Abstract
This multicenter study of 813 consecutive patients with hip fracture was performed to estimate the effectiveness and reproducibility of the Estimation of Physiologic Ability and Surgical Stress (E-PASS) scoring system to assess postoperative risk in patients with hip fracture. E-PASS is comprised of a preoperative risk score, a surgical stress score, and a comprehensive risk score based on the preoperative risk score and surgical stress score. Postoperative complications developed in 163 patients (20.0%); 13 (1.6%) died. Hospital postoperative morbidity and mortality rates increased linearly with the preoperative risk score and comprehensive risk score; the correlation was significant. The severity of postoperative complications and the incidence of higher grades of complications increased significantly with rising preoperative risk score and comprehensive risk score. Each E-PASS score also was related significantly with the length of postoperative hospitalization and costs. These results suggest E-PASS is useful for predicting postoperative risk, estimating costs, and for comparing the outcome in patients having surgical treatment of hip fractures.
Level of Evidence: Level II, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The number of patients with hip fractures is increasing worldwide, and these fractures are an important cause of mortality and loss of function among the elderly [6]. Management of these patients can be problematic because concomitant medical conditions increase operative risk. Physical reserve capacity is normally decreased in the elderly, and many patients admitted with hip fractures present with systemic complications and require physical assistance.
Factors associated with increased risk for mortality after hip fracture and predictive scores for postoperative risk have been reported [2, 5, 7, 8, 16]. Postoperative morbidity and mortality rates can be estimated by quantifying the patient’s physiologic status and surgical stress. However, there are few predictive surgical scores that consider the patient’s preoperative condition and the stress of surgical invasion, and there currently is no practical scoring system to predict postoperative risk in patients with hip fractures. The E-PASS score includes preoperative and intraoperative parameters, and is comprised of a preoperative risk score (PRS), a surgical stress score (SSS), and a comprehensive risk score (CRS) that is determined by the PRS and SSS (Appendix 1) [3]. E-PASS has been used with patients scheduled for gastrointestinal surgery, and the score was found to correlate with mortality and morbidity rates [3]. Using this scoring system to predict the postoperative risk in an emergency hospital, we previously analyzed data from a group of patients operated on for hip fractures for the first time [4]. We found the postoperative hospital morbidity and mortality rates increased linearly with the PRS and CRS and there was a significant correlation in patients who had osteosynthesis or hip arthroplasty [4].
The purpose of the current multicenter study was to investigate the effectiveness and reproducibility of the E-PASS score in the assessment of postoperative risk in patients with hip fractures. Our study addressed the relationship between the E-PASS score and the incidence of postoperative morbidity and mortality, the severity of potential postoperative complications, and the correlation between E-PASS scores and the length and cost of hospitalization. Furthermore, we compared characteristic data and the postoperative course of patients subjected to osteosynthesis and hip arthroplasty and between patients with and without postoperative complications.
Materials and Methods
We retrospectively reviewed 813 consecutive patients (179 men, 634 women) operated on between September 2003 and December 2005 for hip fractures at seven member hospitals of the Japanese National Hospital Organization. Their mean age was 79.7 years (range, 27–100 years). The surgical procedure was osteosynthesis in 497 patients (intramedullary hip screws, n = 249; sliding hip screws, n = 182; intramedullary nails, n = 3; multiple pinning with screws or nails, n = 63). Hip arthroplasty was performed in 316 patients; 312 underwent hemiarthroplasty and four had THAs.
The patients’ age and body weight, operation time, intraoperative blood loss, and postoperative course, eg, the hospital morbidity and mortality rates and the length and cost of hospitalization, were derived from medical records at each participating hospital. The preoperative status and surgical stress parameters were analyzed at each hospital with an electronic E-PASS chart used to calculate the E-PASS score (Appendix 1) [3]. We collected all data and assessed the correlation between each score comprising the E-PASS system and the postoperative course. We also evaluated the relationship between the severity of complications and the E-PASS score. Furthermore, these variables were compared including each E-PASS score between patient groups treated by osteosynthesis or arthroplasty, between patients with and without postoperative complications, and among the seven participating hospitals. The end point of our study was discharge from or predischarge death at the hospital at which the patients had their surgery.
Algorithms and definitions used to calculate individual E-PASS scores were formulated (Appendix 1) [3]. Postoperative complications were included only if the patient had received medical or interventional treatment; these complications were recorded in the patient’s medical record. Complications developed in 163 (20.0%) of the 813 patients (Table 1). The crude mortality rate was 1.6% (13 of 813 patients). Morbidity was scored as Grade 0 (no complications), Grade 1 (mild, nonlife-threatening complications), Grade 2 (moderate complications that were potentially life-threatening unless adequate treatment was initiated), or Grade 3 (organ failure with a sequential organ failure assessment score [15], 3 points or greater or sepsis). Hospitalization costs included all charges for the operation, laboratory tests, diagnostic imaging, ward costs, and expenditures incurred for treatment of postoperative complications. These costs were converted from Japanese to US currency.
