003, 2004, and 2005, the numbers of persons who were not carriers were 53 (67.9%), 85 (78.0%), and 60 (67.6%), respectively. Patients screened in 2005 were more likely to be carriers than expected. In 2003, 2004, and 2005, the numbers of persons who were MSSA carriers did not increase (p = 0.09): 25 (32.1%), 23 (21.1%), and 33 (34.0%), respectively; neither did the numbers of persons who were MRSA carriers increase (p = 0.08) in those same years: 0 (0.0%), one (0.9%), and four (4.1%), respectively.
Fig. 1.
The percentages of orthopaedic preoperative outpatients colonized with SA between 2003−2005 are shown (MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive Staphylococcus aureus).
We observed no correlations between nasal carriage of MRSA and any of the demographic data or surgical procedure variables examined. The odds ratios and p values for each variable examined were obtained for association with colonization status (Table 1). Even when combining patients up to 40 years of age, no associations were found in carriage rate.
Table 1.
Odds ratios and 95% exact confidence limits for potential risk factors
| Demographics | Carrier (n = 86) | Noncarrier (n = 198) | Odds ratio | 95% exact confidence limit* | p Value* | ||
|---|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | ||||
| Gender | |||||||
| Female | 43 | 50.0% | 123 | 62.1% | 0.61 | 0.366−1.016 | 0.058 |
| Male | 43 | 50.0% | 75 | 37.9% | 1.64 | 0.984−2.734 | 0.058 |
| Ethnicity | |||||||
| White | 36 | 41.9% | 68 | 34.3% | 1.376 | 0.819−2.313 | 0.228 |
| Black | 12 | 14.0% | 42 | 21.2% | 0.602 | 0.300−1.211 | 0.155 |
| Hispanic | 36 | 41.9% | 81 | 40.9% | 1.04 | 0.622−1.738 | 0.881 |
| Native American | 0 | 0.0% | 3 | 1.5% | |||
| Asian | 2 | 2.3% | 2 | 1.0% | 2.333 | 0.323−16.842 | 0.401 |
| Other | 0 | 0.0% | 2 | 1.0% | |||
| Age (years) | |||||||
| < 21 | 0 | 0.0% | 2 | 1.0% | |||
| 21−30 | 10 | 11.6% | 14 | 7.1% | 1.729 | 0.756−4.064 | 0.209 |
| 31−40 | 12 | 14.0% | 16 | 8.1% | 1.845 | 0.832−4.088 | 0.132 |
| 41−50 | 21 | 24.4% | 61 | 30.8% | 0.726 | 0.407−1.292 | 0.276 |
| 51−60 | 22 | 25.6% | 49 | 24.7% | 1.045 | 0.584−1.871 | 0.881 |
| > 60 | 21 | 24.4% | 56 | 28.3% | 0.819 | 0.458−1.465 | 0.501 |
| Surgery type | |||||||
| Arthroplasty | 33 | 38.4% | 61 | 30.8% | 1.388 | 0.818−2.357 | 0.224 |
| Arthroscopy | 17 | 19.8% | 46 | 23.2% | 0.809 | 0.433−1.511 | 0.506 |
| Podiatry | 13 | 15.1% | 45 | 22.7% | 0.621 | 0.316−1.222 | 0.168 |
| Open reduction and internal fixation | 6 | 7.0% | 6 | 3.0% | 2.39 | 0.747−7.626 | 0.142 |
| Hardware removal | 6 | 7.0% | 17 | 8.6% | 0.794 | 0.302−2.089 | 0.64 |
| Other | 10 | 11.6% | 23 | 11.6% | 0.995 | 0.452−2.193 | 0.991 |
| Hand surgery | 1 | 1.2% | 1 | 0.5% | 2.306 | 0.148−37.298 | 0.556 |
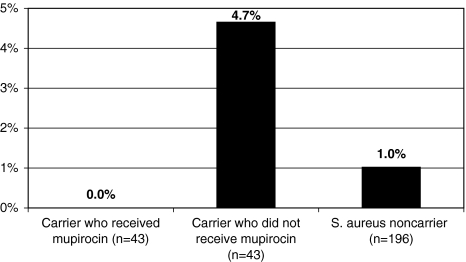
Of the 282 patients in whom we were able to assess SSI, 180 nine (3.2%) developed infections. Five of 9 infections occurred among patients in the arthroplasty group. Four of the 5 arthroplasty infections were cultured, and all 4 grew SA (three 182 MSSA and one MRSA). Two of the other 4 nonarthroplasty infections were cultured, and none grew SA. SSIs resulting from SA were significantly higher (p = 0.02) among arthroplasty vs other surgeries. In two of the patients who had SA SSIs develop, the SSIs were the result of SA phenotypically similar to the strain they carried in the anterior nares preoperatively (both MSSA). The other two patients who had cultivable SSIs resulting from SA were not carriers (Fig. 2). Overall, of the 86 patients colonized with SA in their nares, 43 opted for intranasal decolonization with mupirocin before surgery. A similar proportion of carriers who declined intranasal mupirocin preoperatively experienced SSI from SA (4.7%) compared with noncarriers (1.0%) and carriers decolonized preoperatively (0.0%).
