Abstract
Polyethylene insert backside surface wear is implicated in osteolysis and failure of total knee arthroplasty. Manufacturing and sterilization methods reduce articular-sided wear. We questioned whether manufacturing technique influences the severity of backside wear. We examined 39 explanted tibial bearings in a blinded fashion using visual, stereomicroscopic, and scanning electron microscopic techniques. We examined 26 direct compression molded components and 13 nondirect compression molded components and applied a new backside wear severity score. The score characterized the magnitude of the various modes of wear with severity ranging from 0 (no wear) to 27 (severe wear). Time in vivo, tibial baseplate material, and manufacturing technique were used as variables for comparison. Backside wear was related to polyethylene manufacturing process with direct compression molded implants having a wear score of 2.3 and nondirect compression molded a score of 5.7. Time in vivo influenced backside wear, although direct compression molded predicted decreased backside wear independent of time in vivo. The data suggest manufacturing technique influences backside wear in total knee arthroplasty polyethylene inserts.
Introduction
Polyethylene wear is one of the leading causes for failure of TKA [23, 33]. Considered the “weakest link” in TKA, submicron polyethylene wear particles induce a cytokine-mediated phenomenon leading to periprosthetic osteolysis accounting for 25% of revision TKA [15, 18, 19]. Polyethylene wear in modular TKA occurs at the articular surface and backside of the insert. Although the articular surface of the polyethylene insert is the predominant source of wear debris, a growing number of reports have implicated backside wear as a major source of polyethylene wear [7, 13, 22, 28, 37].
Polyethylene is a semicrystalline polymer that provides a low-friction articular interface between metallic femoral and tibial components in modular TKA. As a modular component, polyethylene inserts afford great adaptability in customizing total knee implants for each patient while also providing excellent biocompatibility, low cost, and an effective bearing surface. The continual advancements in polyethylene technology have led to enhanced manufacturing techniques, which have resulted in improved wear performance characteristics [27]. Currently, polyethylene inserts are manufactured either by machining or by compression molding. Machined inserts are fashioned from an original bar stock into individual inserts of predetermined geometry. Compression-molded inserts are fabricated by directly conforming polyethylene resin to molds of predetermined geometry. Although earlier reports indicated compression-molded inserts had better articular-sided wear characteristics than machined components, limited data are available characterizing the impact of manufacturing technique on backside polyethylene wear [12, 35, 38]. An improved understanding of the relationship between polyethylene manufacturing and backside wear may facilitate improvements in polyethylene bearing design and development, which would improve implant survival and functional outcomes for patients with TKAs.
Our first objective was to develop a system of characterization that comprehensively analyzes the various modes of wear as well as the severity of wear using direct visualization, stereomicroscopy, and scanning electron microscopy. We then asked whether (1) the patterns and modes of backside polyethylene wear would be different between direct compression molded (DCM) and non-DCM inserts; (2) whether inserts with greater duration of implantation would demonstrate more extensive patterns of backside wear than inserts with less time in vivo; and (3) whether DCM inserts would incur less backside wear than non-DCM inserts regardless of time in vivo.
Materials and Methods
We examined 39 explanted tibial bearings of a single locking mechanism, fixed-bearing knee design (Maxim or Vanguard Complete Knee System; Biomet, Warsaw, IN). Twenty-nine polyethylene inserts were retrieved from components of the Maxim Complete Knee System and 10 inserts were from Vanguard Complete Knee System components. Varying levels of implant constraint were represented by cruciate retaining (n = 14), posterior stabilized (n = 17), and posterior stabilized-constrained (n = 8). Twenty-six of the inserts were fabricated with DCM, whereas 13 of the inserts were manufactured by sheet compression molding (non-DCM). DCM polyethylene is an insert fashioned by direct compression molding composed of very high-molecular-weight calcium stearate-free, medical-grade 1900H resin for Maxim designs (16 inserts) and GUR1050 resin for Vanguard designs (10 inserts). The mean thickness for all inserts was 12.6 mm and the mean duration of implantation for all inserts was 53.4 months. The mean duration of implantation was shorter (p < 0.0001) for DCM inserts (23.1 months) when compared with non-DCM inserts (114 months).