Table 1.
Patients with hip fractures (n = 163 [20.2%]) with postoperative complications*
| Complication | Number |
|---|---|
| Respiratory | |
| Pneumonia | 29 |
| Respiratory failure | 5 |
| Influenza | 3 |
| Hypoxia | 3 |
| Chronic obstructive pulmonary disease | 1 |
| Cardiovascular | |
| Hypotension | 9 |
| Stroke/seizure | 8 |
| Arrhythmia | 4 |
| Heart failure | 3 |
| Deep vein thrombosis | 3 |
| Myocardial infarction | 2 |
| Pulmonary embolism | 2 |
| Gastrointestinal disease | |
| Gastroduodenal ulcer | 12 |
| Enterocolitis/cholecystitis | 8 |
| Bleeding | 4 |
| Ascites | 1 |
| Liver dysfunction | 3 |
| Delirium | 27 |
| Infection | |
| Urinary tract | 8 |
| Pyrexia of unknown origin | 6 |
| Deep infection | 4 |
| Wound infection | 1 |
| Disseminated intravascular coagulation | 1 |
| Renal dysfunction | 6 |
| Hypoglycemia | 1 |
| Fracture-related problem | |
| Cutting out of lag screw | 7 |
| Hip dislocation | 6 |
| Femoral shaft fracture | 3 |
| Femoral head avascular necrosis | 1 |
| Femoral malalignment | 1 |
| Peripheral nerve palsy | 1 |
| Decubitus ulcer | 11 |
| Wound bleeding | 2 |
*Some patients had multiple complications.
Using the Mann-Whitney U-test for continuous variables, we determined the significance of differences in the examined data. These included intraoperative and postoperative parameters, the E-PASS scores of patients treated by osteosynthesis or arthroplasty, and the E-PASS scores of patients with and without postoperative complications. Intergroup differences in the severity of postoperative complications were assessed with the chi square test with Yates’ correction for continuity in categorical variables. The correlation between different continuous variables of the E-PASS scores and the length and cost of hospitalization was quantified by Pearson’s correlation coefficient. We analyzed the correlation between the E-PASS scores and the incidence of postoperative morbidity or mortality with the ranked Spearman correlation coefficient. The significance of the multiple correlation coefficient (R) among the seven hospitals and the severity of complications was quantified by analysis of variance (ANOVA) and Fisher’s protected least significant difference (PLSD) or multivariate logistic regression using PRS and SSS data. Differences of p < 0.05 were considered statistically significant.
Results
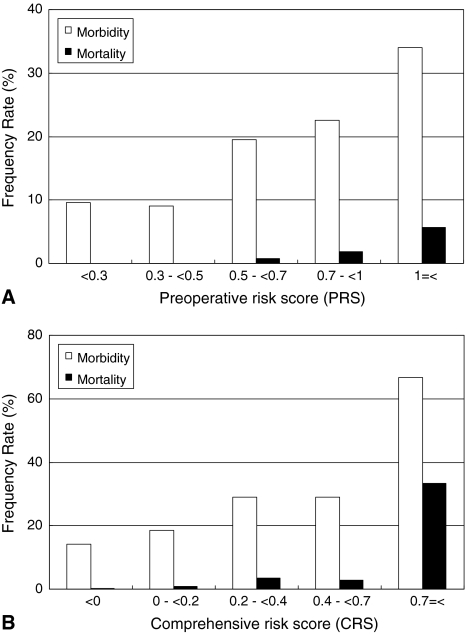
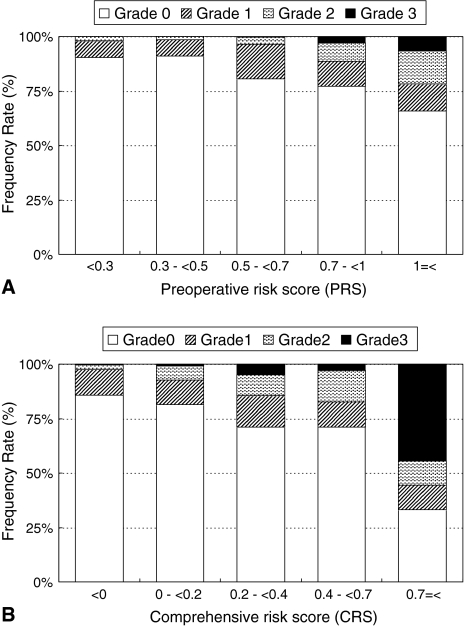
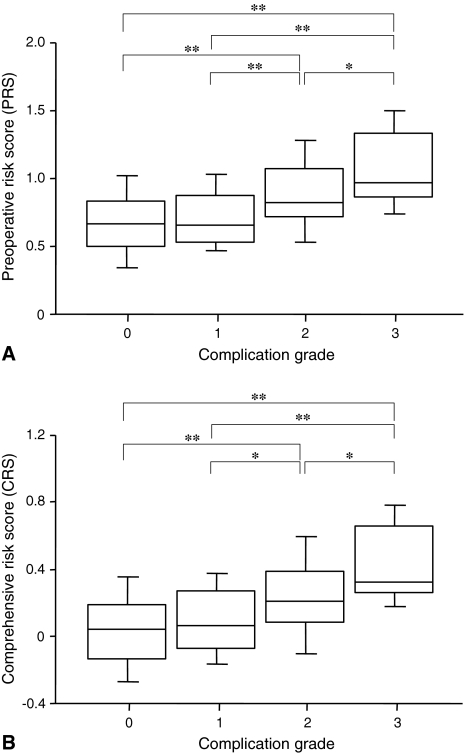