Fig. 2.
The percentages of patients with postoperative SSIs resulting from SA according to nasal colonization status are shown.
Discussion
Staphylococcus aureus is among the most common causes of SSI in orthopaedic patients, and nasal colonization results in increased risk of SSI [1]. MRSA, the prevalence of which has been increasing in the community, is believed to add to the burden of SA colonization [13, 15]. Therefore, we sought to determine the prevalence of asymptomatic nasal colonization with SA, including MRSA, among healthy preoperative orthopaedic surgery outpatients at an urban hospital. We also assessed trends in methicillin resistance with time, examined possible risk factors for nasal colonization with SA by demographic data and elective procedure, and correlated the development of SSI to nasal colonization status and procedure.
A major limitation to our study is that it was underpowered to accomplish our secondary objectives. We were unable to do a comparative analysis of rates of SSIs and effect of decolonization, as only four SA SSIs were recorded. The small number of SSIs as an outcome variable and the nonrandomized choice of SA carriers who were decolonized preclude us making a correlation between the development of SSI to nasal colonization status. Achieving adequate power would have required an extraordinarily large patient population that would not have been feasible with resources available to us. In addition, there is potential underestimation of SA carrier state owing to the focus solely on anterior nares swabs, and not including other sites such as the throat, axilla, groin, and/or rectum. However, previous studies documenting a linkage between colonization and infection have cited nasal colonization, and not reservoirs from these other sites, as the risk factor for infection [1, 4, 12, 14, 17, 23, 24]. Finally, failure to genotypically relate the SA strains causing SSIs with the corresponding preoperative nasal strains makes definitive conclusions about clonality impossible. Although these strains appeared similar phenotypically based on susceptibility profile, it is possible molecular typing studies would reveal two distinct strains.
The overall nasal carriage rate of SA of 30.3% in our study population was within the range of nasal carriage rates reported in other studies [5, 13, 15]. The Centers for Disease Control and Prevention reports approximately 25% to 30% of the United States population is colonized with SA based on epidemiologic evaluations compiled since the 1950s [5]. Studies from recent years trend toward higher reported rates [13, 15]. In a nationally representative US survey of noninstitutionalized individuals 1 year or older, the prevalence of SA nasal colonization was 32.4% in 2001–2002 [15]. However, the overall rate of MRSA in our orthopaedic population of 1.8% was somewhat higher than the 0.8% reported by Kuehnert et al. [15]. In another study, MSSA colonization was present in 153 (38%) of the 404 asymptomatic outpatients, and MRSA colonization was present in eight (2%) [13]. Thus preoperative orthopaedic outpatient colonization rates with MSSA and MRSA in our study population seem to reflect the rates of the general population from other studies during similar periods.
Eighty percent of our patients colonized with MRSA were identified in the final year of the study period. However, the increase from 0% during the first year to 4% during the last year was not significant as we had statistical power of only approximately 40% to detect an effect of time on increased MRSA colonization rates. As with infections, other data indicate the prevalence of MRSA colonization is increasing in some community settings, even in patients who lack traditional or any identifiable risk factors for MRSA [6, 11, 16]. We presume the emergence of MRSA in the community is producing an additive effect to total colonization rates, ie, MRSA is not simply replacing MSSA, but rather adding to the burden of SA colonization. When looking at the overall population in our study, patients screened in 2005 were more likely to be carriers than statistically expected. Owing to the constantly changing nature of antimicrobial susceptibility profiles and regional epidemiology differences, it seems that knowledge of colonization status with this most common cause of infection in orthopaedic patients will be important in choosing perioperative antibiotic and empiric treatment regimens.