Non-DCM polyethylene is an insert fashioned by the machining of extruded bar stock of very high-molecular-weight calcium stearate-free, medical-grade 1900H resin. All DCM and non-DCM inserts were sterilized in an inert argon chamber. The tibial baseplate was composed of titanium with a matte finish and a modular keel design in 20 retrievals or composed of cobalt-chromium with a polished finish with a nonmodular keel design in 19 cases. The manufacturing technique, baseplate modularity, constraint, and knee design system were characterized for each retrieval insert (Fig. 1). Each insert was retrieved during revision arthroplasty in a standardized fashion from a single institution by the two senior authors (KRB, AVL). The inserts were obtained from 38 patients, 25 women and 13 men with an average age of 62.7 years at the time of implantation. The reasons for revision included infection (n = 19 [48.7%]), instability (n = 6 [15.4%]), patellar loosening (n = 5 [12.8%]), polyethylene wear (n = 3 [7.7%]), patella fracture (n = 1 [4%]), patella subluxation (n = 2 [5.1%]), tibial loosening (n = 1 [4%]), locking bar failure (n = 1 [4%]), and periprosthetic fracture (n = 1 [4%]). The mean weight of the patients at the time of implantation was 213.3 pounds and the mean height was 65.6 inches, yielding a mean body mass index of 34.9 kg/m2.
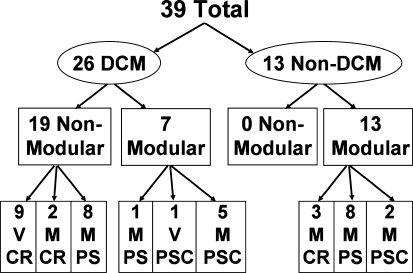
Fig. 1.
Thirty-nine retrieved bearings were examined. Twenty-six bearings were direct compression molded (DCM) and 13 bearings were non-DCM. Of the DCM bearings, there were 19 retrieved from nonmodular (Non-Mod) cobalt-chromium baseplates. Seven DCM bearings were retrieved from modular titanium tibial baseplates (Modular). Of the Non-Mod DCM bearings, nine were from Vanguard (V) and were all cruciate retaining (CR). Ten Non-Mod DCM bearings were from Maxim articulations (M). Of these, two were CR and eight were posterior stabilized (PS). Of the modular baseplate DCM bearings, one was M-PS, one V posterior stabilized-constrained (PSC), and five M-PSC. All 13 non-DCM bearings were retrieved from modular baseplates. Of these, there were three M-CR, eight M-PS, and two M-PSC.
A single modular polyethylene insert locking mechanism design was uniform among all samples with an anterior and posterior linear tongue-and-groove modular locking design (Fig. 2). Each fixed-bearing tibial baseplate contained a slide-in bar that compressed the polyethylene insert within the locking mechanism. All of the polyethylene insert samples were manufactured either by DCM or by sheet compression molding. Direct compression molding involves converting polyethylene resin into a finished component within an individual mold through heat and pressure consolidation. Sheet compression subjects polyethylene resin to heat and pressure consolidation; then, after a cooling process, large flats of polyethylene are machined into individual components. The backside locking mechanism of both DCM and non-DCM components are machined before sterilization and packaging. All inserts were composed of calcium stearate-free polyethylene, gamma-irradiated for sterilization, and packaged in an inert argon environment.
Fig. 2.
The Maxim and Vanguard Complete Knee System tibial baseplate incorporates a novel polyethylene locking mechanism. The anterior locking bar is designed to force molding of the polyethylene bearing into the locking mechanism, thus reducing backside wear.
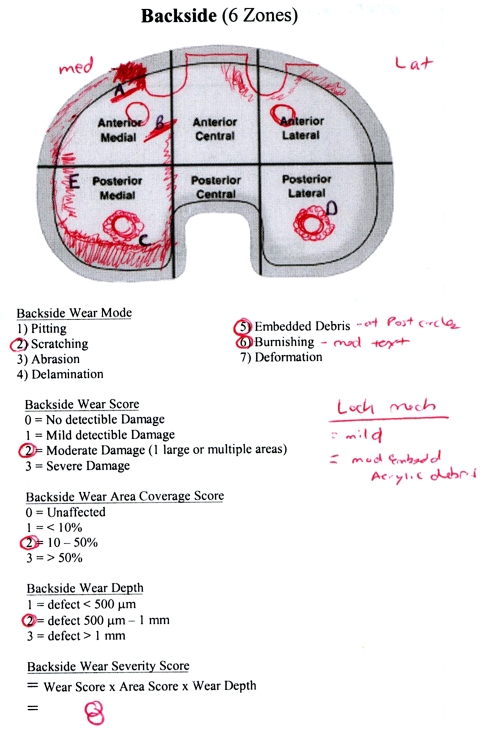
The protocol for backside wear assessment was developed to be as comprehensive as possible, synthesizing several previously described methods into a composite system of visual analysis and polyethylene wear characterization. The division of the baseplate into six topographic zones was originally outlined in an earlier report [29]. Briefly, this divides the backside of the polyethylene insert into six zones through a horizontal line dividing the baseplate into latitudinal halves and into thirds longitudinally (Fig. 3) [29]. Characterization of the various modes of wear was based on previous work that defined and described (1) pitting; (2) scratching; (3) abrasion; (4) delamination; (5) embedded debris; (6) burnishing; (7) deformation; (8) discoloration; and (9) fraying of locking mechanism [8–10, 20, 21, 25, 29, 30]. Pitting was defined as shallow, irregular voids. Scratching was defined as thin, shallow linear defects. Abrasion was defined as a shredded appearance. Delamination was defined as thin sheets of polyethylene separated from the surface. Embedded debris was identified when irregular material became pressed into the polyethylene surface. Burnishing was defined as heavily polished areas with loss of machining marks or text. Deformation was identified as irreversible cold flow structural changes or irregularities. Discoloration was defined as dark yellow tint representative of polyethylene oxidation. Fraying of the locking mechanism was defined by linear defects peeling off tongue-and-groove aperture.
