Abstract
Headache is a common complaint that affects the majority of the population at some point in their lives. The underlying pathological bases for headache symptoms are many, diverse, and often difficult to distinguish. Classification of headache is principally based on the evaluation of headache symptoms as well as clinical testing. Although manual therapy has been advocated to treat a variety of different forms of headache, the current evidence only supports treatment for cervicogenic headache (CGH). This form of headache can be identified from migraine and other headache forms by a comprehensive musculoskeletal examination. Examination and subsequent diagnosis is essential not only to identify patients with headache where manual therapy is appropriate but also to form a basis for selection of the most appropriate treatment for the identified condition. The purpose of this paper is to outline, in clinical terms, the classification of headache, so that the clinician can readily identify those patients with headache suited to manual therapy.
Keywords: Diagnosis, Headache Disorders, Physical Examination, Post-Traumatic Headache
Headache is the most prevalent pain disorder, affecting 66% of the global population1, and thereby it represents a major health problem, disturbing both quality of life and work productivity2,3. It was reported in 1999 that in the US alone, migraine headache cost American employers about $US13 billion per year because of missed workdays and impaired work function4.
The International Headache Society (IHS)5 has classified headaches as primary, where there is no other causative factor, or secondary, where the headache occurs in close temporal relationship to another disorder to which it is attributed. A list of 14 different headache forms have been documented by the IHS5. Further sub-classification is possible; for example, migraine can be sub-classified as migraine with or without aura, and again even further sub-classified. Since each form of headache has a different pathological basis and incorrect differential diagnosis will often lead to treatment failure, it is critical to correctly diagnosis the type of headache. This is of particular importance for manual therapy interventions as they are unlikely to be effective for the majority of headache forms. It should also be noted that different forms of headache may co-exist6, further presenting a challenge for differential diagnosis.
The most common form of headache is tension-type headache with a global prevalence of 38%1, whereas migraine has a prevalence of 10%1, chronic daily headache 3%1, and CGH 2.5–4.1%7,8. Prevalence alone, however, does not provide a complete picture of the disability associated with different forms of headache, as it does not include factors such as the frequency of attacks and intensity of symptoms. Although the prevalence of CGH is considerably lower than tension-type headache and migraine, patients with CGH have a substantial quality-of-life burden, comparable to patients with migraine and tension-type headache9.
The pathophysiological mechanisms underlying many of the classifications of headache are not well understood. In terms of research evaluation, migraine has received the most attention, and it is believed to involve abnormal brain function10, but the pathophysiology is still not clearly defined11.
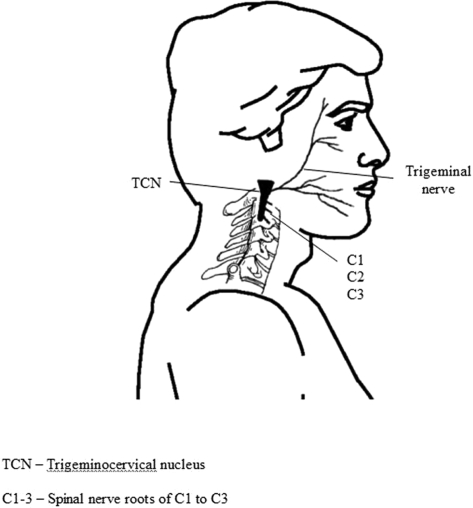
CGH arises primarily from musculoskeletal dysfunction in the upper three cervical segments12. The pathway by which pain originating in the neck can be referred to the head is the trigeminocervical nucleus (Figure 1), which descends in the spinal cord to the level of C3/4, and is in anatomical and functional continuity with the dorsal gray columns of these spinal segments13. Hence, input via sensory afferents principally from any of the upper three cervical nerve roots may mistakenly be perceived as pain in the head12, a concept known as convergence.
Figure 1.
Diagrammatic representation of the anatomical basis for convergence of sensory input from the upper 3 cervical nerve roots with the trigeminal nerve in the trigeminocervical nucleus.
Manual therapists have for some time treated the cervical spine in efforts to relieve headache14, but it is only recently that researchers have evaluated the effectiveness of such intervention for specific headache disorders. For example, Jull et al15, in a randomized controlled trial of high methodological quality, showed that manual therapy was an effective form of management for CGH. Furthermore, Bronfort et al16 reviewed the evidence for non-invasive physical treatments for five types of headache including “migraine,” “tension-type,” “CGH,” a mix of “migraine and tension-type,” and “post-traumatic” headache. They found evidence that both neck exercise (low-intensity endurance training) and spinal manipulation were effective in the short-term and long-term for CGH. In contrast, their review did not support the use of manual therapy for the long-term management of migraine or other headache forms. Similar reviews came to the same conclusions for the treatment of migraine and tension-type headache17,18. In one review, it was proposed that since the aetiology of tension-type headache, CGH, and migraine are different, manual therapy approaches should be different18, but this has not been investigated. When reviewing these data, the reader must take into consideration the relative paucity and low-level of evidence available19. There is an ongoing need for high-quality randomized controlled trials investigating the effectiveness of manual therapies for headache management before it will be possible to form firm conclusions.
