Abstract
Attachment of HeLa cells to gelatin induces the release of arachidonic acid (AA), which is essential for cell spreading. HeLa cells spreading in the presence of extracellular Ca2+ released more AA and formed more distinctive lamellipodia and filopodia than cells spreading in the absence of Ca2+. Addition of exogenous AA to cells spreading in the absence of extracellular Ca2+ restored the formation of lamellipodia and filopodia. To investigate the role of cytosolic phospholipase A2 (cPLA2) in regulating the differential release of AA and subsequent formation of lamellipodia and filopodia during HeLa cell adhesion, cPLA2 phosphorylation and translocation from the cytosol to the membrane were evaluated. During HeLa cell attachment and spreading in the presence of Ca2+, all cPLA2 became phosphorylated within 2 min, which is the earliest time cell attachment could be measured. In the absence of extracellular Ca2+, the time for complete cPLA2 phosphorylation was lengthened to <4 min. Maximal translocation of cPLA2 from cytosol to membrane during adhesion of cells to gelatin was similar in the presence or absence of extracellular Ca2+ and remained membrane associated throughout the duration of cell spreading. The amount of total cellular cPLA2 translocated to the membrane in the presence of extracellular Ca2+ went from <20% for unspread cells to >95% for spread cells. In the absence of Ca2+ only 55–65% of the total cPLA2 was translocated to the membrane during cell spreading. The decrease in the amount translocated could account for the comparable decrease in the amount of AA released by cells during spreading without extracellular Ca2+. Although translocation of cPLA2 from cytosol to membrane was Ca2+ dependent, phosphorylation of cPLA2 was attachment dependent and could occur both on the membrane and in the cytosol. To elucidate potential activators of cPLA2, the extracellular signal-related protein kinase 2 (ERK2) and protein kinase C (PKC) were investigated. ERK2 underwent a rapid phosphorylation upon early attachment followed by a dephosphorylation. Both rates were enhanced during cell spreading in the presence of extracellular Ca2+. Treatment of cells with the ERK kinase inhibitor PD98059 completely inhibited the attachment-dependent ERK2 phosphorylation but did not inhibit cell spreading, cPLA2 phosphorylation, translocation, or AA release. Activation of PKC by phorbol ester (12-O-tetradecanoylphorbol-13-acetate) induced and attachment-dependent phosphorylation of both cPLA2 and ERK2 in suspension cells. However, in cells treated with the PKC inhibitor Calphostin C before attachment, ERK2 phosphorylation was inhibited, whereas cPLA2 translocation and phosphorylation remained unaffected. In conclusion, although cPLA2-mediated release of AA during HeLa cell attachment to a gelatin substrate was essential for cell spreading, neither ERK2 nor PKC appeared to be responsible for the attachment-induced cPLA2 phosphorylation and the release of AA.
INTRODUCTION
Arachidonic acid (AA) and its respective eicosonoid metabolites play a crucial role in a diverse set of signaling processes, including inflammation and cell migration (Piomelli, 1993). Preferentially incorporated into the sn2 position of glycerophospholipids, AA can be released by the phospholipase A2 (PLA2) family of enzymes. In mammalian cells there are currently three known classes of PLA2 (Dennis, 1994). One class is the 14-kDa secretory PLA2 (sPLA2) that is expressed in a variety of cell types (Kudo et al., 1993). It has no preference for AA at the sn2 position, requires millimolar amounts of Ca2+ for activity, and is sensitive to reducing agents (Kramer et al., 1990a,b). The second class of PLA2 is the recently discovered Ca2+-independent PLA2 (iPLA2). This enzyme prefers plasmalogen substrates and does not appear to have a preference for the type of fatty acid at the sn2 position (Gross et al., 1993; Ackermann et al., 1994; Tang et al., 1997). Furthermore, iPLA2 does not require Ca2+ for catalytic activity (Ross et al., 1985; Wolf and Gross, 1985). The modes of regulation and physiological importance of iPLA2 have not been elucidated. A third class of PLA2 is the Ca2+-dependent cytosolic PLA2 (cPLA2) (Kramer, 1994; Leslie, 1997). The structure of cPLA2 is unique and reveals no sequence homology to sPLA2 (Clark et al., 1991; Sharp et al., 1991) or iPLA2 (Tang et al., 1997). cPLA2 provides a likely mode of AA release in many systems, because it preferentially cleaves AA at the sn2 position of phospholipids (Clark et al., 1990; Diez and Mong, 1990; Gronich et al., 1990; Kim et al., 1991; Wijkander and Sundler, 1991).
Calcium and phosphorylation play important roles in the regulation of cPLA2. Calcium appears to enhance AA release by facilitating cPLA2 association with membrane phospholipids (Channon and Leslie, 1990; Diez and Mong, 1990; Clark et al., 1991; Rehfeldt et al., 1993). In addition to translocation, cPLA2 is regulated by phosphorylation (Kramer et al., 1993; Currie et al., 1994), particularly on serine residues (Lih-ling et al., 1992, 1993; Kramer et al., 1995; Schalkwijk et al., 1995). cPLA2 has consensus sites for phosphorylation by protein kinase C (PKC), protein kinase A, casein kinase II, and tyrosine kinases (Kemp and Pearson, 1990; Sharp et al., 1991). In Chinese hamster ovary (CHO) cells, extracellular-related protein kinase 2 (ERK2) has been shown to phosphorylate cPLA2 at Ser505 in vivo and in vitro, increasing its activity by ∼1.5-fold (Kramer et al., 1995). Although ERK2 is responsible for the phosphorylation of cPLA2 in many systems (Nemenoff et al., 1993; Durstin et al., 1994; Clark and Hynes, 1996; Kan et al., 1996), there have also been reports of ERK2-independent pathways of cPLA2 activation (Kemp and Pearson, 1990; Qiu and Leslie, 1994; Waterman and Sha’afi, 1995). In addition to ERK2, cPLA2 can be activated by PKC-dependent (Lih-ling et al., 1993; Durstin et al., 1994; Kramer et al., 1995; Waterman and Sha’afi 1995; Xing and Insel, 1996; Teslenko et al., 1997) and PKC-independent (Durstin et al., 1994; Börsch-Haubold et al., 1995; Waterman and Sha’afi, 1995) mechanisms.
Cell adhesion plays an important role in migration, angiogenesis, and tumor metastasis (Huttenlocher et al., 1995). These complex interactions are initiated in part by receptor-mediated events involving integrin (Hynes, 1992) as well as non-integrin cell surface molecules (Lu et al., 1992). Previous studies have shown that integrin clustering during HeLa cell attachment to a gelatin substrate induces a release of AA (Chun and Jacobson, 1992). AA release is essential for cell spreading on a collagen or gelatin substrate, because inhibition of PLA2-dependent spreading can be overcome by the addition of exogenous AA (Chun and Jacobson, 1992; Auer and Jacobson, 1995). Furthermore, the percent of HeLa cells that spread on a gelatin substrate is proportional to the amount of AA released (Chun and Jacobson, 1993). Although it is evident that cPLA2 is involved in the intracellular release of AA in response to soluble agonists, little is known about its regulation in response to cell–substrate adhesion. To address this question, the role of cPLA2 translocation and phosphorylation in the modulation of AA release upon HeLa cell attachment and spreading on a gelatin substrate was investigated. The involvement of cPLA2 on HeLa cell spreading was selectively investigated based on the following observations: 1) HeLa cells spread in the absence of millimolar Ca2+ (Beacham and Jacobson, 1990) that is required for sPLA2 activity (Kramer et al., 1990a,b); and 2) unlike iPLA2 or sPLA2, cPLA2 preferentially hydrolyzes arachidonyl-containing phospholipids (Clark et al., 1990; Gronich et al., 1990; Diez and Mong, 1990; Kim et al., 1991; Wijkander and Sundler, 1991) to release AA required for cell spreading (Chun and Jacobson, 1992).
The data show that in the presence of extracellular Ca2+ more AA is released than in Ca2+-free medium. Although HeLa cells are able to spread on gelatin in the absence of extracellular Ca2+, the increased amount of AA released in the presence of Ca2+ had distinct morphological consequences in that more filopodia and lamellipodia were observed. In part, this can be explained by an increase in the amount of cPLA2 that translocated to the membrane and an increased rate of phosphorylation. Calcium by itself, however, was not able to promote cPLA2 phosphorylation; instead phosphorylation of cPLA2 appeared to be attachment dependent and could occur when the enzyme was membrane bound or in the cytosol. Additional data indicate ERK2 was phosphorylated upon attachment to gelatin, but unlike other systems (Nemenoff et al., 1993; Durstin et al., 1994; Kan et al., 1996), ERK2 did not appear to be related to cPLA2 activation, translocation, or cell spreading in HeLa cells. Stimulation of PKC by phorbol ester was found to phosphorylate cPLA2 in suspension cells; however, the adhesion-induced phosphorylation and translocation of cPLA2 was PKC independent. Regulation of cPLA2 activity by translocation to the membrane and phosphorylation that is not dependent on ERK2 or PKC seems to be critical for the regulation of HeLa cell adhesion to gelatin and the morphology of the spread cells.
MATERIALS AND METHODS
Materials
Tritiated AA was purchased from American Radiolabeled Chemicals (St. Louis, MO). ERK kinase (MEK) kinase inhibitor PD98059 was obtained from Calbiochem (La Jolla, CA). Ionomycin and bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM) were purchased from Biomol (Plymouth Meeting, PA). Mouse anti-human cPLA2 was from Santa Cruz Biotechnology (Santa Cruz, CA), and mouse anti-human ERK2 was from Transduction Laboratories (Lexington, KY). Mouse anti-human phospho-ERK was purchased from New England Biolabs (Beverly, MA). Purified cPLA2 was generously donated by Dr. Ruth Kramer (Eli Lilly Research Laboratories, Indianapolis, IN). ECL reagent was purchased from Amersham (Arlington Heights, IL). Polystyrene dishes for AA release and spreading assays were from Fisher Scientific (Medford, MA). Nitrocellulose for immunoblotting was obtained from Schleicher & Schuell (Keene, NH). Electrophoresis grade acrylamide and bisacrylamide were purchased from ICN (Costa Mesa, CA). All other chemicals including goat anti-mouse IgG-HRP and potato acid phosphatase were obtained from Sigma (St. Louis, MO).