Fig. 3.
Backside wear severity scores were calculated in a blinded fashion. The bearing is divided into six areas and the type and severity of wear recorded.
Topographic maps, as diagramed per Rao et al. [29] were developed for the backside of each polyethylene insert to characterize the location and magnitude of the various modes of wear. The overall severity of backside polyethylene wear was graded by direct visual analysis during blinded (BSE) inspection. Grades were assigned based on the overall severity of wear as negligible (0), mild (1), moderate (2), and severe (3) [10, 20, 25, 36]. Using the six zones, the area of polyethylene wear was then graded as negligible (0), less than 10% (1), between 10% and 50% (2), or greater than 50% (3) [14, 29, 36]. The depth of the major wear defect was then graded as less than 500 μm (1), between 500 μm and 1 mm (2), or greater than 1 mm (3) [8, 9, 25]. Depth measurements were obtained using stereomicroscopy and scanning electron microscopy for specific defects containing volumetric loss, specifically pitting, scratching, abrasion, delamination, and burnishing. Finally, a backside wear severity score (BWSS) was obtained for each polyethylene insert by multiplying all of the grades together (eg, overall wear grade × wear area grade × wear depth grade) [8, 9, 29, 34, 36]. By quantifying the overall BWSS for each polyethylene insert, numeric values could be ascribed to each insert (Fig. 3). The BWSS could then be compared with other variables associated with polyethylene wear to determine if relationships existed. Each mode was then expressed as a percentage representing the number of inserts depicting this mode of wear from the DCM or non-DCM insert subgroups. Backside wear was categorized as mild (BWSS 4 or less), moderate (BWSS greater than 4 and 8 or less), or severe (BWSS greater than 8).
The stereomicroscopic analysis was conducted in conjunction with the Center of Optics and Microscopy facility at The Ohio State University Department of Materials Sciences and Engineering. An Olympus high-resolution optical stereomicroscope connected to color videographic printers and imaging analysis system was used for microscopic measurements and characterization of polyethylene wear modes. Inspection with scanning electron microscopy (SEM) was performed using a Field Emission Imaging Quanta scanning electron microscope (FEI Company, Hillsboro, OR) that used a heated tungsten filament electron source operating within accelerating voltage ranges of 200 V to 30 kV in a vacuum environment. Electron backscatter pattern mapping was used to provide ultrahigh-resolution imaging analysis of different modes of backside polyethylene wear. The modes of wear characterized included pitting, scratching, abrasion, delamination, and burnishing. Measurements of width and depth were obtained to define volumetric loss using metric scale preconfigured for designated magnification using the scanning electron microscope. Stereomicroscopic and SEM analyses of the various modes of wear were based on techniques previously described [8–10, 21, 29, 34].
A grouped linear regression analysis was performed to determine the level of association among independent variables, including manufacturing technique, baseplate modularity, and time in vivo. Specifically, time in vivo was used for covariance to obviate the effect of differing in vivo times. Multiple linear regression analysis and covariance analysis were conducted to determine independence of variables, including manufacturing technique, baseplate modularity, and time in vivo, that were associated with backside wear. A Student paired t test was used to determine differences in the mean BWSS between DCM and non-DCM inserts and between modular and nonmodular baseplates. A power analysis was performed to determine adequate sample size at a power of 80% and p = 0.05 for each comparison. Post hoc power analysis was performed to estimate the actual beta error of each statistical analysis that did not achieve statistical significance. Sample sizes and effect size from the test results were evaluated and a percentage beta error was calculated and is reported. Stats Direct software (StatsDirect, Cheshire, UK) was used to perform these functions.