Nonetheless, currently available evidence suggests that manual therapy is ineffective for some forms of headache. It follows that correct classification of the headache disorder is very important so that appropriate treatment can be given, and it could be argued that manual therapists have an ethical obligation to make an accurate diagnosis so resources are not wasted on physical treatment when patients would be better directed to more appropriate therapy.
One of the common diagnostic challenges in headache evaluation is to distinguish CGH from other headache forms20,21. Indeed, studies have shown that an incorrect headache diagnosis may occur in more than 50% of cases22. Diagnosis is essentially based on the presenting symptoms, together with the clinical physical examination findings23. Similarities of signs and symptoms among the many types of headache24 undoubtedly contribute to the challenge of differentiating between some headache forms, whereas those forms with unique characteristics are more readily identified.
Examination
Subjective Examination
It is of primary concern to exclude serious or life-threatening pathology such as cranial tumors, meningitis, giant cell arteritis, sub-arachnoid hemorrhage, carotid artery, or vertebral artery dissection, among others. Although these are relatively rare25, the clinician should be vigilant for historical features or “red flags” suggestive of such disorders. “Red flags” include (1) sudden onset of a new severe headache; (2) a worsening pattern of a pre-existing headache in the absence of obvious predisposing factors; (3) headache associated with fever, neck stiffness, skin rash, and with a history of cancer, HIV, or other systemic illness; (4) headache associated with focal neurologic signs other than typical aura; (5) moderate or severe headache triggered by cough, exertion, or bearing down; and (6) new onset of a headache during or following pregnancy26. Patients with one or more red flags should be referred for an immediate medical consultation and further investigation.
Some headaches are easy to differentiate from CGH due to their distinctive subjective characteristics. For example, cluster headaches, paroxysmal hemicranias, and other trigeminal autonomic cephalalgias typically present with very severe unilateral but short-lasting headache. According to the IHS5, headache duration may be as short as 2 minutes with a frequency of 5 per day for paroxysmal hemicrania. Typically, the pain is associated with autonomic features of eye tearing, nasal stuffiness, facial sweating, and ptosis. Taken as a whole, these characteristics are not consistent with CGH and patients presenting with such symptoms should seek medical consultation, as they are unlikely to respond to manual therapy.
Migraine with aura also has distinctive symptoms27, such as flickering lights or spots in the field of vision, numbness, or pins and needles, all of which are fully reversible, lasting less than 60 minutes. Typical characteristics of migraine without aura include pain of unilateral location, pulsating quality, moderate or severe intensity, lasting a fixed time period of 24–72 hours, aggravated by routine physical activity such as stair-climbing, and associated with nausea, photophobia, or phonophobia5.
Characteristics of chronic tension-type headache include headache lasting from 30 minutes to 7 days, of pressing or tightening quality, mild to moderate intensity, bilateral location, and no aggravation by physical activity. In addition, there should be no associated features of nausea, vomiting, photophobia, or phonophobia5.
The profile of sufferers of CGH varies according to the population under review. Hospital-based studies reveal an 85–88% female preponderance8,28; in contrast, a large-scale community-based study revealed a 71% male preponderance8. This difference was explained by the reluctance of males to seek treatment. Mean age at onset has been reported as 33–43 years, and a mean duration of symptoms of 7–17 years7,8. Chronicity appears to develop through increasing frequency of short-lasting headache attacks, rather than continuous unrelenting pain.
Characteristics of CGH according to the Cervicogenic Headache International Study Group23,29 are shown in Table 1. Unfortunately, a number of headache characteristics are shared between the common headache forms and CGH, except for the presence of non-throbbing pain that usually starts in the neck, with episodes of varying duration23.
TABLE 1.