Cell Culture and Spreading Assay
HeLa-S3 cells were grown in suspension to midlog phase (2–3 × 105 cells/ml) in RPMI 1640 medium (Sigma) supplemented with 0.3% NaHCO3, 100 μg/ml dihydrostreptomycin, 60 μg/ml penicillin, 0.002% butyl parahydroxybenzoate, and 5% normal calf serum (Intergen, Purchase, NY). For spreading assays HeLa cells were centrifuged at 300 × g for 5 min, washed twice, and resuspended in PBS containing 1 mM MgCl2 (PBS-Mg), plus either 1 mM CaCl2 or 2 mM EGTA for calcium-free conditions. After the appropriate treatment, cells were plated onto polystyrene dishes covalently coupled to gelatin at 37°C as previously described (Lu et al., 1992). At the appropriate time, HeLa cells were observed by phase-contrast microscopy (Nikon Diaphot-TMD inverted microscope) at 40×, and pictures were taken using an attached Nikon N6000 camera. For determination of spreading rate, cells (at least 50) were counted in triplicate at the indicated times from no less than three fields of view. A spread cell is defined as one in which the cell size is twice that of the nucleus. For determination of cell perimeter and cell area, digital images were analyzed using NIH Image software (National Institutes of Health, Bethesda, MD).
Cell Attachment Assay
HeLa cells were centrifuged at 300 × g for 5 min, washed twice, and resuspended in PBS-Mg plus either 1 mM CaCl2 or 2 mM EGTA for calcium-free conditions. Before plating cells were treated with 1 mg/ml BSA to prevent nonspecific binding. HeLa cells (∼0.5 × 106) were allowed to attach at room temperature to 35-mm gelatinized dishes of varying concentrations. At 10 min the cells were agitated for 5 s, the medium was decanted, and cells were scored for attachment. The data are normalized to cells attached to dishes that were coated with gelatin at a concentration of 1 mg/ml. This concentration of gelatin is sufficient to saturate the dish surface (Lu et al. 1992).
AA Release Assay
HeLa cells at a density of 2–3 × 105 cells/ml were labeled with [3H]AA at a final concentration of 0.25 μCi/ml for 12–16 h in serum-containing medium. It has been previously shown that >90% of the AA is incorporated into cellular phospholipids under these conditions (Chun and Jacobson, 1993). Cells were centrifuged at 300 × g for 5 min and washed with PBS-Mg plus 2 mM EGTA or 1 mM Ca2+. After two rounds of washing, cells were plated onto 60-mm gelatinized polystyrene dishes at a concentration of 2 × 106 cells per dish at 37°C. At the indicated times, 300-μl aliquots were taken from each dish and replaced with 300 μl of fresh buffer. Aliquots were microfuged for 2 min, and three 40-μl samples were taken for scintillation counting (LS3801 counter; Beckman Instruments, Palo Alto, CA). Raw data are represented as counts per minute per dish with SDs from four independent experiments. The zero time point reflects background counts before attachment and spreading. At the end of each experiment, dishes were observed by phase-contrast microscopy to ensure that similar amounts of cells from each treatment were attached and spread.
cPLA2 Mobility Shift and Translocation Assay
HeLa cells were washed once in PBS-Mg buffer plus 2 mM EGTA or 1 mM Ca2+ and plated onto 100-mm gelatinized polystyrene dishes at a concentration of 1 × 107 cells per dish at 37°C. At the indicated times each dish was washed two times with PBS-Mg containing 1 mM EGTA to remove excess Ca2+ and unbound cells. One hundred microliters of ice-cold extraction buffer A (25 mM Tris, pH 7.4, 2 mM EGTA, 2 mM EDTA) with protease inhibitors (1 mM PMSF, 20 μM leupeptin, 7.2 μM pepstatin A) were added to the dishes, and cells were scraped into microfuge vials. Two dishes were combined for each time point. Zero time samples represent pelleted cells (2 × 107) that were suspended in buffer A plus protease inhibitors after the first wash. After 30 1-s bursts (40 W per burst) of sonication (model 185 sonicator; Branson, Plainview, NY), cells were transferred to the appropriate tubes for ultracentrifugation at 40,000 rpm (Beckman 50Ti rotor and L5–50B centrifuge) for 60 min. These extraction conditions induced neither the binding nor the release of cPLA2 from the membrane. Supernatants (cytosolic fraction) from the centrifugation were diluted with 2× sample buffer without bromophenol blue or β-mercaptoethanol, and to the pellet, 200 μl of ice-cold buffer B (buffer A plus 1% Nonidet P-40 [NP-40]) containing protease inhibitors was added. Pellets were agitated on ice for 15 min to extract membrane-bound cPLA2 before a second centrifugation at 20,000 rpm for 20 min. The NP-40–soluble supernatant (membrane fraction) was similarly diluted in 2× sample buffer as described above. As a control to indicate that the mobility shift was due to a phosphorylation, cytosol or membrane fractions (250–500 μg of total protein) were treated with 30 μg/ml potato acid phosphatase at 30°C for 30 min (our unpublished observations). Control cells incubated at 30°C for 30 min without enzyme showed no change in cPLA2 mobility (our unpublished observations). Total concentration of protein was determined using the BCA method. After addition of bromophenol blue and β-mercaptoethanol, 60 μg of protein were loaded on to a 10% polyacrylamide gel (Mini-PROTEAN; Bio-Rad, Hercules, CA). Samples were electrophoresed for 4 h at 115 V to obtain optimal separation between the phosphorylated and unphosphorylated forms of cPLA2. After transfer of proteins to nitrocellulose (60 V, 1.5 h), blots were blocked overnight in 5% nonfat dry milk in PBS. Membranes were incubated with mouse anti-human cPLA2 (1:500 dilution) for 2 h followed by treatment with goat anti-mouse IgG-HRP (1:500 dilution) for 2 h. Chemiluminescent detection by ECL was used to visualize bands.
To test the effects of ERK2 on cPLA2 activation, HeLa cells were treated with the MEK kinase inhibitor PD98059 (32 μM) or left untreated for 10 min. Cells were spread on 60-mm gelatinized dishes at 37°C for the indicated times before lysis. Forty micrograms of total cell lysates were run on 10% polyacrylamide gels and detected for cPLA2 activation as described above. To test the activation of PKC on cPLA2 activation, HeLa cells in suspension were treated with 1 μM 12-O-tetradecanoylphorbol-13-acetate (TPA) or left untreated for 10 min before assaying for cPLA2 activation. Additionally, HeLa cells in suspension were treated with the PKC inhibitor Calphostin C (1 μM) or left untreated for 10 min and were allowed to attach to gelatin for the indicated times at 37°C before detection of cPLA2 phosphorylation.
Quantitation of cPLA2 Translocation
HeLa cell cytosol and membrane fractions were separated by ultracentrifugation as described above, except that a careful recording of all volumes for each cytosol and membrane fraction was taken. Sixty micrograms of cytosol and membrane protein from each of the three samples (suspension, spread 15 min with +Ca2+, and spread 15 min −Ca2+) were electrophoresed on the same gel and probed with mouse anti-human cPLA2 as mentioned above. Optical densities of the bands were measured using NIH Image software and the values were normalized to achieve an optical density value per total microgram of protein in each cytosol and membrane sample. Results are expressed as the fraction of total cPLA2 within each of the three time points with SDs from four experiments. To determine the effects of ERK2 and PKC on cPLA2 translocation, HeLa cells were first treated with PD98059 (32 μM) or Calphostin C (1 μM) for 10 min before plating on gelatinized dishes plus extracellular calcium. Cells were allowed to attach for 15 min before extraction as described above.
Attachment-induced cPLA2 Phosphorylation Assay
HeLa cells in suspension were incubated with or without 1 μM ionomycin or 20 μM BAPTA-AM for 10 min in PBS (1 mM Mg, 0.5 mM Ca2+). Cells incubated without ionomycin or BAPTA-AM were either homogenized in 25 mM Tris buffer (pH 7.4) minus (1 mM EGTA) or plus 0.5 mM Ca2+. After ionomycin or BAPTA-AM treatment, one sample was allowed to attach and spread on gelatin for 15 min while the other remained in suspension. Both ionomycin- and BAPTA-AM-treated samples were extracted in the same buffer as the control. After the separation of the cell lysates into soluble and NP-40 soluble fractions, 60 μg of protein were electrophoresed in each lane, and the blots were probed with mouse anti-human cPLA2 IgG as described above.
ERK2 Activation Assay
HeLa cells were prepared before spreading as described above. Cells (2 × 106) were plated onto 60-mm gelatinized dishes at 37°C. At the indicated times 100 μl of buffer A (with protease inhibitors) and 100 μl of 2× sample buffer were added to each dish before scraping. Zero time samples represent extractions of cells in suspension after the first wash. Forty micrograms of total cell lysates were electrophoresed on 10% polyacrylamide gels and blotted onto nitrocellulose for 3 h at 60 V. Blots were blocked overnight before incubation with a 1:500 dilution of mouse anti-human ERK2 IgG for 1 h. After incubation with a 1:500 dilution of goat anti-mouse IgG-HRP for a second hour, an ERK2 mobility shift was detected by ECL. Bands were quantitated using NIH Image and expressed as a percentage of ERK2 phosphorylated. ERK2 phosphorylation was alternatively detected using a 1:500 dilution of mouse anti-human phospho-ERK antibody for 1 h followed by secondary antibody incubation and detection as described above. To test the roles of ERK2 on spreading, HeLa cells were pretreated with PD98059 at various concentrations for 10 min before plating onto gelatinized dishes at 37°C. After 30 min dishes were analyzed for percent cells spread as previously described. To test whether PD98059 was affecting ERK2 activation, HeLa cells were treated with PD98059 at 32 μM or left untreated for 10 min. Cells were then allowed to attach to 60-mm gelatinized dishes at 37°C for 5 min before extraction. Forty micrograms of total cell lysate were electrophoresed on 10% polyacrylamide gels as described. The role of PKC in the activation of ERK2 was tested by treating suspension HeLa cells with 1 μM TPA or 1 μM Calphostin C for 10 min. Cells treated with Calphostin C were allowed to attach to gelatinized dishes for 10 min before extraction. ERK2 phosphorylation was assayed using the mobility shift assay as described above.