Results
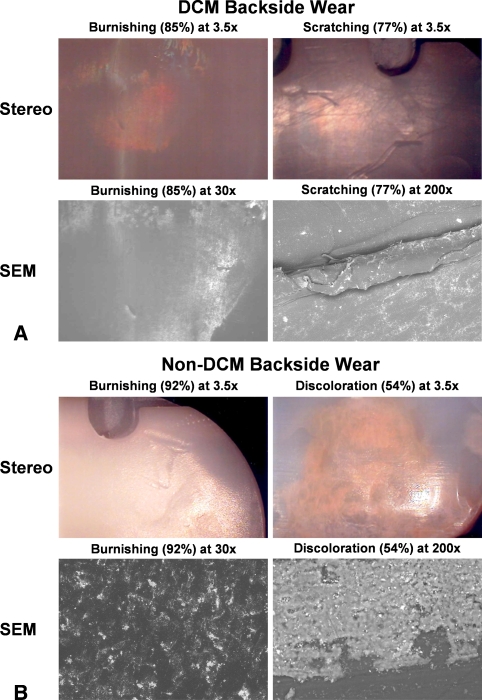
The modes and patterns of backside wear were distinctly different for DCM and non-DCM inserts. The typical pattern and geographic distribution of backside wear for the DCM polyethylene insert was primarily fine burnishing localized to the central-most regions with relative peripheral sparing or absence of wear (Fig. 4A). Overall, the predominant modes of backside wear for DCM inserts were burnishing (84.6%), scratching (76.9%), fraying of the locking mechanism (69.2%), and pitting (26.9%). A minimal amount of abrasion and embedded debris was identified and no delamination, deformation, or discoloration was identified for DCM inserts. In contrast, the typical pattern and geographic distribution of backside wear for non-DCM inserts was more evenly distributed across central and peripheral zones with apparent combined modes of wear, specifically course burnishing, peripheral cold wear deformation at edges circumferentially, more yellowish discoloration, and more extensive fraying of the locking mechanism (Fig. 4B). Neither DCM nor non-DCM inserts demonstrated appreciable differences between medial- and lateral-sided wear. For non-DCM inserts, the predominant modes of wear were burnishing (92.3%), fraying of the locking mechanism (69.2%), scratching (61.5%), and discoloration (53.8%). A minimal amount of pitting (15.4%), embedded debris (15.4%), and deformation (7.7%) was observed and no abrasion or delamination was identified for the non-DCM inserts. Although scratching was identified as linear striations of an approximate width of 60 to 80 μm and was apparent for both DCM and non-DCM inserts, this was difficult to interpret because scratching was more likely a consequence of extraction technique during revision surgery rather than a true mode of backside wear. However, because true cause and effect could not be determined, scratching was included for our analyses. With advanced backside wear, the linear striations were obliterated and supplanted by irregular foci of adhesive wear patterns as a result of contact interference with the tibial baseplate. For DCM inserts, the predominant mode of wear was burnishing, which under SEM appeared as groupings of circular adhesive wear patterns. As was identified with lower magnification, a relative peripheral-sparing pattern was observed with greater representation of wear localized more centrally. For non-DCM inserts, the linear striations were more greatly disrupted by relatively large areas of irregular-shaped adhesive wear patterns arranged in a more diffuse pattern than observed with DCM inserts. With more advanced backside wear identified in the non-DCM inserts, the flat surface became supplanted by coarse speculated arrangements of polyethylene.
Fig. 4A–B.
(A) An example of a direct compression molded (DCM) retrieval is shown. Stereomicroscopic images (stereo) and scanning electron microscopic images (SEM) demonstrating burnishing and scratching are shown. (B) An example of a nondirect compression molded component is shown. Burnishing and discoloration were prevalent in the non-DCM bearings. Examples of these wear types are given.
Using direct visual analysis, DCM inserts incurred less (p = 0.03) backside wear than non-DCM inserts regardless of time in vivo. With stereomicroscopic analysis, the overall severity of backside wear was also increased (p = 0.04) among non-DCM inserts with a mean BWSS of 5.7 compared with 2.3 for DCM inserts. Mild wear was demonstrated by 100% of DCM inserts examined by direct visualization and 96.6% of the DCM bearings examined by stereomicroscopy. For non-DCM inserts, mild backside wear was identified for 61.5% of samples examined by direct visualization and stereomicroscopy. Moderate backside wear was observed for 15.4% of non-DCM inserts examined by direct visualization and stereomicroscopy. Severe backside wear was observed for 23.1% of non-DCM inserts examined by direct visualization and stereomicroscopy. Collectively, the backside wear severity scores with this novel polyethylene locking mechanism were generally mild for both DCM and non-DCM inserts, demonstrating excellent prevention of backside micromotion.