We observed no correlations between SA colonization rates and any of the demographic data or surgical procedure variables examined. Previous large, population-based surveillance studies report colonization varies between different ethnic groups, with higher rates in whites, in men [9, 25], and patients with extremes of age [2]. Dividing our population into these subgroups made each sample size too small to be able to duplicate detection of such differences; in addition, patients with extremes of age were not well represented in our population, and no one younger than 21 years presented for surgery in our clinic during the study period. It is likely our healthy preoperative outpatient population mirrors that of the general adult population, and unless the previously described risk factors for carriage were disproportionately represented in the various surgery types, it would be unlikely to see significant differences based on surgical procedure.
Greater than half of the SSIs that occurred were in patients having arthroplasties, and all SSIs attributable to SA were in patients having arthroplasties. Half the patients undergoing arthroplasties who were infected with SA were infected with the same phenotypic strain as carried in the infected patients’ nares. All of these infections were significant and the patients underwent additional surgery for treatment of the infections. Although this study lacks power to detect a difference in SSI rates resulting from SA between nasal carriers and noncarriers, and a benefit from nasal decolonization in reducing SA SSIs, our trends are similar to those of others who have attempted to show elimination of carriage in the anterior nares, the principle reservoirs of SA, reduces the incidence of SA SSI [11, 20, 21]. In one large study, application of prophylactic intranasal mupirocin ointment decreased the rate of all nosocomial SA infections among patients who were SA carriers (48% risk reduction); the 37% reduction in SA SSI overall was not significant [20]. In another study, patients undergoing elective orthopaedic surgery during which prosthetic implant material was used and who received mupirocin had rates of endogenous SA infections that were five times less than in the placebo group, but this difference was not statistically significant [11]. Although such trials have failed to show reductions of SSIs among patients receiving mupirocin, the trend toward lower SSI rates has remained consistent. Adequate power required to show a reduction in SSI from a baseline of 3.2%, as in our study, would require thousands of patients, as SA nasal colonization is not invariably present in individuals with an active SA SSI [8], and decolonization is not 100% successful.
The prevalence of asymptomatic nasal colonization with SA, including MRSA, among healthy preoperative orthopaedic surgery outpatients is similar to what has been reported in the general community. Therefore, the changing epidemiology of SA in the community is important to preoperative orthopaedic surgery outpatients. In 1974, Patzakis et al. reported a reduction of infections in patients with open fractures or gunshot wounds who received prophylactic antibiotics active against SA [19]. MRSA now comprises greater than half of all SA isolates in some communities. However, CDC guidelines for the use of antimicrobial prophylaxis in surgery in 1999 cautioned against routine use of vancomycin for perioperative antimicrobial prophylaxis but encouraged the decision to involve consideration of local frequencies of MRSA isolates [11]. French guidelines for use of antimicrobial prophylaxis in surgery in 1999 recommended the use of vancomycin in patients with MRSA colonization [22]. To allow for compliance with these guidelines, particularly in arthroplasties when SSI results in a relatively higher morbidity, routine screening may be warranted to determine optimal perioperative antibiotic and empiric treatment regimens and consideration for perioperative nasal decolonization for nasal carriers.
Acknowledgments
We thank the Denver Health Orthopedic Clinic nursing staff for assistance in performing anterior nares swabs; our microbiology laboratory staff for processing the large volume of surveillance cultures; and Marybeth Delassandro, BS M(ASCP), for critical review of the manuscript.
Footnotes
One of the authors (CSP) has received funding from GlaxoSmithKline Pharmaceuticals.
References
- 1.Arciola CR, Cervellati M, Pirini V, Gamberini S, Montanaro L. Staphylococci in orthopaedic surgical wounds. New Microbiol. 2001;24:365–369. [PubMed]
- 2.Armstrong-Esther CA. Carriage patterns of Staphylococcus aureus in a healthy non-hospital population of adults and children. Ann Hum Biol. 1976;3:221–227. [DOI] [PubMed]
- 3.Brown DF, Edwards DI, Hawkey PM, Morrison D, Ridgway GL, Towner KJ, Wren MW; Joint Working Party of the British Society for Antimicrobial Chemotherapy; Hospital Infection Society; Infection Control Nurses Association. Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA). J Antimicrob Chemother. 2005;56:1000–1018. [DOI] [PubMed]
- 4.Calia FM, Wolinsky E, Mortimer EA Jr, Abrams JS, Rammelkamp CH Jr. Importance of the carrier state as a source of Staphylococcus aureus in wound sepsis. J Hyg (Lond). 1969;67:49–57. [DOI] [PMC free article] [PubMed]
- 5.Centers for Disease Control, Prevention DoHQPD. Community-associated MRSA information for clinicians. Vol 2006. Atlanta, GA: Centers for Disease Control and Prevention. 2005. http://www.cdc.gov/ncidod/dhqp/ar_mrsa_ca_clinicians.html. Accessed June 6, 2008.