Postoperative morbidity rates increased linearly with the PRS and CRS and correlated significantly with the PRS (ρ = 0.16, p < 0.0001; Fig. 1A) and CRS (ρ = 0.18, p < 0.0001; Fig. 1B). However, the SSS was not correlated with postoperative morbidity rates (ρ = 0.06, p = 0.07). The mortality rates correlated with the PRS (ρ = 0.14, p = 0.0001; Fig. 1A) and CRS (ρ = 0.14, p = 0.0001; Fig. 1B), but not with the SSS (ρ = −0.03, p = 0.4). The incidence of higher-grade postoperative complications significantly increased with an increase in the PRS (p < 0.0001; Fig. 2A) and CRS (p < 0.0001; Fig. 2B). Furthermore, patients with higher grades of postoperative complications manifested a higher PRS (p < 0.0001; Fig. 3A) and CRS (p < 0.0001; Fig. 3B).
Fig. 1A–B.
(A) The bar chart shows the relationship between the preoperative risk score (PRS) and postoperative morbidity and mortality rates. The PRS correlated significantly with morbidity rates (ρ = 0.16, p < 0.0001) and mortality rates (ρ = 0.14, p = 0.0001). (B) The bar chart shows the relationship between the comprehensive risk score (CRS) and postoperative morbidity and mortality rates. The CRS correlated significantly with morbidity rates (ρ = 0.18, p < 0.0001) and mortality rates (ρ = 0.14, p = 0.0001).
Fig. 2A–B.
(A) The bar chart shows the relationship between the severity of complications and the preoperative risk score (PRS). The incidence of higher-grade postoperative complications significantly increased with an increase in the PRS (p < 0.0001). (B) The bar chart shows the relationship between the severity of complications and the comprehensive risk score (CRS). The incidence of higher-grade postoperative complications significantly increased with an increase in the CRS (p < 0.0001).
Fig. 3A–B.
(A) The bar chart shows the relationship between the severity of complications and the preoperative risk score (PRS). Patients with higher-grade postoperative complications manifested a higher PRS (p < 0.0001). *p < 0.05; **p < 0.01. (B) The bar chart shows the relationship between the severity of complications and the comprehensive risk score (CRS). Patients with higher-grade postoperative complications manifested a higher CRS (p < 0.0001). *p < 0.05; **p < 0.01.
Examination of the correlation between E-PASS scores and other examined parameters related to the postoperative course showed the length of postoperative hospitalization was significantly correlated with the PRS (r = 0.09, p = 0.009), SSS (r = 0.17, p < 0.0001), and CRS (r = 0.13, p = 0.0001). The cost of hospitalization also was significantly related to the PRS (r = 0.12, p = 0.0007), SSS (r = 0.44, p < 0.0001), and CRS (r = 0.23, p < 0.0001).
There was no difference in the morbidity and mortality rates of patients treated by osteosynthesis or arthroplasty. Based on the E-PASS scoring system, the SSS and CRS were higher in the hip arthroplasty than the osteosynthesis group (p < 0.0001); the two groups did not differ with respect to the PRS (Table 2).
Table 2.