CGH: Cervicogenic Headache International Study Group diagnostic criteria23.
| Major criteria | |
|---|---|
|
|
| Head pain characteristics | |
|
|
| Other characteristics of some importance | |
|
|
| Other features of lesser importance | |
|
|
Vincent and Luna28 examined the validity of Sjaastad et al's 1990 diagnostic criteria in patients with CGH, tension-type headache, and migraine. Patients with CGH met significantly more criteria than those with tension-type headache or migraine. However, 30% of patients with CGH met the IHS criteria for migraine, whereas only 3% of patients with CGH met the criteria for tension-type headache; the remaining 66% of patients could not be classified according to IHS criteria as having either migraine or tension-type headache. Antonaci et al30 reported that at least five items of Sjaastad et al's 1990 diagnostic criteria must be present in order to establish a diagnosis of CGH. Furthermore, Vincent31 has shown that if seven or more of these criteria were present, then cervicogenic headache could be distinguished from migraine and tension-type headache with a high level of sensitivity and a moderate level of specificity. Moreover, if pain is first experienced in the neck and then spreads to the frontal region and is unilateral, the chance of correctly identifying patients as CGH increases significantly. While unilaterality is a strong diagnostic indicator of CGH, in clinical practice bilateral symptoms do not preclude CGH32, as there is a strong case for a “unilaterality of two sides”33.
A recent community-based study revealed differences in headache profile between sufferers of relatively “pure” CGH and “pure” migraine8,34. Migraine sufferers are more likely to be female, and more frequently report nausea, photophobia, phonophobia, and throbbing pain. In addition, headache onset is in the anterior head and is infrequently brought on by mechanical provocative activity (sustained or awkward neck positioning) and exacerbated by a change in spatial orientation (standing from lying, or forward bending to upright)34.
While efforts have been made to try to isolate patients with “pure” CGH from other headache forms, it is apparent that there is a significant proportion of headache sufferers who cannot be categorized into such a group as they have mixed features of CGH, migraine, tension-type headache, or other headache forms. Indeed Fishbain et al6 in their study of pain clinic patients presenting with headache found that 84% had neck pain. The most common predictor of headache onset across diagnostic groups was severe headache beginning in the neck, and 44% had more than one headache form. This suggests that there are patients with either concurrent headache diagnoses35 or perhaps there is a continuum across different headache forms.
Taken as a whole, the information from the subjective examination should point towards the possible involvement of the cervical spine in headache pathogenesis and that further physical examination is required to confirm the diagnosis. A summary of the subjective criteria is shown in Table 2.
TABLE 2.
| Migraine | CGH | |
|---|---|---|
| Gender ratio | 1.69 female/male | 0.71 female/male |
| Age at onset | 18 years | 33 years |
| Headache onset | Anterior head | Posterior head/neck |
| Pain area | 50% unilateral | Predominately unilateral |
| Nausea | Frequent | Infrequent |
| Photo/phonophobia | Very frequent | Infrequent |
| T robbing pain | Frequent | Infrequent |
| Pain increases when bending forward | Very frequent | Infrequent |
| Migraine medication | Usually helpful | Not helpful |
| Sustained/awkward neck position provokes pain | Rare | Universal |
Physical Examination
Although up to 70% of individuals with frequent intermittent headache report accompanying neck pain36,37, less than 18% are thought to be symptoms of neck pathology38. One explanation for this may be the convergence of afferent information from the sensitized trigeminal afferents with the upper three cervical nerves in the trigeminocervical nucleus39. In this way, pain arising in the head/face is being perceived as pain in the neck. Consequently, physical examination of the neck is a critical component of differential diagnosis23,35,40,41,41. Physical examination criteria include clinical, laboratory, and/or imaging evidence of a disorder within the cervical spine or soft tissues of the neck known to be a valid cause of headache. In the absence of this, there should be evidence that the headache can be attributed to the neck disorder based on clinical signs that implicate a source of pain in the neck, or abolition of headache following diagnostic blockade (pain-relieving injection) of a cervical structure or its nerve supply5. Essentially, any structure that is innervated by the upper three cervical nerves is a potential source of headache. Hence, the clinical examination must potentially encompass the articular, neural, and myofascial structures shown in Figure 1.
Articular System
The CGH International Study Group considers restricted range of motion of the neck to be one of the major diagnostic criteria for CGH29. Some40,42,43 but not all44,45 studies have reported diminished cervical ROM in subjects with CGH with limitation of active movement in the sagittal plane, in particular extension, as the major loss. Other studies, however, have not found any limitations44,45.