RESULTS
Role of Extracellular Calcium on Morphology of HeLa Cells during Spreading on Gelatin
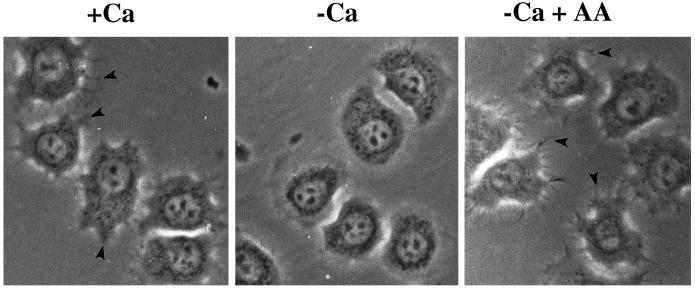
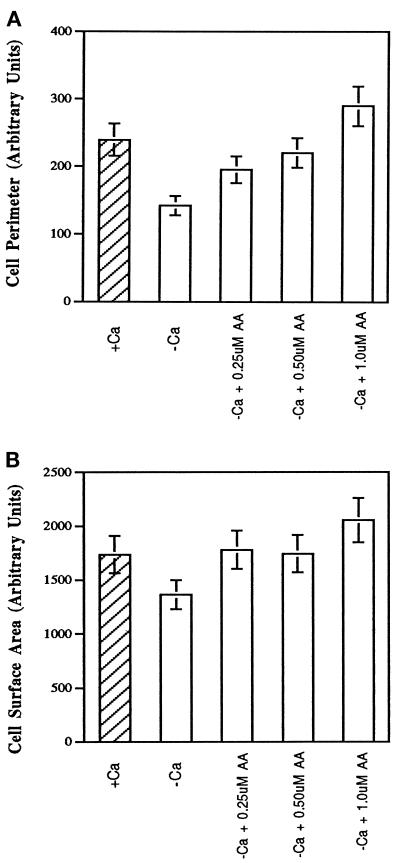
It has been previously shown that Ca2+ is essential for cPLA2 binding to membranes and is essential for translocation from the cytosol to the membrane (Channon and Leslie, 1990; Diez and Mong, 1990; Clark et al., 1991; Rehfeldt et al., 1993). HeLa cells have been shown to spread equally well in the presence or absence of extracellular Ca2+ as long as magnesium is present in the medium (Beacham and Jacobson, 1990). No spike in intracellular calcium [Ca2+]i was observed during attachment or spreading (Chun and Jacobson, 1992); however, a smaller increase was not ruled out. The concentration of [Ca2+]i in cells in suspension or during spreading on gelatin ranged from 80 to 100 nM (Chun and Jacobson, 1992). HeLa cells spread in the presence of extracellular Ca2+ produced more lamellipodia and filopodia (Figure 1). More importantly, the difference in morphology does not appear to be due to the Ca2+ in itself but to an increased production of AA, because addition of exogenous AA to cells in Ca2+-free medium restored the formation of filopodia and lamellipodia. Morphometry of cells shown in Figure 2 revealed that although the total surface area occupied by fully spread HeLa cells in the presence or absence of extracellular Ca2+ on a gelatin substrate was not drastically different, the perimeter of the cells was significantly altered. Furthermore, the addition of exogenous AA showed a concentration-dependent increase in perimeter with little change in area (Figure 2). It should be noted that lamellipodia and filopodia were always greater in cells treated with extracellular Ca2+ or AA regardless of whether the cells were beginning to spread, partially spread, or maximally spread.
Figure 1.
Effects of extracellular Ca2+ on HeLa cell morphology during attachment and spreading on gelatin. HeLa cells were washed twice in PBS-Mg plus or minus Ca2+ before plating onto gelatin-coated polystyrene dishes at 37°C. One sample of cells resuspended in PBS-Mg minus Ca2+ was treated with AA at a final concentration of 1 μM before plating. After 30 min HeLa cells were observed by phase-contrast microscopy using a 40× phase-contrast objective. Arrowheads indicate filopodia seen when HeLa cells are spread in the presence of extracellular Ca2+ or in the absence of extracellular Ca2+ when treated with exogenous AA. Pictures are representative of five independent experiments.
Figure 2.
Quantatative analysis of the effects of extracellular calcium and exogenous AA on HeLa cell area and perimeter. HeLa cells were washed twice in PBS-Mg plus or minus calcium before plating onto gelatin-coated polystyrene dishes at 37°C. Alternatively, cells were resuspended in PBS-Mg minus calcium and treated with AA at 0.25, 0.50, or 1 μM before plating. After 30 min HeLa cells were observed by phase-contrast microscopy using a 40× objective, and pictures were taken. Digital images of cells were analyzed for both cell perimeter (A) and area (B) using NIH Image software. At least 30 cells from three fields of view were analyzed. Data are expressed in terms of total area or perimeter using arbitrary units specified by NIH Image with SDs from three independent experiments.
Effects of Extracellular Calcium on Rate of Spreading, Attachment, and AA Release
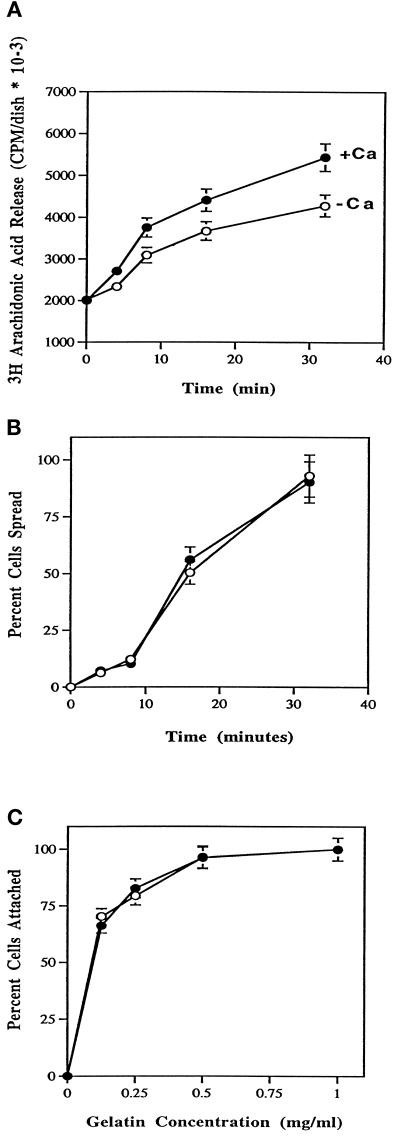
It was next determined whether the amount of AA released during attachment and spreading could be affected by extracellular Ca2+. Release of AA was measured in the presence and absence of Ca2+ at 37°C after the addition of HeLa cells to culture dishes coated with gelatin. Figure 3A shows that more AA was released in the presence of extracellular Ca2+ than in its absence. Although the increase in AA release attributable to extracellular calcium was only 20–30%, the increase was statistically significant and correlates with the morphological changes (Figure 1). Even though the levels of AA release and morphology were different plus and minus calcium, there was no significant difference between the rate and percent of cells that spread (Figure 3B). To test whether the differences in AA release could be due to differences in the avidity of the cells for gelatin, a quantitative adhesion assay was performed using culture dishes coated with gelatin at concentrations suboptimal for cell attachment (Lu et al. 1992). As shown in Figure 3C, extracellular Ca2+ does not play a role in HeLa cell attachment to a wide variety of gelatin concentrations. Therefore, an alternative explanation must exist to explain the increased amount of AA release during spreading in the presence of extracellular calcium.
Figure 3.
Effects of extracellular calcium on release of AA (A), spreading rate (B), and attachment to various concentrations of gelatin (C). (A) HeLa cells at density of 2–3 × 105 were labeled with [3H]AA at a final concentration of 0.25 μCi/ml for 12–16 h in the presence of serum. Cells were washed twice in PBS-Mg plus (•) or minus (○) Ca2+ and plated onto 60-mm gelatin-coated polystyrene dishes at a density of 2 × 106 cells per dish. At the indicated times a 300-μl aliquot was taken from the supernatant and microfuged for 2 min. Three 40-μl fractions were then placed into scintillation vials for counting. Data are expressed as counts per minute per dish with SDs from four experiments. (B) At the indicated times, HeLa cells were assayed for percent cells spread from no less than three fields of view. Cells were spread in PBS-Mg plus (•) or minus (○) Ca2+. Data are represented with SDs from no less than five experiments. (C) HeLa cells were washed twice in PBS-Mg plus (•) or minus (○) Ca2+, treated with 1 mg/ml BSA, and plated at a density of 0.5 × 105 cells per dish onto 35-mm polystyrene dishes coated with gelatin at the indicated concentrations. After 10 min at room temperature, cells were agitated for 5 s, the medium and unattached cells were decanted, and attached cells were scored. Data are expressed as percentage of cells attached and are normalized to cells attached at 1 mg/ml gelatin. Data are represented with SDs from at least five independent experiments.
Phosphorylation of cPLA2 during Adhesion to a Gelatin Substrate
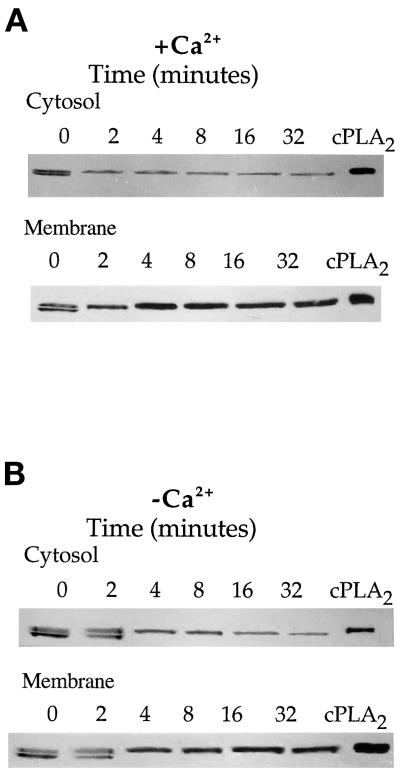
Extracellular Ca2+ could bring about an increase in the activity of cPLA2 to release more AA by inducing phosphorylation of the enzyme or by increasing its binding to membranes where the substrate for the reaction resides (Channon and Leslie, 1990; Diez and Mong, 1990; Clark et al., 1991; Rehfeldt et al., 1993). The role of extracellular Ca2+ on the phosphorylation of cPLA2 was first studied. A convenient and accurate method to ascertain the extent of cPLA2 phosphorylation is by a gel shift method in which the phosphorylated form of the enzyme exhibits a decreased electrophoretic mobility (Kemp and Pearson, 1990; Lih-Ling et al., 1993; Kramer et al., 1995). ECL assays were used to detect the amount of antibody bound to cPLA2 on immunoblots. ECL was done for different lengths of time for each of the membrane or cytosolic fractions to optimize the signal. As shown in Figure 4A, HeLa cells spread on gelatin in the presence of Ca2+ showed that in both the cytosol and membrane fractions there was a complete phosphorylation during the earliest measurable time of attachment that lasted for the duration of cell spreading. Cells spread in the absence of extracellular Ca2+ also exhibited an increase in cPLA2 phosphorylation upon attachment (Figure 4B); however, it took longer for complete phosphorylation to occur. This may in part explain the differences in AA release during spreading in the absence of Ca2+ (Figure 3A).