Clinical data for patients having osteosynthesis or arthroplasty (mean ± standard deviation)
| Variable | Osteosynthesis | Arthroplasty | p Value | Total | |
|---|---|---|---|---|---|
| Number of patients | 497 | 316 | 813 | ||
| Male/female | 116/381 | 63/253 | 179/634 | ||
| Number of patients with morbidity | 92 | 71 | 0.4 | 163 | |
| Number of patients with mortality | 8 | 5 | 0.5 | 13 | |
| Age in years (range) | 80.4 ± 11.6 (32–100) | 78.6 ± 10.6 (27–97) | 0.0007 | 79.7 ± 11.2 (27–100) | |
| Body weight in kilograms (range) | 46.5 ± 13.3 (24–170) | 47.2 ± 12.7 (26–156) | 0.2 | 46.8 ± 13.1 (24–170) | |
| Operation time in minutes (range) | 53.0 ± 30.9 (15–310) | 131.6 ± 841.3 (30–400) | < 0.0001 | 65.0 ± 37.6 (15–400) | |
| Intraoperative blood loss in grams (range) | 58.4 ± 72.4 (0–480) | 212.4 ± 205.7 (9–1970) | < 0.0001 | 117.9 ± 158.7 (0–1970) | |
| Postoperative hospitalization in days (range) | 30.5 ± 21.6 (1–142) | 35.7 ± 34.4 (2–365) | 0.02 | 32.5 ± 27.4 (1–365) | |
| Cost of hospitalization in 103 US dollars (range) | 12.8 ± 6.6 (2.8–64.9) | 20.6 ± 9.3 (5.3–103.7) | < 0.0001 | 15.8 ± 8.6 (2.8–103.7) | |
| E-PASS | PRS (range) | 0.704 ± 0.279 (0.056–1.582) | 0.688 ± 0.240 (0.177–1.579) | 0.6 | 0.698 ± 0.264 (0.056–1.582) |
| SSS (range) | −0.289 ± 0.040 (−0.330–0.037) | −0.192 ± 0.555 (−0.319–0.445) | < 0.0001 | −0.264 ± 0.065 (−0.330–0.445) | |
| CRS (range) | 0.049 ± 0.262 (−0.516–0.914) | 0.128 ± 0.562 (−0.418–0.849) | 0.002 | 0.068 ± 0.251 (−0.516–0.914) | |
E-PASS = Estimation of Physiologic Ability and Surgical Stress; PRS = preoperative risk score; SSS = surgical stress score; CRS = comprehensive risk score.
The PRS (p < 0.0001) and CRS (p < 0.0001) were higher in patients with postoperative complications than without (Table 3); the SSS did not differ significantly.
Table 3.
Clinical data for patients with hip fracture (n = 813) without (−) and with (+) postoperative complications*
| Variable | Complications (−) | Complications (+) | p Value | |
|---|---|---|---|---|
| Number of patients | 650 | 163 | ||
| Male/female | 144/506 | 35/128 | ||
| Age in years (range) | 79.2 ± 11.5 (27–100) | 81.6 ± 10.2 (40–97) | 0.01 | |
| Body weight in kilograms (range) | 46.5 ± 12.1 (24–170) | 48.0 ± 16.5 (32–156) | 0.7 | |
| Operation time in minutes (range) | 63.4 ± 36.0 (15–400) | 71.6 ± 42.8 (18–331) | 0.04 | |
| Intraoperative blood loss in grams (range) | 111.3 ± 139.8 (0–1550) | 144.0 ± 217.1 (0–1970) | 0.08 | |
| Postoperative hospitalization in days (range) | 29.1 ± 22.7 (1–365) | 46.2 ± 38.2 (1–250) | < 0.0001 | |
| Cost of hospitalization in 103 US dollars (range) | 14.5 ± 5.8 (2.8–64.9) | 21.3 ± 14.2 (5.2–103.7) | < 0.0001 | |
| E-PASS | PRS (range) | 0.675 ± 0.256 (0.056–1.582) | 0.792 ± 0.276 (0.184–1.553) | < 0.0001 |
| SSS (range) | −0.266 ± 0.060 (−0.330–0.344) | −0.254 ± 0.081 (−0.329–0.445) | 0.07 | |
| CRS (range) | 0.044 ± 0.241 (−0.516–0.891) | 0.166 ± 0.264 (−0.474–0.914) | < 0.0001 | |
*Mean ± standard deviation; E-PASS = Estimation of Physiologic Ability and Surgical Stress; PRS = preoperative risk score; SSS = surgical stress score; CRS = comprehensive risk score.