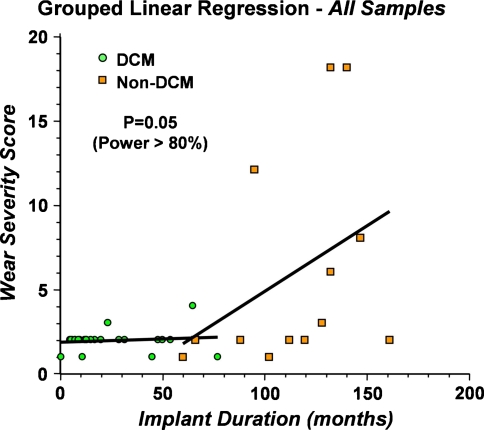
Accounting for time in vivo, a grouped linear regression analysis revealed DCM inserts incurred less backside polyethylene wear than non-DCM inserts independent of duration of implantation. A linear projection of best-fit probability for backside wear revealed two independent slopes for DCM and non-DCM inserts (Fig. 5). The common slope for DCM inserts was 0.004 and the common slope for non-DCM inserts was 0.094, indicating the BWSS was increased (p = 0.05) for non-DCM inserts compared with DCM inserts independent of duration of implantation. Additionally, when time in vivo is taken into account, we observed no difference (p > 0.05) between BWSS between various levels of constraint or baseplate modularity.
Fig. 5.
Grouped linear regression analysis with time as the covariant demonstrates a difference in wear between direct compression molded (DCM) and non-DCM bearings. Adequate power to detect a difference was present.
Discussion
In our study, we developed a new system of polyethylene wear characterization that comprehensively analyzes the various modes of backside wear and applied this technique to compare and contrast DCM and non-DCM polyethylene inserts from retrieved samples. We then asked whether (1) the patterns and modes of backside polyethylene wear would be different between DCM and non-DCM inserts; (2) whether inserts with greater duration of implantation would demonstrate more extensive patterns of backside wear than inserts with less time in vivo; and (3) whether DCM inserts would incur less backside wear than non-DCM inserts regardless of time in vivo.
One of the limitations of our study is the total sample size of our DCM and non-DCM retrieval inserts was relatively small. Also, as a result of the confines of a retrieval polyethylene analysis comparing manufacturing technique, duration of implantation cannot be controlled as part of the experimental design and must be accounted for with statistical models. Although the average time in vivo differed between DCM and non-DCM inserts, a multiple linear regression analysis and covariance analysis were performed to account for this difference in duration of implantation. Specifically, time in vivo was used for covariance to obviate the effect of differing durations of implantation. The lack of DCM inserts with a long-term period of implantation and the lack of non-DCM inserts without a short-term period of implantation does complicate comparative analysis. Although the use of regression and covariance analysis accounts for the discrepancy in duration of implantation for DCM and non-DCM inserts, ideally, retrieval inserts in both DCM and non-DCM categories would be representative of short-term and long-term periods of time in vivo. Without having any DCM inserts with long-term periods of implantation in this study, it is difficult to predict exactly how the improved performance in backside wear observed with DCM inserts with short-term periods of implantation will translate into long-term periods of implantation. It is our expectation, as guided by regression and covariance analysis along with other studies with similar conclusions, DCM inserts will continue to incur less backside wear than non-DCM inserts over time indefinitely [1]. Other factors, including mechanical alignment, component instability patient activity, and surface roughness of the tibial baseplate, likely impact the severity of backside polyethylene wear but were outside the scope of this focused study. One of the particular advantages of the inserts we analyzed is that in sharing a common locking mechanism, the difference in backside wear observed between DCM and non-DCM machined inserts cannot be attributed to differences in locking mechanism design. Rather, it is expected the amount of backside wear for DCM and non-DCM machined inserts from our study was generated with near equivalent levels of micromotion between the insert undersurface and tibial baseplate for each sample.
We found mild backside wear for all DCM inserts and moderate backside wear for all non-DCM inserts. The patterns and modes of backside wear for DCM and non-DCM inserts demonstrated subtle distinctions. Although discoloration was present only in non-DCM inserts, burnishing, scratching, and fraying of the locking mechanism were the three most common modes of backside wear for both DCM and non-DCM inserts. Additionally, only mild backside wear was identified for nearly every DCM insert; however, 38.5% of non-DCM inserts demonstrated moderate or severe wear. In summary, with these results, we conclude DCM polyethylene is superior to non-DCM machined polyethylene in reducing backside wear regardless of time in vivo.