Figure 4.
Effect of extracellular calcium on the rate of phosphorylation of cPLA2 during HeLa cell attachment and spreading on a gelatin substrate. (A) HeLa cells were washed twice in PBS-Mg plus Ca2+ and plated onto 100-mm gelatinized dishes at a density of 8 × 106 cells per dish, or cells remained in suspension (0 min). At the indicated times the excess medium was removed, and cells were washed twice with PBS-Mg containing 2 mM EGTA. Cell lysates were separated into a soluble (cytosolic) and an NP-40-soluble (membrane) fraction. Sixty micrograms of protein from each sample were electrophoresed per lane, and the blots were probed with mouse anti-human cPLA2 IgG. (B) The samples were treated identically to those described in A, except the HeLa cells were initially washed and spread in PBS-Mg containing 2 mM EGTA.
Translocation of cPLA2 during Adhesion of HeLa Cells to a Gelatin Substrate
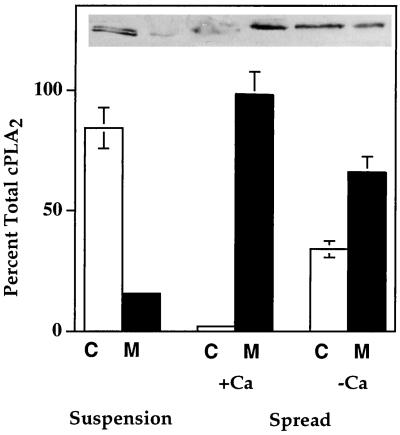
In addition to phosphorylation of cPLA2, Figure 4 also indicates that there is a progressive increase in the relative amount of membrane-associated cPLA2 during the course of spreading. In both the absence and presence of extracellular Ca2+, it took 4 min for maximal translocation to the membrane fraction to occur (Figure 4). To more accurately determine whether extracellular Ca2+influenced the extent to which cPLA2 was translocated, a more quantitative densitometric analysis of cPLA2 translocation was conducted. Cytosol and membrane samples from cells in suspension or cells spread for 15 min on gelatin in the presence or absence of Ca2+ were electrophoresed on the same gel, electroblotted, probed with anti-cPLA2 antibody, and analyzed by ECL during the linear phase of the assay so that the optical density of each band on the exposed x-ray film could be normalized to the amount of total protein in each fraction. This allowed for an accurate determination of the percent of the total cPLA2 in each fraction. The results shown in Figure 5 indicate that essentially all of the cPLA2 in cells in suspension was in the cytosolic fraction, whereas cell adhesion to a gelatin substrate induced an essentially complete translocation of cPLA2 from cytosol to membrane for cells in the presence of extracellular Ca2+. In the absence of extracellular Ca2+, there was much less translocation from the cytosol to the membrane (Figure 5). Maximal translocation of cPLA2 in both experiments (Figures 4 and 5) occurred within 4 min of cell attachment. The amount of cPLA2 that translocated to the membrane in the absence of extracellular Ca2+ was 50–60% of the total cPLA2. In cells in the presence of extracellular Ca2+, >95% of the total cPLA2 was membrane associated after 4 min of attachment. Interestingly, the average increase in AA release (∼30%) for cells in the presence of extracellular Ca2+ (Figure 3A) was similar to the amount of increase in the association of cPLA2 with the membrane induced by Ca2+. This would be sufficient to account for the observed increase of AA released by HeLa cells in the presence of Ca2+ (Figure 3A) without involving phosphorylation. The amount of cPLA2 that translocated in the absence of Ca2+ remains at 40–50% less than that in the presence of Ca2+ throughout the time of spreading. However, complete phosphorylation of cPLA2 is only slightly slowed down. Therefore, it is possible that the sustained decrease in AA release during spreading is due more to the reduction in translocation than to a 2- to 4-min delay in phosphorylation.
Figure 5.
Quantification of cPLA2 translocation from the cytosol to membrane during HeLa cell spreading on gelatin plus and minus extracellular Ca2+. HeLa cells were washed twice in PBS-Mg plus Ca2+ and plated onto 100-mm gelatinized dishes at a density of 8 × 106 cells per dish, or cells remained in suspension. After 15 min the excess medium was removed, and cells were washed twice with PBS-Mg containing 2 mM EGTA. Cell lysates were separated into a soluble cytosolic fraction (C) and an NP-40 solubilized membrane fraction (M). Sixty micrograms of protein from each sample were electrophoresed per lane, and the blots were probed with mouse anti-human cPLA2 IgG. The optical density of each band was used to calculate the total amount of cPLA2 in each cytosol and membrane fraction. Data are expressed as a percentage of total cPLA2 for each treatment with SDs from five experiments.
Independent Regulation of Translocation and Phosphorylation of cPLA2 in HeLa Cells
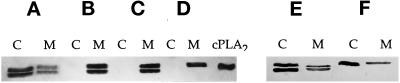
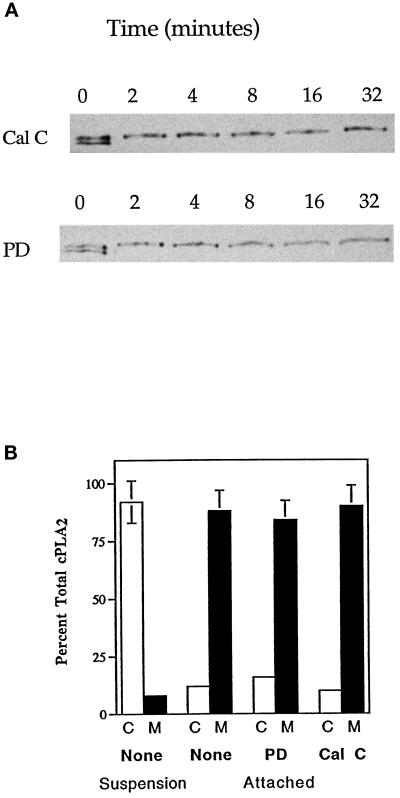
The above results combined indicated that cPLA2 underwent both phosphorylation and translocation during HeLa cell spreading on gelatin. The next experiments were designed to address whether phosphorylation and translocation could act independently. This is an area of current controversy. Some have shown that an increase in the amount of intracellular Ca2+ leads to an increase in cPLA2 phosphorylation (Hirasawa et al., 1995; Nahas et al., 1996), whereas others conclude that the two events can occur independently (Lih-Ling et al., 1993; de Carvalho et al., 1996; Schalkwijk et al., 1996). To test how Ca2+ and phosphorylation were regulated in the HeLa cell system, cells were treated under conditions outlined in Figure 6. When HeLa cells in suspension were lysed and fractionated into membrane and cytosol under conditions that induced neither binding nor release of cPLA2 from the membrane, most of the cPLA2 remained in the cytosolic fraction (Figure 6A). Also, the relative amounts of phosphorylated and nonphosphorylated cPLA2 were approximately the same, as indicated by the two bands on the immunoblot. When lysis and fractionation of the cells were done in the presence of 0.5 mM Ca2+, all of the cPLA2 was chased to the membrane fraction, and there was no change in the state of phosphorylation (Figure 6B). Cells treated with ionomycin (1 μM) in the presence of 0.5 mM extracellular Ca2+ to equilibrate extra- and intracellular Ca2+ (Chun and Jacobson, 1993) showed a complete translocation to the membrane and no change in phosphorylation state (Figure 6C). Therefore, it was of interest to determine whether phosphorylation was attachment dependent and whether in intact cells cPLA2 was phosphorylated in the cytosol or on the membrane. The mobility shift assays (Figure 4) demonstrated that phosphorylated cPLA2 could exist in both the cytosol and on the membrane. However, it is unknown whether the enzyme can be phosphorylated in the cytosol, on the membrane, or both. In an attempt to answer this question, HeLa cells were treated with ionomycin in the presence of extracellular Ca2+ (Figure 6C) to chase both the phosphorylated and nonphosphorylated cPLA2 to the membrane but were then allowed to attach to a gelatin-coated culture dish before lysis and fractionation. As shown in Figure 6D, all of the cPLA2 associated with the membrane fraction upon attachment was in the phosphorylated form. These data are consistent with cPLA2 phosphorylation occurring on the membrane in an attachment-dependent manner. To test whether cPLA2 could become phosphorylated in the cytosol, HeLa cells were treated with BAPTA-AM (20 μM) at a concentration previously shown to chelate intracellular Ca2+ (Chun and Jacobson, 1993). HeLa cells treated in suspension with BAPTA-AM in the absence of extracellular Ca2+ showed that the majority of the cPLA2 was in the cytosolic fraction (Figure 6E). Upon attachment to gelatin, there was a complete phosphorylation of cPLA2 and no translocation to the membrane (Figure 6F). Therefore it appears as if attachment-dependent phosphorylation of cPLA2 can occur on the membrane, in the cytosol, or both.
Figure 6.