Analysis of variance (ANOVA) showed significant differences among the seven participating hospitals with respect to postoperative morbidity rates (p < 0.0001), operation time (p < 0.0001), amount of blood loss during surgery (p = 0.02), length of postoperative hospitalization (p < 0.0001), cost of hospitalization (p < 0.0001), and each score of the E-PASS system (p < 0.0001) (Table 4). Comparison of the morbidity rates at two arbitrarily chosen participating hospitals revealed a different pattern for data obtained with Fisher’s PLSD used for direct comparisons and data obtained by multivariate logistic regression with normalization of the PRS and SSS (Table 5).
Table 4.
Clinical data for 813 patients treated at seven participating hospitals*
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 100 | 99 | 221 | 101 | 111 | 81 | 100 | ||
| Female/male | 21/79 | 15/84 | 45/176 | 25/76 | 24/87 | 21/60 | 28/72 | ||
| Patients with morbidity (%) | 9 (9.0%) | 16 (16.2%) | 40 (18.1%) | 19 (18.8%) | 22 (19.8%) | 19 (23.5%) | 38 (38.0%) | < 0.0001 | |
| Hospital mortality (%) | 1 (1.0%) | 4 (4.0%) | 1 (0.5%) | 0 (0%) | 1 (0.9%) | 2 (2.5%) | 4 (4.0%) | 0.06 | |
| Age in years (range) | 82.1 ± 9.7 (45–99) | 78.3 ± 11.3 (40–96) | 80.4 ± 10.3 (43–97) | 79.2 ± 11.8 (41–100) | 80.2 ± 12.5 (32–98) | 77.9 ± 13.1 (27–95) | 78.8 ± 10.9 (36–97) | 0.1 | |
| Body weight in kilograms (range) | 44.6 ± 9.2 (25–65) | 46.0 ± 10.8 (28–85) | 45.0 ± 7.4 (30–70) | 49.9 ± 17.0 (24–152) | 46.9 ± 10.3 (30–73) | 49.2 ± 19.0 (26–156) | 48.6 ± 18.7 (24–170) | 0.007 | |
| Operation time in minutes (range) | 47.3 ± 25.3 (16–145) | 73.8 ± 27.5 (28–158) | 64.3 ± 37.7 (21–310) | 63.1 ± 44.3 (15–400) | 53.6 ± 22.7 (16–157) | 70.4 ± 42.3 (22–290) | 86.3 ± 44.9 (32–331) | < 0.0001 | |
| Intraoperative blood loss in grams (range) | 67.1 ± 101.9 (5–700) | 141.2 ± 156.3 (10–900) | 127.5 ± 156.2 (10–155) | 119.1 ± 180.1 (5–1300) | 110.4 ± 122.7 (2–530) | 114.4 ± 152.9 (10–1230) | 134.0 ± 214.0 (0 −1970) | 0.02 | |
| Postoperative hospitalization in days (range) | 31.1 ± 14.5 (1–82) | 46.0 ± 41.2 (15–365) | 17.5 ± 11.7 (2–112) | 19.5 ± 9.9 (6–55) | 42.0 ± 24.6 (7–225) | 30.7 ± 24.0 (6–157) | 57.9 ± 33.4 (14–250) | < 0.0001 | |
| Cost of hospitalization in 103 US dollars (range) | 13.6 ± 4.2 (5.1–24.8) | 18.7 ± 9.2 (7.4–69.3) | 12.9 ± 7.3 (6.0–92.2) | 14.6 ± 5.6 (5.2–30.0) | 16.1 ± 6.9 (5.3–61.8) | 16.1 ± 7.9 (6.4–52.6) | 22.4 ± 13.5 (2.8–103.7) | < 0.0001 | |
| E-PASS | PRS (range) | 0.710 ± 0.194 (0.153–1.275) | 0.620 ± 0.237 (0.136–1.145) | 0.632 ± 0.192 (0.205–1.376) | 0.639 ± 0.284 (0.171–1.560) | 0.732 ± 0.346 (0.129–1.553) | 0.884 ± 0.242 (0.258–1.579) | 0.783 ± 0.282 (0.056–1.582) | < 0.0001 |
| SSS (range) | −0.289 ± 0.046 (−0.329–−0.014) | −0.250 ± 0.059 (−0.320–−0.033) | −0.261 ± 0.060 (−0.324–0.132) | −0.265 ± 0.082 (−0.330–−0.344) | −0.275 ± 0.044 (−0.329–−0.115) | −0.260 ± 0.061 (−0.320–0.037) | −0.245 ± 0.085 (−0.321–−0.445) | < 0.0001 | |
| CRS (range) | 0.054 ± 0.180 (−0.492–0.604) | 0.008 ± 0.226 (−0.508–0.576) | 0.008 ± 0.183 (−0.428–0.660) | 0.012 ± 0.280 (−0.465–0.826) | 0.089 ± 0.319 (−0.516–0.804) | 0.245 ± 0.223 (−0.368–0.891) | 0.165 ± 0.273 (−0.467–0.914) | < 0.0001 | |
*Mean ± standard deviation; E-PASS = Estimation of Physiologic Ability and Surgical Stress; PRS = preoperative risk score; SSS = surgical stress score; CRS = comprehensive risk score.