Patterns of backside wear have been described in earlier reports and are distinctly different from the types of polyethylene wear observed at the articular surface. With the contact stresses transmitted during physiological loading, the severity of articular wear is most influenced by the quality of the polyethylene, level of implant constraint, insert thickness, and coronal tibiofemoral alignment [4, 26, 30, 36]. The axial rolling, sliding, and rotation forces generate articular-sided polyethylene wear debris that is relatively larger in size as a byproduct of subsurface cracking, delamination, and pitting [4, 30]. Backside wear patterns are typically different from articular wear patterns in modular TKA. In our study, DCM inserts most often demonstrated burnishing (85%), scratching (77%), and fraying of the locking mechanism (69%) without any overt discoloration. The non-DCM inserts shared the presence of burnishing (92%), scratching (62%), and fraying of the locking mechanism (69%) with the majority of inserts demonstrating a yellow permeative discoloration (54%). These modes of wear have similarly been observed in other reports of backside wear characterized for retrieved inserts from different implants [9–11, 22, 36].
Burnishing was the most pervasive mode of backside wear among DCM and non-DCM explants. The size of the wear debris particles produced by burnishing is much smaller (ranging from 0.32 to 0.35 μm) than articular-sided wear debris, which typically ranges from 1 to 2 mm in diameter [24, 30, 32]. The similarity in size of wear debris at the backside of modular inserts with THA reflects the similar mechanism of adhesive and abrasive wear that occurs under lower contact stresses. In comparing wear debris from failed THAs and TKAs, one report identified a large range in size of polyethylene wear debris [17]. Yet, the mean diameter of particles smaller than 10 μm was 0.72 μm in total knee samples with the number of particles ranging from 6.7 × 108 to 2.1 × 1010 [17]. Although the larger flakes associated with articular-sided wear are visually impressive, other reports have noted the majority of wear particles were submicron [31, 34]. In addition to the smaller size of backside wear debris, the total volume of wear is important. Conditt et al. [10] demonstrated a volumetric wear rate of 138 mm3 per year, which was comparable to severe wear of acetabular components. Thus, with the high volume of submicron wear debris particles that characterizes backside wear, a growing concern is raised about the association of backside wear with periprosthetic osteolysis in modular knee designs.
Processing of polyethylene has undergone critical changes in terms of fabrication and sterilization techniques over the past three decades. Currently, polyethylene in modular TKA is manufactured either by compression molding or machining. From our results, DCM inserts demonstrated less backside wear than non-DCM inserts independent of time in vivo. In several studies, compression-molded polyethylene components have exhibited better articular-sided wear characteristics than machined components, indicating compression-molded polyethylene is better suited for clinical applications [2, 12, 24, 35, 38]. The sterilization process has also improved since Bohl et al. demonstrated gamma irradiation in an oxygen environment resulted in poor wear characteristics [3]. Other studies have likewise demonstrated sterilization techniques that minimize free oxygen radicals using either ethylene oxide gas or gamma irradiation in an inert environment enhance wear resistance [5–7, 16].
Backside polyethylene wear has been identified as a major source of wear debris resulting from progressive polyethylene breakdown secondary to motion between the insert and tibial baseplate. Several factors have been identified that influence backside wear, including insert locking mechanism, tibial baseplate surface, implant duration, patient activity level, and tibiofemoral alignment [6, 7, 26, 29, 34]. Modular insert locking designs may use a mechanism of full peripheral capture, partial capture, full tongue-and-groove, or partial tongue-and-groove. Despite the differences in design, micromotion has been observed with all of these designs of locking mechanisms, at least 500 μm in the transverse plane with a 400 N load [28]. In our study, a single partial tongue-and-groove design intended to partially mold the polyethylene onto the locking mechanism when a locking bar is inserted was characteristic of all of the retrieved inserts (Fig. 2). Previous reports have demonstrated the locking mechanism used by the inserts from our study has the least amount of micromotion as a result of a constant compressive fit of the polyethylene onto the tibial baseplate [28]. This is one possible explanation for the remarkably low backside wear seen in all of the current retrievals.
DCM polyethylene inserts demonstrated less backside wear than non-DCM polyethylene inserts independent of time in vivo. Although the modes of wear at the backside surface were similar for both DCM and non-DCM inserts, the non-DCM inserts demonstrated more pervasive discoloration and overall more severe wear scores. Many improvements in polyethylene processing have occurred in the past decade that undoubtedly have addressed many of the problems associated with backside wear, including better crosslinking, ultrahigh-molecular-weight substrates, inert environment sterilization, and clinically proven locking mechanisms. Yet, backside wear still occurs in modular TKA and concern remains that backside wear may generate polyethylene wear debris that may compromise clinical outcomes for patients undergoing TKA. It would appear the choice of implanting DCM polyethylene in modular TKA is supported by both clinical data and the aforementioned reduction in backside wear.