Attachment induces the phosphorylation of cPLA2 on the membrane independent of Ca2+-mediated translocation. HeLa cells in suspension were treated without (A and B) or with (C and D) 1 μM ionomycin or 20 μM BAPTA-AM (E and F) for 10 min. At this concentration of ionomycin the Ca2+ influx across the plasma membrane was detected and measured in the HeLa cell system (Chun and Jacobson, 1992). Likewise, the concentration of BAPTA-AM used in these experiments has been shown to chelate [Ca2+]i in HeLa cells (Chun and Jacobson, 1992). Cells incubated without ionomycin (A and B) were homogenized in buffer either lacking (A) or containing (B) calcium. Cells treated with ionomycin (C and D) or BAPTA-AM (E and F) were either left in suspension (C and E) or allowed to attach and spread on gelatin for 15 min (D and F). Samples C–F were extracted in Ca2+-free buffer to prevent artificial translocation. After separation of the cell lysates into a soluble cytosolic fraction (C) and NP-40-soluble membrane fraction (M), 60 μg of protein were electrophoresed per lane, and the electroblots were probed with mouse anti-human cPLA2 IgG. A representative gel of three experiments is shown.
Attachment-dependent Activation of ERK2 Occurs via PKC but Is Not Required for HeLa Cell Spreading on Gelatin or cPLA2 Activation
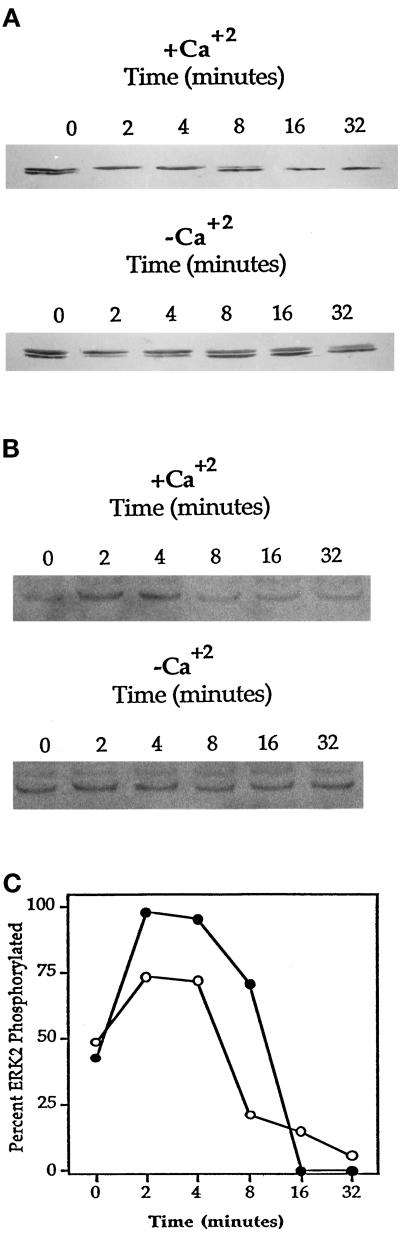
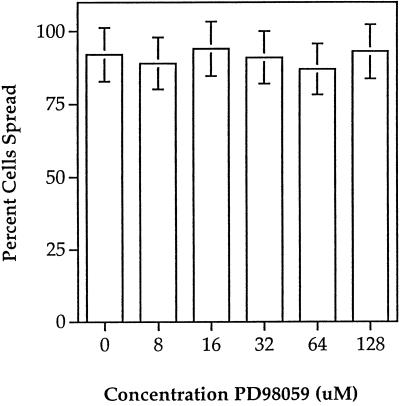
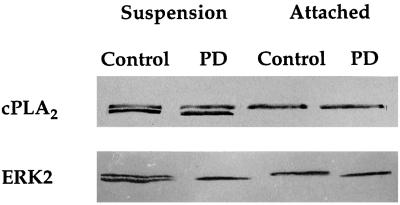
It has been reported by several groups that ERK2 is the kinase responsible for cPLA2 phosphorylation and its activation, in response to a number of stimuli (Nemenoff et al., 1993; Durstin et al., 1994; Qiu and Leslie, 1994). It has also been shown that ERK2 activation is mediated in part by integrin–matrix interactions (Morino et al., 1995; Zhu and Assoian, 1995; Chen et al., 1996; Renshaw et al., 1996). Because HeLa cell spreading is β1-integrin dependent (Auer and Jacobson, 1995), it was of interest to test whether ERK2 activation was involved in cell spreading and whether extracellular Ca2+ played a role in its activation. Mobility shift assays allow the observation of both phosphorylated and nonphosphorylated forms of ERK2 on immunoblots; the higher molecular weight represents the phosphorylated form. ERK2 was rapidly phosphorylated upon cellular attachment, followed by a dephosphorylation for the remainder of cell spreading (Figure 7A). ERK2 was similarly activated and deactivated during cell spreading in the absence of extracellular Ca2+ (Figure 7A); however, the maximal level of phosphorylation was less than that of cells spread with Ca2+ (Figure 7C). Figure 7B shows similar results when HeLa lysates were treated with an antibody that recognizes the phosphorylated form of ERK2. To test the biological significance of attachment-dependent ERK2 activation on cell spreading, the MEK inhibitor PD98059 was used (Lazar et al., 1994). PD98059 did not inhibit spreading at concentrations up to 128 μM (Figure 8) and did not greatly alter cellular morphology (our unpublished observations). However, treatment of HeLa cells with PD98059 was capable of inhibiting the attachment-induced phosphorylation of ERK2 (Figure 9). To study the dependence of PKC on ERK2 activation, HeLa cells were treated with the PKC inhibitor Calphostin C before attachment. As shown in Figure 10, Calphostin C completely inhibited the attachment-induced ERK2 activation and cell spreading (our unpublished observations). However, ERK2 phosphorylation could occur independent of attachment, because suspension cells treated with TPA showed a complete phosphorylation of ERK2. Interestingly, inhibition of an ERK2 mobility shift with PD98059 had no effect on cPLA2 phosphorylation, translocation (Figures 9 and 11), or AA release (our unpublished observations). Taken together the above results indicate that cPLA2 activation by phosphorylation and translocation during HeLa cell attachment to gelatin was independent of ERK2 phosphorylation and ERK2 phosphorylation is not necessary for cell spreading.
Figure 7.
Differential activation and deactivation of ERK2 during HeLa cell attachment and spreading on gelatin plus and minus extracellular Ca2+. (A) Mobility shift assay. HeLa cells were washed twice in PBS-Mg plus or minus calcium and plated onto 60-mm gelatinized dishes. At the indicated times the excess medium was removed, and cells were washed twice with PBS-Mg containing 2 mM EGTA. After scraping, cells were lysed by the addition of 2× sample buffer. Sixty micrograms of protein from total cell lysates were loaded per well, and the electroblots were probed with mouse anti-human ERK2 IgG, The top band represents the phosphorylated form of ERK2. (B) Anti-phospho-ERK assay. HeLa cells were treated as described in A, except the electroblots were probed with a mouse anti-human phospho-ERK antibody. (C) Percent ERK2 activation plus (•) and minus (○) Ca2+ in A was quantified at each time point by densitometry. Data are expressed as a percentage of the total amount of ERK2 that becomes phosphorylated. A representative gel and scan from three independent experiments are shown.
Figure 8.
Effects of the MEK inhibitor PD98059 on HeLa cell spreading. Cells in suspension were treated with PD98059 at the indicated concentrations for 10 min before plating onto gelatinized polystyrene dishes at 37°C. Percent cells spread was scored at 30 min as described in MATERIALS AND METHODS. Data are representative of no less than five independent experiments.
Figure 9.
Effects of the MEK inhibitor PD98059 on activation of cPLA2 and ERK2 on attachment of HeLa cells to gelatin. HeLa cells were incubated in suspension with 32 μM PD98059 (PD) or left untreated (Control) for 10 min. After incubation the cells were either plated onto gelatinized dishes for 5 min at 37°C (attached) or directly lysed (suspension). Sixty micrograms of protein from each of the total cell lysates were loaded per well, and blots were probed with mouse anti-human cPLA2 IgG or mouse anti-human ERK2 IgG.
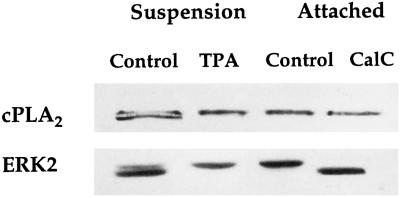
Figure 10.
Effects of PKC on the activation of cPLA2 and ERK2. HeLa cells were incubated in suspension (Suspension) without (Control) or with 1 μM phorbol ester (TPA) and then lysed, and 60 μg of protein from each of the total cell lysates were loaded per well for SDS-PAGE and immunoblotted with either mouse anti-human cPLA2 or mouse anti-human ERK2 IgG. To test the effects of PKC inhibition on the activation of cPLA2 and ERK2, HeLa cells were incubated in suspension with 1 μM Calphostin C (CalC) or left untreated for 10 min (Control) before attachment (Attached) to gelatin for 5 min at 37°C. Cells were then lysed and treated as described above. Calphostin C completely inhibits spreading.
Figure 11.
Effects of PKC and ERK2 inhibition on cPLA2 translocation and phosphorylation. (A) Cells in suspension were treated with PD98059 (32 μM) or Calphostin C (1 μM) 10 min before plating onto gelatinized polystyrene dishes at 37°C. At the indicated times the excess medium was removed, and cells were washed twice with PBS-Mg containing 2 mM EGTA. After scraping, cells were lysed by the addition of 2× sample buffer. Eighty micrograms of protein from total cell lysates were loaded per well, and the electroblots were probed with mouse anti-human cPLA2 IgG. (B) After treatment with PD98059 (32 μM) or Calphostin C (1 μM) for 10 min, HeLa cells were washed twice in PBS-Mg plus Ca2+ and plated onto 100-mm gelatinized dishes at a density of 8 × 106 cells per dish. After 15 min the excess medium was removed, and cells were washed twice with PBS-Mg containing 2 mM EGTA. Cell lysates were separated into a soluble cytosolic fraction (C) and an NP-40-solubilized membrane fraction (M). Sixty micrograms of protein from each sample were electrophoresed per lane, and the blots were probed with mouse anti-human cPLA2 IgG. The optical density of each band was used to calculate the total amount of cPLA2 in each cytosol and membrane fraction. Data are expressed as a percentage of total cPLA2 for each treatment with SDs from five experiments.