Table 5.
p Values for morbidity rates at the seven participating hospitals
| Statistics | Multivariate logistic regression | |||||||
|---|---|---|---|---|---|---|---|---|
| Hospital | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Fisher’s PLSD | 1 | — | 0.5 | 0.002 | 0.007 | 0.2 | 0.07 | 0.01 |
| 2 | 0.2 | — | 0.01 | 0.03 | 0.5 | 0.2 | 0.04 | |
| 3 | 0.06 | 0.7 | — | 0.9 | 0.03 | 0.2 | 0.6 | |
| 4 | 0.08 | 0.6 | 0.9 | — | 0.08 | 0.3 | 0.7 | |
| 5 | 0.05 | 0.5 | 0.7 | 0.9 | — | 0.5 | 0.1 | |
| 6 | 0.01 | 0.2 | 0.3 | 0.4 | 0.5 | — | 0.5 | |
| 7 | < 0.0001 | 0.0001 | < 0.0001 | 0.0006 | 0.0009 | 0.01 | — | |
PLSD = protected least significant difference.
Discussion
In aged patients, operative risk evaluation and treatment selection are major determinants of the postoperative course. Although postoperative morbidity and mortality rates can be estimated not only by quantifying a patient’s physiologic status, but also by the level of surgical stress, there are few predictive scores that include the patient’s preoperative condition and the stress induced by surgical invasion. The E-PASS scoring system originally was developed for gastrointestinal surgery to predict postoperative risk; it considered the patient’s preoperative condition and intraoperative variables [3]. Respectively, the PRS and SSS can express the reserve capacity of individual patients and the degree of surgical stress as continuous variables. These scores require no special examinations [3]. and can be recorded at any hospital [9]. The E-PASS system is a good predictor of the outcome of surgery in patients with hip fractures and can be used at an emergency hospital [4].
Our multicenter study of patients undergoing surgery for hip fractures showed the E-PASS score exhibited a good correlation with the postoperative course. As a primary result, the E-PASS score correlated with the incidence of postoperative morbidity and mortality and the severity of potential postoperative complications. It also correlated with the length and cost of hospitalization. Our results show the E-PASS system is effective and reproducible for prediction of the postoperative course at surgical hospitals. We also found marked differences in several parameters when we compared patients treated with hip arthroplasty or osteosynthesis, and patients with and without postoperative complications, and among the seven participating hospitals. These differences may reflect differences in the E-PASS scores and postoperative course. However, because there was no difference in the complication rate of patients subjected to the two different surgical procedures, we analyzed the correlation between the E-PASS scores and parameters related to the patients’ postoperative course using combined data from the arthroplasty and osteosynthesis groups. This study was limited to the length of hospitalization at the participating hospital where the patients underwent surgery.
Surgical stress that greatly exceeds the patient’s reserve capacity often disrupts homeostasis of the respiratory, circulatory, metabolic, or immune system, leading to postoperative complications that reflect the patient’s physiologic status and surgical stress. In our multicenter study, the PRS was significantly correlated with the hospital morbidity and mortality rate. The linear increase in the incidence of complications with an increasing PRS was consistent with earlier findings of patients with hip fractures undergoing surgery at an emergency hospital [4]. Furthermore, the severity of complications and the hospital mortality rate increased with the PRS. These results suggest the PRS can be used at hospitals and that it may be a predictor of progression to multiple organ failure in patients scheduled for hip fracture surgery.
In contrast, the SSS was not correlated with postoperative morbidity rates and it did not differ among patients with and without postoperative complications. Although it was greater in patients treated by hip arthroplasty than osteosynthesis, the two treatment groups did not differ with respect to morbidity and mortality rates. However, the PRS was greater in patients with postoperative complications than without, although it did not differ between the two treatment groups. These findings indicate the postoperative risk of patients with hip fractures was affected more by their physiologic status than the degree of surgical stress induced by either surgical procedure. According to some reports, when the patients’ preoperative physiologic condition was used to obtain predictive scores for postoperative risk, there was an association with an increased risk of mortality after a hip fracture [2, 5, 7, 8]. The SSS is comprised of the operation time, the blood loss per body weight, and the extent of the skin incision [3]. The higher SSS scores in patients subjected to hip arthroplasty may reflect longer operation times and greater intraoperative blood loss. The size of the skin incision is not relevant in patients undergoing surgery for hip fracture. Compared with patients without postoperative complications, only the operation time was longer in patients with postoperative morbidity; there was no correlation with the amount of intraoperative blood loss. It is unclear whether surgical stress affects the postoperative results [7, 12–14], and studies examining this issue are being done.