Footnotes
One or more of the authors (AVL, KRB) has received institutional research funding, consulting income, and/or royalties from a commercial interest related to the subject of the manuscript (Biomet, Inc). One or more of the authors (BSE) has received institutional research or grant funding from the Ohio State University Department of Orthopaedics in support of this research.
Each author certifies that his institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bankston AB, Cates H, Ritter MA, Keating EM, Faris PM. Polyethylene wear in total hip arthroplasty. Clin Orthop Relat Res. 1995;317:7–13. [PubMed]
- 2.Benson LC, DesJardins JD, LaBarge M. Effects of in vitro wear machined and molded UHMWPE tibial inserts on TKR kinematics. J Biomed Mater Res. 2001;58:496–504. [DOI] [PubMed]
- 3.Bohl JR, Bohl WR, Postak PD, Greenwald AS. The Coventry Award: the effects of shelf life on clinical outcome for gamma sterilized polyethylene tibial components. Clin Orthop Relat Res. 1999;367:28–38. [DOI] [PubMed]
- 4.Collier JP, Mayor MB, McNamara JL, Surprenant VA, Jensen RE. Analysis of the failure of 122 polyethylene inserts from uncemented tibial knee components. Clin Orthop Relat Res. 1991;273:232–242. [PubMed]
- 5.Collier JP, Sutula LC, Currier BH, Currier JH, Wooding RE, Williams IR, Farber KB, Mayor MB. Overview of polyethylene as a bearing material: comparison of sterilization methods. Clin Orthop Relat Res. 1996;333:76–86. [DOI] [PubMed]
- 6.Collier MB, Engh CA Jr, McAuley JP, Engh GA. Factors associated with the loss of thickness of polyethylene tibial bearings after total knee arthroplasty. J Bone Joint Surg Am. 2007;89:1306–1314. [DOI] [PubMed]
- 7.Collier MB, Engh CA Jr, McAuley JP, Ginn SD, Engh GA. Osteolysis after total knee arthroplasty: influence of tibial baseplate surface finish and sterilization of polyethylene insert. J Bone Joint Surg Am. 2005;87:2702–2708. [DOI] [PubMed]
- 8.Conditt MA, Ismaily SK, Alexander JW, Noble PC. Backside wear of ultrahigh molecular weight polyethylene tibial inserts. J Bone Joint Surg Am. 2004;86:1031–1037. [DOI] [PubMed]
- 9.Conditt MA, Stein JA, Noble PC. Factors affecting the severity of backside war of modular tibial inserts. J Bone Joint Surg Am. 2004;86:306–311. [DOI] [PubMed]
- 10.Conditt MA, Thompson MT, Usrey MM, Ismaily SK, Noble PC. Backside wear of polyethylene tibial inserts: Mechanism and magnitude of material loss. J Bone Joint Surg Am. 2005;87:326–331. [DOI] [PubMed]
- 11.Cuckler JM, Lemons J, Tamarapalli JR, Beck P. Polyethylene damage on the nonarticular surface of modular total knee prostheses. Clin Orthop Relat Res. 2003;410:248–253. [DOI] [PubMed]
- 12.Currier BH, Currier JH, Collier, Mayor MB. Effect of fabrication method and resin type on performance of tibial bearings. J Biomed Mater Res. 2000;53:143–151. [DOI] [PubMed]
- 13.Engh GA, Ammeen DJ. Epidemiology of osteolysis: backside implant wear. Instr Course Lect. 2004;53:243–249. [PubMed]
- 14.Engh GA, Dwyer KA, Hanes CK. Polyethylene wear of metal-backed tibial components in total and unicompartmental knee prostheses. J Bone Joint Surg Br. 1992;74:9–17. [DOI] [PubMed]
- 15.Engh GA, Lounici S, Rao AR, Collier MB. In vivo deterioration of tibial baseplate locking mechanisms in contemporary modular total knee components. J Bone Joint Surg Am. 2001;83:1660–1665. [DOI] [PubMed]
- 16.Fehring TK, Murphy JA, Hayes TD, Roberts DW, Pomeroy DL, Griffin WL. Factors influencing wear and osteolysis in press-fit condylar modular total knee replacements. Clin Orthop Relat Res. 2004;428:40–50. [DOI] [PubMed]
- 17.Hirakawa K, Bauer TW, Stulberg BN, Wilde AH. Comparison and quantitation of wear debris of failed total hip and knee arthroplasty. J Biomed Mater Res. 1996;31:257–263. [DOI] [PubMed]