- 6.Creech CB 2nd, Kernodle DS, Alsentzer A, Wilson C, Edwards KM. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr Infect Dis J. 2005;24:617–621. [DOI] [PubMed]
- 7.Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39-7:971–979. [DOI] [PubMed]
- 8.Frazee BW, Lynn J, Charlebois ED, Lambert L, Lowery D, Perdreau-Remington F. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann Emerg Med. 2005;45:311–320. [DOI] [PubMed]
- 9.Herwaldt LA, Cullen JJ, French P, Hu J, Pfaller MA, Wenzel RP, Perl TM. Preoperative risk factors for nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol. 2004;25:481–484. [DOI] [PubMed]
- 10.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606–608. [PubMed]
- 11.Kalmeijer MD, Coertjens H, van Nieuwland-Bollen PM, Bogaers-Hofman D, de Baere GA, Stuurman A, van Belkum A, Kluytmans JA. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis. 2002;35:353–358. [DOI] [PubMed]
- 12.Kalmeijer MD, van Nieuwland-Bollen E, Bogaers-Hofman D, de Baere GA. Nasal carriage of Staphylococcus aureus is a major risk factor for surgical-site infections in orthopedic surgery. Infect Control Hosp Epidemiol. 2000;21:319–323. [DOI] [PubMed]
- 13.Kenner J, O’Connor T, Piantanida N, Fishbain J, Eberly B, Viscount H, Uyehara C, Hospenthal D. Rates of carriage of methicillin-resistant and methicillin-susceptible Staphylococcus aureus in an outpatient population. Infect Control Hosp Epidemiol. 2003;24:439–444. [DOI] [PubMed]
- 14.Kluytmans J. Reduction of surgical site infections in major surgery by elimination of nasal carriage of Staphylococcus aureus. J Hosp Infect. 1998;40(suppl B):S25–29. [DOI] [PubMed]
- 15.Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK, Killgore G, Tenover FC. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. J Infect Dis. 2006;193:172–179. [DOI] [PubMed]
- 16.Lu PL, Chin LC, Peng CF, Chiang YH, Chen TP, Ma L, Siu LK. Risk factors and molecular analysis of community methicillin-resistant Staphylococcus aureus carriage. J Clin Microbiol. 2005;43:132–139. [DOI] [PMC free article] [PubMed]
- 17.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132; quiz 133–134; discussion 96. [DOI] [PubMed]
- 18.National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard M7-A6. 6th ed. Wayne PA: NCCLS. 2003.
- 19.Patzakis MJ, Harvey JP Jr, Ivler D. The role of antibiotics in the management of open fractures. J Bone Joint Surg Am. 1974;56:532–541. [PubMed]
- 20.Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, Twombley J, French PP, Herwaldt LA. Mupirocin and the risk of Staphylococcus aureus study team. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346:1871–1877. [DOI] [PubMed]
- 21.Roche SJ, Fitzgerald D, O’Rourke A, McCabe JP. Methicillin-resistant Staphylococcus aureus in an Irish orthopaedic centre: a five-year analysis. J Bone Joint Surg Br. 2006;88:807–811. [DOI] [PubMed]
- 22.The French Society of Anesthesia and Resuscitation. Recommendations for the practice of antibiotic prophylaxis in surgery: current status 1999. Chirurgie 1999;124:441–447. [DOI] [PubMed]
- 23.Weinstein HJ. The relation between the nasal-staphylococcal-carrier state and the incidence of postoperative complications. N Engl J Med. 1959;260:1303–1308. [DOI] [PubMed]
- 24.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5–12:751–762. [DOI] [PubMed]
- 25.Williams RE. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev. 1963;27:56–71. [DOI] [PMC free article] [PubMed]