The E-PASS score exhibited a significant correlation with the length of hospitalization. In Japan, patients treated by uneventful acute phase surgery for hip fracture at emergency hospitals are transferred for rehabilitation or discharged to nursing homes. Our previous study showed no correlation between the E-PASS score and the length of stay at an emergency hospital [4]. The current study included various types of surgical hospitals and patients treated in the subacute and chronic phases. Some institutions discharge surgical patients directly to their homes. The longer the hospital stay, the more significant the correlation among the E-PASS score, the length of hospitalization, and treatment costs. The E-PASS system may be applicable to the Diagnostic Procedure Combination and the Japanese Diagnosis-Related Groups/Prospective Payment System that are widely used in Japan and it may be useful in the area of hospital management.
Among the seven participating hospitals, there were significant differences with respect to some parameters, including the E-PASS score. These differences may account for the divergent postoperative complication rates recorded at these hospitals. However, a direct comparison of morbidity and mortality rates is not warranted because they can be expected to be higher in high-risk than in low-risk patients. There are few clinical outcome studies that normalized patients and surgical risk in comparisons involving different operative methods, surgeons, or hospitals. We applied different statistical methods to compare surgical outcomes at two arbitrarily selected participating hospitals. Different results were obtained when we applied ANOVA with Fisher’s PLSD in simple comparisons and multivariate logistic regression analysis with normalized PRS and SSS scores in standardized comparisons. For example, Hospital 7 had significantly higher morbidity rates than the other six hospitals when we performed simple comparison using Fisher’s PLSD. However, multivariate logistic regression analysis in standardized comparisons showed significant differences only at Hospitals 1 and 2 vis-a-vis Hospital 7 (Table 5). The E-PASS scoring system may be useful for standardization of patient backgrounds in the analysis of surgical outcomes.
Our multicenter study showed the value of the E-PASS scoring system in patients with hip fracture scheduled for surgical treatment. Scoring requires little time, is simple, and the results are easily calculable and can be used by orthopaedic surgery departments in any hospital. The E-PASS scoring system is useful for estimating the postoperative risk and medical expenses incurred by treating patients with hip fracture, for standardizing patient populations in surgical audits, and for obtaining better treatment outcomes.
Acknowledgments
We thank Drs. Hiroshi Usui, Yuri Yabuki (NHO Tokyo Medical Center), Yukio Nakatsuchi, Yutaka Tateiwa (NHO Nagano National Hospital), Toshiaki Miyahara, Taro Mawatari (NHO Kyusyu Medical Center), Satoshi Motokawa, Kiyofumi Mitsutake (NHO Nagasaki Medical Center), Kazuhiko Ihara (NHO Beppu Medical Center), Satoshi Maeda (NHO Kumamoto Medical Center), and Tateki Segata (NHO Kumamoto Saisyunso National Hospital) for supplying patient data.
Appendix
Appendix 1.
Algorithms used to calculate the estimation of Physiologic Ability and Surgical Stress (E-PASS) Scores
| Preoperative risk score (PRS) = −0.0686 + 0.00345X1 + 0.323X2 + 0.205X3 + 0.153X4 + 0.148X5 + 0.0666X6, where |
| X1 is the patient age, X2 the presence (1) or absence (0) of severe heart disease, X3 the presence (1) or absence (0) of severe pulmonary disease, X4 the presence (1) or absence (0) of diabetes mellitus, X5 the performance status index (0–4), and X6 the American Society of Anesthesiologists (ASA) physiologic status classification (1–5) |
| Severe heart disease was defined as heart failure corresponding to Class III or IV of the New York Heart Association (NYHA) classification or severe arrhythmia requiring mechanical support. Severe pulmonary disease was defined as any condition with a percent vital capacity of less than 60% and/or a forced expiratory volume in one second of less than 50%, or an arterial blood oxygen level of less than 60 mm Hg without oxygen being supplied to patients in whom pulmonary function could not be measured. Diabetes mellitus was based on the definition of the World Health Organization (WHO) criteria [1]. The performance status index was defined according to Eastern Cooperative Oncology Group (ECOG) criteria [10] in which Grade 0 = fully active and able to perform all predisease activities without restriction, Grade 1 = restricted strenuous physical activity but ambulatory and able to perform work of a light or sedentary nature (eg, light house and office work), Grade 2 = ambulatory and capable of all self-care but unable to perform any work activities for up to or more than 50% of waking hours, Grade 3 = capable of only limited self-care and confined to bed or chair for more than 50% of waking hours, and Grade 4 = completely disabled, unable to perform any self-care and totally confined to bed or chair. The ASA classification was as previously described [11], ie, Class 1 = normally healthy, Class 2 = mild systemic disease, Class 3 = severe systemic disease that is not incapacitating, Class 4 = incapacitating systemic disease that is a constant threat to life, and Class 5 = moribund, not expected to survive for 24 hours with or without surgery. |
| Surgical stress score (SSS) = −0. 342 + 0.0139X1 + 0.0392X2 + 0.352X3, where |
| X1 is the amount of blood loss per kilogram body weight (g/kg), X2 the operation time (hours), and X3 the extent of skin incision (0, minor incision without laparotomy or and thoracotomy; 1, laparotomy or thoracotomy alone; 2, laparotomy and thoracotomy) |
| Comprehensive risk score (CRS) = −0.328 + 0.936 (PRS) + 0.976 (SSS) |
Footnotes
One of the authors (JH) has received funding from a research grant for the policy-based medical services network for musculoskeletal diseases (Number 771).