- 18.Holding CA, Findlay DM, Stamenkov R, Neale SD, Lucas H, Dharmapatni AS, Callary SA, Shrestha KR, Atkins GJ, Howie DW, Haynes DR. Correlation of RANK, RANKL, and TNF-alpha expression with bone loss volume and polyethylene wear debris around hip implants. Biomaterials. 2006;27:5212–5219. [DOI] [PubMed]
- 19.Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;460:240–252. [DOI] [PubMed]
- 20.Hood RW, Wright TM, Burstein AH. Retrieval analysis of total knee prosthesis. J Biomed Mater Res. 1983;17:829–842. [DOI] [PubMed]
- 21.Landy MM, Walker PS. Wear of ultra-high-molecular-weight polyethylene components of 90 retrieved knee prostheses. J Arthroplasty. 1998;3:S73–85. [DOI] [PubMed]
- 22.Li S, Scuderi G, Furman BD, Bhattacharyya S, Schmieg JJ, Insall JN. Assessment of backside wear from the analysis of 55 retrieved tibial inserts. Clin Orthop Relat Res. 2002;404:75–82. [DOI] [PubMed]
- 23.Lonner JH, Siliski JM, Scott RD. Prodromes of failure of total knee arthroplasty. J Arthroplasty. 1999;14:488–492. [DOI] [PubMed]
- 24.Mueller-Rath R, Kleffner B, Andereya S, Mumme T, Wirtz DC. Measures for reducing ultra-high-molecular-weight polyethylene wear in total knee replacement: a simulator study. Biomed Tech (Berl). 2007;52:295–300. [DOI] [PubMed]
- 25.Muratoglu OK, Ruberti J, Melotti S, Spiegelberg SH, Greenbaum BA, Harris WH. Optical analysis of surface changes on early retrievals of highly cross-linked and conventional polyethylene tibial inserts. J Arthroplasty. 2003;18:42–47. [DOI] [PubMed]
- 26.Naudie DD, Ammeen DJ, Engh GA, Rorabeck CH. Wear and osteolysis around total knee arthroplasty. J Am Acad Orthop Surg. 2007;15:53–64. [DOI] [PubMed]
- 27.Naudie DD, Rorabeck CH. Sources of osteolysis around total knee arthroplasty: wear of the bearing surface. Instr Course Lect. 2004;53:251–259. [PubMed]
- 28.Parks NL, Engh GA, Topoleski LD, Emperado J. The Coventry Award: modular tibial insert micromotion. A concern with contemporary knee implants. Clin Orthop Relat Res. 1998;356:10–15. [PubMed]
- 29.Rao AR, Engh GA, Collier MB, Lounici S. Tibial interface wear in retrieved total knee components and correlations with modular insert motion. J Bone Joint Surg Am. 2002;84:1849–1855. [DOI] [PubMed]
- 30.Schmalzried TP, Callaghan JJ. Wear in total hip and knee replacements. J Bone Joint Surg Am. 1999;81:115–136. [DOI] [PubMed]
- 31.Schmalzried TP, Campbell P, Schmitt AK, Brown IC, Amstutz HC. Shapes and dimensional characteristics of polyethylene wear particles in vivo by total knee replacements compared to total hip replacements. J Biomed Mater Res. 1997;38:203–210. [DOI] [PubMed]
- 32.Shanbhag AS, Bailey HO, Hwang DS, Cha CW, Eror NG, Rubash HE. Quantitative analysis of ultrahigh molecular weight polyethylene (UHMWPE) wear debris associated with total knee replacements. J Biomed Mater Res. 2000;53:100–110. [DOI] [PubMed]
- 33.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM: Insall Award Paper: why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. [DOI] [PubMed]
- 34.Taki N, Goldberg VM, Kraay MJ, Rimnac CM. Backside wear of Miller-Galante I and Insall-Burstein II tibial inserts. Clin Orthop Relat Res. 2004;428:198–206. [DOI] [PubMed]
- 35.Tanner MG, Whiteside LA, White SE. Effect of polyethylene quality on wear in total knee arthroplasty. Clin Orthop Relat Res. 1995;317:83–88. [PubMed]
- 36.Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31–43. [PubMed]
- 37.Wasielewski RC, Parks N, Williams I, Suprenant H, Collier JP, Engh G. Tibial insert undersurface as a contributing source of polyethylene wear debris. Clin Orthop Relat Res. 1997;345:53–59. [DOI] [PubMed]
- 38.Won CH, Rohatagi S, Kraay MJ, Goldberg VM, Rimnac CM. Effect of resin type and manufacturing method on wear of polyethylene tibial components. Clin Orthop Relat Res. 2000;376:161–171. [DOI] [PubMed]