PKC-dependent and -independent Pathways of cPLA2 Activation
The ability of PD98059 to block ERK2 phosphorylation without inhibiting spreading, cPLA2 phosphorylation, translocation, or AA release suggested that there may be another kinase involved in the activation of cPLA2. It has been well established that PKC is required for HeLa cell spreading on gelatin (Chun and Jacobson, 1993). It was found that PKC activation induces an attachment-independent phosphorylation of cPLA2, evident by an enhanced mobility shift upon addition of TPA to cells in suspension (Figure 10). On the other hand, in cells treated with Calphostin C, cell spreading was completely abolished, with no effect on cPLA2 phosphorylation or translocation (Figure 11). In fact, inhibition of PKC and cell spreading with Calphostin C causes a modest reduction of AA release (Chun and Jacobson, 1993). Thus phosphorylation of cPLA2 and subsequent AA release appeared to be necessary but not sufficient for HeLa cell spreading. These results are consistent with our previous findings that suggest PKC, which is activated in response to AA production during cell attachment, can act to amplify AA release by a positive feedback mechanism (Chun and Jacobson, 1993). In conclusion, the initial activation of the cytoskeletal machinery by the production of AA was independent of a PKC-mediated phosphorylation of cPLA2.
DISCUSSION
Upon attachment of HeLa cells to a gelatin matrix, cPLA2 underwent a rapid phosphorylation and translocation from a cytosolic fraction to a membrane fraction (Figures 4 and 5). The consequence is the release of AA from membrane phospholipids. As previously demonstrated, the AA is metabolized in a lipoxygenase-dependent manner resulting in the activation of PKCε and the subsequent polymerization of actin to induce spreading of the attached cells (Chun and Jacobson, 1993, 1996; Chun et al., 1997). The results also indicated that extracellular Ca2+ regulates cPLA2 activity by increasing the rate of phosphorylation and translocation from the cytosol to the membrane during the attachment and spreading (Figures 4 and 5). Although HeLa cells spread equally well in terms of percent cells spread (Figure 3B) whether extracellular Ca2+ was present, more lamellipodia and filopodia (Figure 1) and a greater release of AA (Figure 3C) were observed during cell spreading in the presence of extracellular Ca2+. It has been previously shown by several workers that extracellular Ca2+ can act to enhance formation of lamellipodia and cell migration (Jaconi et al., 1991; Marks et al., 1991; Grzesiak et al., 1992; Leavesley et al., 1993; Stossel, 1993). Furthermore, agonist-induced AA release in macrophages can be modulated by levels of extracellular Ca2+ (Qiu et al. 1998). The enhanced morphology seen in HeLa cells was quantitated in terms of cell perimeter (Figure 2A) and was most likely due to the increased release of AA observed during attachment and spreading in Ca2+-containing medium (Figure 3A), because formation of lamellipodia and filopodia could be restored when exogenous AA was added to cells spread in the absence of extracellular Ca2+ (Figure 1). In addition, the degree of lamellipodia and filopodia formation to enhance cell perimeter is proportional to the amount of AA added to the medium (Figure 2A). It appears as if Ca2+ is acting intracellularly because extracellular Ca2+ does not affect the rate of attachment of cells to gelatin (Figure 3C). These results are in agreement with the work of others who have shown that AA is able to induce Ca2+ release from internal stores (Wolf et al. 1986). Interestingly, addition of thapsigargin to HeLa cells spread in the absence of extracellular Ca2+ partially restores lamellipodia and filopodia formation (our unpublished observations). Results reported here indicate that AA not only might act to enhance filopodia and lamellipodia formation but also may have additional roles yet to be determined.
It is not known how the presence of extracellular Ca2+ in the medium increased the output of AA by HeLa cells during attachment to gelatin. Because Ca2+ has been shown to enhance the association of cPLA2 with membranes (Clark et al., 1991), it is possible that the increase in AA output is simply a matter of a cell–substrate attachment-induced influx of the extracellular Ca2+ to raise [Ca2+]i. Previously, it was shown that p11 (a member of the S100 family of calcium-binding proteins) interacts and inhibits cPLA2 activity (Wu et al. 1997a,b). It is possible that p11 may play a role in HeLa cell spreading by modulating cPLA2 activity. It is interesting that during HeLa cell attachment and spreading on gelatin in the presence of extracellular Ca2+ there is no major rise or transient spike of [Ca2+]i (Chun and Jacobson, 1992). This is also the case with adhesion of endothelial cells to collagen (Leavesley et al., 1993). However, adhesion of neutrophils and endothelial cells to fibronectin or vitronectin shows a transient increase in [Ca2+]i that is abolished when the cells spread in the absence of extracellular Ca2+ (Jaconi et al., 1991; Marks et al., 1991; Leavesley et al., 1993; Schwartz, 1993). The absence of a definitive increase in [Ca2+]i during cell attachment and spreading, as seen in HeLa cells and endothelial cells on collagen, does not necessarily contradict a role for Ca2+ influx as the basis for the greater output of AA. It has previously been demonstrated in macrophages treated with PMA or okadaic acid that increasing [Ca2+]i is not necessary for AA release (Qiu et al. 1998). Currently, it is not known what level of increase in [Ca2+]i above the basal concentration is essential for translocation. In HeLa cells the basal level is 80–100 nM (Chun and Jacobson, 1992), and this seems to be sufficient to translocate most of the cPLA2 to the membrane (Figures 4 and 5). Furthermore, a small or localized rise in [Ca2+]i in cells could have gone undetected by the methods used with HeLa cells (Chun and Jacobson, 1992). The stoichiometric relationship among the [Ca2+]i, translocation of the cPLA2 to the membrane, and regulation of AA production needs to be examined.
The role of Ca2+ in the phosphorylation of cPLA2 is an area of current controversy. It is well known that the structure of cPLA2 reveals an N-terminal Ca2+ lipid binding domain homologous to PLC, GAP, p65, and the C2 region of PKC (Clark et al., 1991). The Ca2+ lipid binding domain is functionally distinct, because its deletion does not effect the catalytic ability of the molecule (Nalefski et al., 1994). Treatment of cells with Ca2+ ionophores is known to increase AA release, presumably by facilitating its association with membrane phospholipids. Some groups have reported that in addition to translocation, ionophore treatment results in an increase in cPLA2 phosphorylation (Hirasawa et al., 1995; Nahas et al., 1996; Qiu et al., 1998). To determine whether Ca2+ affects phosphorylation, HeLa cells were treated in suspension with or without the Ca2+ ionophore ionomycin. Without ionomycin most of the cPLA2 was in the cytosol. Essentially all of the cPLA2 was chased from the cytosol to the membrane when homogenized cells were treated with Ca2+ (Figure 6). This occurred without a change in the state of phosphorylation. When cells were treated with ionomycin in the presence of extracellular Ca2+, all of the cPLA2 was chased to the membrane, and no detectable changes in the state of cPLA2 phosphorylation were observed. Thus, Ca2+ did not appear to affect cPLA2 phosphorylation. Interestingly, HeLa cells treated with ionomycin in the absence of extracellular Ca2+ still showed a modest translocation of cPLA2 to the membrane fraction (our unpublished observations). This finding can be explained by previous studies in which ionomycin is also capable of releasing Ca2+ from intracellular stores (Moolenaar et al., 1986).
As indicated above, Ca2+ alone did not induce phosphorylation of cPLA2 in HeLa cells. This poses the questions of whether phosphorylation is cell–substrate attachment dependent and whether in vivo cPLA2 can be phosphorylated in the cytosol or on the membrane. As shown in Figures 4 and 6, cPLA2 could stably exist both in the cytosol and on the membrane. However, it is not known whether the enzyme is phosphorylated in the cytosol, on the membrane, or both. When HeLa cells were treated in suspension with ionomycin in the presence of extracellular Ca2+, all of the cPLA2, including both the phosphorylated and nonphosphorylated forms, was chased to the membrane (Figure 6). If the same ionomycin treatment was done to first chase all of the cPLA2 to the membrane, and the cells were then allowed to attach to gelatin, all of the cPLA2 was on the membrane and in the phosphorylated form. This indicates that cPLA2 phosphorylation can occur on the membrane in an attachment-dependent manner. HeLa cells pretreated with BAPTA-AM before attachment in calcium-free medium show a complete phosphorylated cPLA2 that resides predominantly in the cytosolic fraction (Figure 6). Therefore it appears as if attachment-induced cPLA2 phosphorylation can occur in both the cytosol and on the membrane.
Activation of ERK2 MAP kinase occurred during HeLa cell attachment and spreading on a gelatin substrate but did not appear to be required for HeLa cell spreading, cPLA2 phosphorylation, translocation, or AA release during adhesion. In CHO cells, ERK2 has been shown to phosphorylate cPLA2 at Ser505 in vivo and in vitro, increasing its activity by ∼1.5-fold (Lih-Ling et al., 1993). Later work revealed that the ERK2 is responsible for the phosphorylation of cPLA2 in some systems (Nemenoff et al., 1993; Durstin et al., 1994; Kan et al., 1996). However, there have also been reports of ERK2-independent pathways of cPLA2 activation (Qiu and Leslie, 1994; Kramer et al., 1995; Waterman and Sha’afi, 1995). More recently it has been demonstrated that ERK2 actually inhibits CHO cell spreading (Reszka et al., 1997). Additionally, inhibition of ERK1 and ERK2 by antisense oligonucleotide treatment or inhibition of MEK activity with PD98059 does not prevent attachment and spreading of FG carcinoma cells (Klemke et al., 1997). The above clearly supports the data that ERK2 is not involved in HeLa cell attachment and spreading. Whether ERK2 is involved in chemotactic cell migration is still controversial. CHO cell migration seems to be inhibited by ERK2, whereas migration of FG carcinoma cells seems to require active ERK2 (Klemke et al., 1997; Reszka et al., 1997).