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus—provisional report of a WHO consultation. Diabetes Med. 1998;15:539–553. [DOI] [PubMed]
- 2.Elliott J, Beringer T, Kee F, Marsh D, Willis C, Stevenson M. Predicting survival after treatment for fracture of the proximal femur and the effect of delays to surgery. J Clin Epidemiol. 2003;56:788–795. [DOI] [PubMed]
- 3.Haga Y, Ikei S, Ogawa M. Estimation of Physiologic Ability and Surgical Stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today. 1999;29:219–225. [DOI] [PubMed]
- 4.Hirose J, Mizuta H, Ide J, Nomura K. Evaluation of estimation of physiologic ability and surgical stress (E-PASS) to predict the postoperative risk for hip fracture in elder patients. Arch Orthop Trauma Surg. 2008 Jan 4 [Epub ahead of print]. [DOI] [PubMed]
- 5.Jiang HX, Majumdar SR, Dick DA, Moreau M, Raso J, Otto DD, Johnston DW. Development and initial validation of a risk score for predicting in-hospital and 1-year mortality in patients with hip fractures. J Bone Miner Res. 2005;20:494–500. [DOI] [PubMed]
- 6.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. [DOI] [PubMed]
- 7.Kenzora JE, McCarthy RE, Lowell JD, Sledge CB. Hip fracture mortality: relation to age, treatment, preoperative illness, time of surgery, and complications. Clin Orthop Relat Res. 1984;186:45–56. [PubMed]
- 8.Masuda T, Miura N, Ishii S, Hibino Y, Beppu M. New preoperative evaluation system of the physical findings of aged patients with femoral neck fracture. J Orthop Sci. 2004;9:434–439. [DOI] [PubMed]
- 9.Oka Y, Nishijima J, Oku K, Azuma T, Inada K, Miyazaki S, Nakano H, Nishida Y, Sakata K, Izukura M. Usefulness of an Estimation of Physiologic Ability and Surgical Stress (E-PASS) scoring system to predict the incidence of postoperative complications in gastrointestinal surgery. World J Surg. 2005;29:1029–1033. [DOI] [PubMed]
- 10.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [DOI] [PubMed]
- 11.Owens WD, Felts JA, Spitznagel EL Jr. ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239–243. [DOI] [PubMed]
- 12.Puolakka TJ, Laine HJ, Tarvainen T, Aho H. Thompson hemiarthroplasty is superior to Ullevaal screws in treating displaced femoral neck fractures in patients over 75 years: a prospective randomized study with two-year follow-up. Ann Chir Gynaecol. 2001;90:225–228. [PubMed]
- 13.van Dortmont LM, Douw CM, van Breukelen AM, Laurens DR, Mulder PG, Wereldsma JC, van Vugt AB. Cannulated screws versus hemiarthroplasty for displaced intracapsular femoral neck fractures in demented patients. Ann Chir Gynaecol. 2000;89:132–137. [PubMed]
- 14.van Vugt AB, Oosterwijk WM, Goris RJ. Osteosynthesis versus endoprosthesis in the treatment of unstable intracapsular hip fractures in the elderly: a randomised clinical trial. Arch Orthop Trauma Surg. 1993;113:39–45. [DOI] [PubMed]
- 15.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. [DOI] [PubMed]
- 16.Wehren LE, Hawkes WG, Orwig DL, Hebel JR, Zimmerman SI, Magaziner J. Gender differences in mortality after hip fracture: the role of infection. J Bone Miner Res. 2003;18:2231–2237. [DOI] [PubMed]