Although the evidence strongly suggests that cPLA2 is a major source of AA release in the HeLa cell system, potential contributions made by sPLA2 or iPLA2 cannot be excluded. Work by others reveals the possibility of cross talk among the various types of PLA2 in the modulation of AA release (Börsch-Haubold et al., 1995; Balsinde and Dennis 1996; Huwiler et al., 1997; Murakami et al., 1998). The observation that HeLa cells released AA and spread in the absence of millimolar amounts of extracellular Ca2+ is a good indication that sPLA2 is not functional, because sPLA2 is secreted and millimolar Ca2+ is needed for it to be active. Furthermore, sPLA2 is generally involved in the long-term potentiation of AA, unlike the AA release that occurs during early stages of HeLa cell attachment. In mouse macrophages it has been shown that cPLA2 is responsible for the early events of AA release that lead to spreading (Börsch-Haubold et al., 1995). Conversely, iPLA2 appears to be responsible for the long-term maintenance of spreading (Teslenko et al., 1997), as is the case with sPLA2. Previous work on the physiological role of iPLA2 comes from the use of the pharmacological inhibitor HELSS (Hazen and Gross, 1991a,b), whose selectivity among the various PLA2s is questionable. An alternative approach using iPLA2 overexpression in human embryonic kidney fibroblasts suggests that iPLA2 is not involved in agonist-stimulated AA release (Murakami et al. 1998). However, more details regarding the biochemical and structural properties of iPLA2 are still needed before one can accurately investigate its expression and possible role in the spreading of HeLa cells on gelatin.
Cell spreading on a matrix is a complex phenomenon involving a multitude of regulatory components. Although HeLa cell attachment is accompanied by cPLA2 phosphorylation, translocation, and AA release, these events are not sufficient for spreading on gelatin. It is known that β1-integrin cell surface receptors are involved in the adhesion of HeLa cells to gelatin and that ligation of the β1-integrins into large clusters activates AA release (Auer and Jacobson, 1995). The release of AA results in the activation of PKCε by inducing its translocation from the cytosol to the membrane and a particulate fraction containing the cytoskeletal components (Chun et al., 1996). More recently it has been shown that PKCε translocates first to the membrane then to filaments via an actin binding motif (Prekeris et al., 1996) in response to treating cells with AA (Huang et al., 1997). The AA-induced translocation of PKC in HeLa cells is not just to the membrane contacting the matrix and the cytoskeleton but to intracellular membranes and the apical membrane exposed to the culture medium as well (Renshaw et al., 1996; Chun et al., 1997). This suggests that PKC is activated throughout the cell to globally turn on the polymerization of actin rather than just acting at the adhesion zones where actin stress fibers terminate into integrin clusters ligated to the matrix. A global translocation of PKC would require diffusible second messengers that could move throughout the cell. It is possible that the activation of cPLA2 on cell attachment to release AA and/or its lipoxygenase metabolites could act in this capacity.
ACKNOWLEDGMENTS
We thank Audrey Eisen for performing the cell attachment assays. This research was supported in part by a grant from the National Institutes of General Medical Science (GM29127).
REFERENCES
- Ackermann EJ, Kempner ES, Dennis EA. Ca(2+)-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells. Isolation and characterization. J Biol Chem. 1994;269:9227–9233. [PubMed] [Google Scholar]
- Auer KL, Jacobson BS. Beta 1 integrins signal lipid second messengers required during cell adhesion. Mol Biol Cell. 1995;6:1305–1313. doi: 10.1091/mbc.6.10.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham DA, Jacobson BS. Mg++ mediates the cell-substratum interaction of Arg-Gly-Asp dependent HeLa cell collagen receptors. Exp Cell Res. 1990;189:69–80. doi: 10.1016/0014-4827(90)90258-c. [DOI] [PubMed] [Google Scholar]
- Börsch-Haubold A, Kramer RM, Watson SP. Cytosolic phospholipase A2 is phosphorylated in collagen- and thrombin-stimulated human platelets independent of protein kinase C and mitogen-activated protein kinase. J Biol Chem. 1995;270:25885–25892. doi: 10.1074/jbc.270.43.25885. [DOI] [PubMed] [Google Scholar]
- Channon JY, Leslie CC. A calcium-dependent mechanism for associating a soluble arachidonyl-hydrolyzing phospholipase A2 with membrane in the macrophage cell line RAW 264.7. J Biol Chem. 1990;265:5409–5413. [PubMed] [Google Scholar]
- Chen Q, Lin TH, Der CJ, Juliano RL. Integrin-mediated activation of MEK and mitogen-activated protein kinase in independent of RAS. J Biol Chem. 1996;271:18122–18127. doi: 10.1074/jbc.271.30.18122. [DOI] [PubMed] [Google Scholar]
- Chun JS, Auer KA, Jacobson BS. Arachidonate initiated protein kinase C activation regulates HeLa cells spreading on a gelatin substratum by the induction of F-actin formation and the exocytotic upregulation of β1 integrin. J Cell Physiol. 1997;173:361–370. doi: 10.1002/(SICI)1097-4652(199712)173:3<361::AID-JCP8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chun JS, Jacobson BS. Spreading of HeLa cells on a collagen substratum requires a second messenger formed by the lipoxygenase metabolism of arachidonic acid released by collagen receptor clustering. Mol Biol Cell. 1992;3:481–492. doi: 10.1091/mbc.3.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JS, Jacobson BS. Requirement for diacylglycerol and protein kinase C in HeLa cell-substratum adhesion and their feedback amplification of arachidonic acid production for optimum cell spreading. Mol Biol Cell. 1993;4:271–281. doi: 10.1091/mbc.4.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JS, Ha MJ, Jacobson BS. Differential translocation of protein kinase C ε during HeLa cell adhesion to a gelatin substratum. J Biol Chem. 1996;271:13008–13012. doi: 10.1074/jbc.271.22.13008. [DOI] [PubMed] [Google Scholar]
- Clark EA, Hynes RO. Ras activation is necessary for integrin-mediated activation of extracellular signal-related kinase 2 and cytosolic phospholipase A2 but not for cytoskeletal organization. J Biol Chem. 1996;271:14814–14818. doi: 10.1074/jbc.271.25.14814. [DOI] [PubMed] [Google Scholar]
- Clark JD, Lih-Ling L, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. A novel arachidonic acid-selective cytosolic phospholipase A2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Clark JD, Milona N, Knopf JL. Purification of a 110-kilodalton cytosolic phospholipase A2 from the human monocytic cell line U937 Proc. Natl Acad Sci USA. 1990;87:7708–7712. doi: 10.1073/pnas.87.19.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie S, Roberts EF, Spaethe SM, Roehm NW, Kramer RM. Phosphorylation and activation of Ca2+-sensitive cytosolic phospholipase A2 in MCII mast cells mediated by high-affinity Fc receptor for IgE. Biochem J. 1994;304:923–928. doi: 10.1042/bj3040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana R, Leto TL, Malech HL, Levy R. Essential requirement of cytosolic phospholipase A2 for activation of the phagocyte NADPH oxidase. J Biol Chem. 1998;273:441–445. doi: 10.1074/jbc.273.1.441. [DOI] [PubMed] [Google Scholar]
- de Carvalho MGS, McCormack AL, Olson E, Ghomashchi F, Gelb M, Yates JR, III, Leslie CC. Identification of phosphorylation sites of human 85-kD cytosolic phospholipase A2 expressed in insect cells and present in human monocytes. J Biol Chem. 1996;271:6987–6997. doi: 10.1074/jbc.271.12.6987. [DOI] [PubMed] [Google Scholar]
- Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- Diez E, Mong S. Purification of phospholipase A2 from human monocytic leukemic U937 cells. J Biol Chem. 1990;265:14654–14661. [PubMed] [Google Scholar]
- Durstin M, Durstin S, Molski TFP, Becker EL, Sha’afi RI. Cytoplasmic phospholipase A2 translocates to membrane fraction in human neutrophils activated by stimuli that phosphorylate mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1994;91:3142–3146. doi: 10.1073/pnas.91.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronich JH, Bonventre JV, Nemenoff RA. Purification of a high-molecular mass form of phospholipase A2 from rat kidney activated at physiological calcium concentrations. Biochem J. 1990;271:37–43. doi: 10.1042/bj2710037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RW, Ramanadham S, Kruska KK, Han X, Turk J. Rat and human pancreatic islet cells contain a calcium ion independent phospholipase A2 activity selective for hydrolysis of arachidonate which is stimulated by adenosine triphosphate and is specifically localized to islet β-cells , Biochemistry. 1993;32:327–336. doi: 10.1021/bi00052a041. [DOI] [PubMed] [Google Scholar]
- Grzesiak JJ, Davis GE, Kirchhofer D, Pierschbacher MD. Regulation of α2β1-mediated fibroblast migration on type I collagen by shifts in the concentrations of extracellular Mg2+ and Ca2+ J Cell Biol. 1992;117:1109–1117. doi: 10.1083/jcb.117.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa N, Santini F, Beaven MA. Activation of the mitogen-activated protein kinase/cytosolic phospholipase A2 pathway in a rat mast cell line. J Immunol. 1995;154:5391–5402. [PubMed] [Google Scholar]
- Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Staudt G, Kramer RM, Pfeilschifter J. Cross-talk between secretory phospholipase A2 and cytosolic phospholipase A2 in rat renal mesangial cells. Biochim Biophys Acta. 1997;1348:257–272. doi: 10.1016/s0005-2760(97)00073-8. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jaconi ME, Theler JM, Schlegel W, Appel RD, Wright SD, Lew PD. Multiple elevations of cytosolic free calcium in human neutrophils: initiation by adherence receptors of the integrin family. J Cell Biol. 1991;112:1249–1257. doi: 10.1083/jcb.112.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan H, Ruan Y, Malik KU. Involvement of mitogen-activated protein kinase and translocation of cytosolic phospholipase A2 to the nuclear envelope in acetylcholine-induced prostacyclin synthesis in rabbit coronary endothelial cells. Mol Pharmacol. 1996;50:1139–1147. [PubMed] [Google Scholar]
- Kemp BE, Pearson RP. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990;15:342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kim DK, Kudo I, Inoue K. Purification and characterization of rabbit platelet cytosolic phospholipase A2. Biochim Biophys Acta. 1991;1083:80–88. doi: 10.1016/0005-2760(91)90127-4. [DOI] [PubMed] [Google Scholar]
- Kramer, R.M. (1994). Structure and function of Ca2+-sensitive cytosolic phospholipase A2 signal-activated phospholipases, ed. M. Liscovitch, M.
- Kramer RM, et al. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1990a;264:5768–5775. [PubMed] [Google Scholar]
- Kramer RM, Johansen B, Hession C, Pepinsky RB. Structure and properties of a secretable phospholipase A2 from human platelets. Adv Exp Med Biol. 1990b;275:35–54. doi: 10.1007/978-1-4684-5805-3_3. [DOI] [PubMed] [Google Scholar]
- Kramer RM, Roberts EF, Manetta JV, Hyslop PA, Jakubowski JA. Thrombin-induced phosphorylation and activation of Ca2+-sensitive cytosolic phospholipase A2 in human platelets. J Biol Chem. 1993;268:26796–26804. [PubMed] [Google Scholar]
- Kramer RM, Roberts EF, Hyslop PA, Utterback BG, Hui KY, Jakubowski JA. Differential activation of cytosolic phospholipase A2 (cPLA2) by thrombin and thrombin receptor agonist peptide in human platelets. J Biol Chem. 1995;270:14816–14823. doi: 10.1074/jbc.270.24.14816. [DOI] [PubMed] [Google Scholar]
- Kudo I, Murakami M, Hara S, Inoue K. Mammalian nonpancreatic phospholipases A2. Biochim Biophys Acta. 1993;1170:217–231. doi: 10.1016/0005-2760(93)90003-r. [DOI] [PubMed] [Google Scholar]
- Lazar DF, Wiese RJ, Brady MJ, Mastick CC, Waters SB, Yamauchi K, Pessin JE, Cuatrecasas P, Saltiel AR. Mitogen activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J Biol Chem. 1994;270:20801–20807. doi: 10.1074/jbc.270.35.20801. [DOI] [PubMed] [Google Scholar]
- Leavesley DI, Scwartz MA, Rosenfeld M, Cheresh DA. Integrin β1 -and β3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol. 1993;121:163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie CC. Properties and regulation of cytosolic phospholipase A2. J Biol Chem. 1997;272:16709–16712. doi: 10.1074/jbc.272.27.16709. [DOI] [PubMed] [Google Scholar]
- Lih-Ling L, Lin A, Knopf JL. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci USA. 1992;89:6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lih-Ling L, Wartmann M, Lin AY, Knopf JL, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- Locati M, Lamorte G, Luini W, Introna M, Bernasconi S, Mantovani A, Sozzani S. Inhibition of monocyte chemotaxis to c-c chemokines by antisense oligonucleotide for cytosolic phospholipase A2. J Biol Chem. 1996;271:6010–6016. doi: 10.1074/jbc.271.11.6010. [DOI] [PubMed] [Google Scholar]
- Lu ML, McCarron RJ, Jacobson BS. Initiation of HeLa cell adhesion to collagen is dependent upon collagen receptor upregulation, segregation to the basal plasma membrane, clustering and binding to the cytoskeleton. J Cell Sci. 1992;101:873–883. doi: 10.1242/jcs.101.4.873. [DOI] [PubMed] [Google Scholar]
- Marks PW, Hendey B, Maxfield FR. Attachment to fibronectin or vitronectin makes human neutrophil migration sensitive to alterations in cytolic free calcium concentrations. J Cell Biol. 1991;112:149–158. doi: 10.1083/jcb.112.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino N, Mimura T, Hamasaki K, Tobe K, Ueki K, Kikuchi K, Takehara K, Kadowaki T, Yazaki Y, Nojima Y. Matrix/integrin interaction activates the mitogen-activated protein kinase, p44erk-1 and p42erk-2. J Biol Chem. 1995;270:269–273. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, Tischfield JA, Kudo I. J. Biol Chem. 1998;273:14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- Nahas N, Waterman WH, Sha’afi RI. Granulocyte-macrophage colony-stimulating factor (GM-CSF) promotes phosphorylation and an increase in the activity of cytosolic phospholipase A2 in human neutrophils. Biochem J. 1996;313:503–508. doi: 10.1042/bj3130503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalefski EA, Sultzman LA, Martin DM, Kriz RW, Towler PS, Knopf JL, Clark JD. Delineation of two functionally distinct domains of cytosolic phospholipase A2, a regulatory Ca2+-dependent lipid-binding domain and a Ca2+-independent catalytic domain. J Biol Chem. 1994;269:18239–18249. [PubMed] [Google Scholar]
- Nemenoff RA, Winitz S, Qian NX, Van Putten V, Johnson GL, Heasley LE. Phosphorylation and activation of a high molecular weight form of phospholipase A2 by p42 microtubule associated protein 2 kinase and protein kinase C. J Biol Chem. 1993;268:1960–1964. [PubMed] [Google Scholar]
- Piomelli D. Arachidonic acid in cell signaling, Curr. Opin Cell Biol. 1993;5:274–280. doi: 10.1016/0955-0674(93)90116-8. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Gijon MA, de Carvalho MS, Spencer DM, Leslie CC. The role of calcium and phosphorylation of cytosolic phospholipase A2 in regulating arachidonic acid release in macrophages. J Biol Chem. 1998;273:8203–8211. doi: 10.1074/jbc.273.14.8203. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Leslie CC. Protein kinase C-dependent and independent pathways of mitogen activated protein kinase activation in macrophages by stimuli that activate phospholipase A2. J Biol Chem. 1994;269:19480–19487. [PubMed] [Google Scholar]
- Rehfeldt W, Resch K, Goppelt-Struebe M. Cytosolic phospholipase A2 from human monocytic cells: characterization of substrate specificity and Ca2+-dependent membrane association. Biochem J. 1993;293:255–261. doi: 10.1042/bj2930255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw MW, Toksoz D, Schwartz MA. Involvement of the small GTPase rho in integin-meditated activation of mitogen-activated protein kinase. J Biol Chem. 1996;271:21691–21694. doi: 10.1074/jbc.271.36.21691. [DOI] [PubMed] [Google Scholar]
- Ross MI, Deems RA, Jesaitis AJ, Dennis EA, Ulevitch RJ. Phospholipase activities of the P388D1 macrophage-like cell line. Arch Biochem Biophys. 1985;238:247–258. doi: 10.1016/0003-9861(85)90162-6. [DOI] [PubMed] [Google Scholar]
- Schalkwijk CG, Spaargaren M, Defize LH, Verkleij AJ, van den Bosch H, Boonstra J. Epidermal growth factor (EGF) induces serine phosphorylation-dependent activation and calcium-dependent translocation of cytosolic phospholipase A2. Eur J Biochem. 1995;231:593–601. doi: 10.1111/j.1432-1033.1995.tb20737.x. [DOI] [PubMed] [Google Scholar]
- Schalkwijk CG, van der Heijden MAG, Bunt G, Maas R, Tertoolen LGJ, van Bergen en Henegouwen PMP, Verkleij AJ, van den Bosch H, Boonstra J. Maximal epidermal growth factor-induced cytosolic phospholipase A2 activation in vivo requires phosphorylation followed by an increased intracellular calcium concentration. Biochem J. 1996;313:91–96. doi: 10.1042/bj3130091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA. Transmembrane signalling by integrins. Trends Cell Biol. 1993;2:304–307. doi: 10.1016/0962-8924(92)90120-c. [DOI] [PubMed] [Google Scholar]
- Sharp JD, et al. Molecular cloning and expression of human Ca2+-sensitive cytosolic phospholipase A2. J Biol Chem. 1991;266:14850–14853. [PubMed] [Google Scholar]
- Stossel TP. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Tang J, Kriz RW, Wolfman N, Shaffer M, Seehra J, Jones SS. A novel cytosolic calcium-independent phospholipase A2 contains eight ankyrin motifs. J Biol Chem. 1997;272:8567–8575. doi: 10.1074/jbc.272.13.8567. [DOI] [PubMed] [Google Scholar]
- Teslenko V, Rogers M, Lefkowith JB. Macrophage arachidonate release via both the cytosolic Ca+2-dependent and -independent is necessary for cell spreading. Biochim Biophys Acta. 1997;1344:189–199. doi: 10.1016/s0005-2760(96)00137-3. [DOI] [PubMed] [Google Scholar]
- Waterman WH, Sha’afi RI. A mitogen-activated protein kinase independent pathway involved in the phosphorylation and activation of cytosolic phospholipase A2 in human neutrophils stimulated with tumor necrosis factor-181. Biochem Biophys Res Commun. 1995;209:271–278. doi: 10.1006/bbrc.1995.1499. [DOI] [PubMed] [Google Scholar]
- Wijkander J, Sundler R. A 100-kDa arachidonate-mobilizing phospholipase A2 in mouse spleen and the macrophage cell line. Eur J Biochem. 1991;202:873–880. doi: 10.1111/j.1432-1033.1991.tb16445.x. [DOI] [PubMed] [Google Scholar]
- Wolf BA, Turk J, Sherman WR, McDaniel ML. Intracellular Ca2+ mobilization by arachidonic acid. J Biol Chem. 1986;261:3501–3511. [PubMed] [Google Scholar]
- Wolf RA, Gross RW. Identification of neutral active phospholipase C which hydrolyzes choline glycerophospholipids and plasmalogen selective phospholipase A2 in canine myocardium. J Biol Chem. 1985;260:7295–7303. [PubMed] [Google Scholar]
- Wu T, Angus W, Yao X, Logun C, Shelhamer JH. p11, a unique member of the S100 family of calcium-binding proteins, interacts with and inhibits the activity of the 85kD cytosolic phospholipase A2. J Biol Chem. 1997a;272:17145–17153. doi: 10.1074/jbc.272.27.17145. [DOI] [PubMed] [Google Scholar]
- Wu T, Levine SJ, Cowan M, Logun C, Angus CW, Shelhamer JH. Antisense inhibition of 85kDa cPLA2 blocks arachidonic acid release from airway epithelial cells. Am J Physiol. 1997b;273:L331–L338. doi: 10.1152/ajplung.1997.273.2.L331. [DOI] [PubMed] [Google Scholar]
- Xing M, Insel PA. Role of extracellular-signal related kinase and PKC alpha in cytosolic PLA2 activation by bradykinin in MDCK-D1 cells. J Clin Invest. 1996;97:1302–1310. doi: 10.1152/ajpcell.1997.272.4.C1380. [DOI] [PubMed] [Google Scholar]
- Zhu X, Assoian RK. Integrin-dependent activation of MAP Kinase: a link to shape-dependent cell proliferation. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]