As previously stated, CGH arises primarily from musculoskeletal dysfunction in the upper three cervical segments12. Manual examination has high sensitivity and specificity to detect the presence or absence of cervical joint dysfunction in neck pain and headache patients46,47,48. Moreover, Zito et al42 determined that the presence of upper cervical joint dysfunction measured by manual examination, in comparison to measures of posture, range of motion, cervical kinaesthesia, and craniocervical muscle function49, most clearly identified CGH sufferers. The term manual examination incorporates tests of passive physiological intervertebral motion, as well as passive accessory intervertebral motion, such as posteroanterior pressures. Motion restriction and symptom responses indicate the most painful dysfunctional cervical motion segment46,47,48. However, these tests require a high degree of skill on the part of the therapist, and their reliability has been questioned50. It has been suggested though that this may be a reflection of poor research methods rather than being an unreliable test50. More recently, Jull et al40 and Amiri et al35 have condensed the manual examination procedure to include only passive accessory intervertebral motion assessed using posteroanterior pressure. Identification of a pain response, rather than joint hyper- or hypomobility, simplifies the identification of cervical dysfunction, thereby reducing the skill required. However, this information on its own is not sufficient to provide adequate sensitivity and specificity to identify CGH; other forms of assessment are required40.
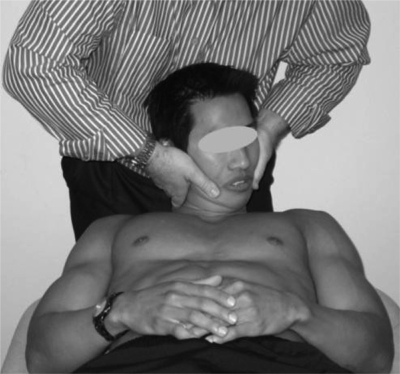
The cervical flexion-rotation test (FRT) is an objective method of determining upper cervical joint dysfunction that is showing promise in the identification of patients with CGH. The FRT (Figure 2) is a simplified form of manual examination developed to identify C1/2 dysfunction51. In this test procedure, the cervical spine is fully flexed and should allow unrestricted motion at C1/2, which has a unique ability to rotate in any cervical posture. As movement at other cervical segments would be constrained by this end-range position, movement is isolated to the C1/2 segment.
Figure 2.
The cervical flexion-rotation test.
Range of rotation in end-range flexion is normally 40–44° to each side45,52. In contrast, subjects with C1/2 dysfunction have significantly less rotation20,45,53,54. When administered by highly trained manual therapists, the FRT has high sensitivity (91%) and specificity (90%) in differentiating subjects with CGH from asymptomatic controls or subjects with migraine with aura20. Data from the same study demonstrated that a range limited to 32° or less may be considered positive.
Subjects in that study were selected according to strict criteria to ensure that the comparative groups were discrete and that there was no cervical involvement in the migraine or control groups. Hence the reliability, sensitivity, and specificity determined in this study may be higher than levels occurring in a clinical environment where patients are likely to be more heterogenous. However, similar values (diagnostic accuracy = 89%; kappa = 0.85, positive cut-off value = 33°) have been reported when the FRT was evaluated in a more heterogenous sample including subjects with CGH arising from levels other than C1/255. This study also demonstrated that inexperienced examiners could use the FRT test. Although inexperienced examiners recorded larger ranges of motion for the FRT, the sensitivity (>83%), specificity (>83%), and agreement (kappa >0.67) were still within acceptable values.
Another advantage of the FRT is that it is independent of other physiological and lifestyle factors56. In a cross-sectional study, whole cervical cardinal plane active movement, sleeping posture, age, gender, hand-dominant recreation, or occupation (activity involving repetitive use of one side of the body) were correlated with FRT mobility. Multiple linear regression analysis demonstrated that 59% of the variance in the FRT (ROM) was explained by the presence of pain and cervical lateral flexion measures. Consequently, the test has utility regardless of the age, gender, or lifestyle of the patient.
Muscle System
Muscle dysfunction has also been identified as an important feature of CGH40,42,57,58. It has been suggested that dysfunction may include loss of postural alignment and neuromuscular control as well as muscle weakness, endurance, and extensibility59. A reflection of the importance of the muscle system to CGH is shown by the long-term improvement in headache symptoms as a result of exercise designed to retrain the muscle system in patients with CGH15.
Posture is an indirect measure of the functional status of the neuromuscular system. While one early study found an association between forward head posture and CGH58, which has been cited in the literature, this association has not been substantiated by more recent studies, and postural change is not a unique feature to sufferers of CGH42,44,60.
Impairments in muscle strength and endurance of the deep neck flexors appear to be one of the defining features of CGH42,57,58. Similar impairments were not present in migraine or tension-type headache40. The craniocervical flexion test indirectly measures deep neck flexor function61, and it has been shown to have good reliability57. This test is performed in crook-lying, and it requires the patient to perform upper cervical flexion in five stages of increasing range, holding each position for up to 10 seconds. The range of upper cervical spine flexion has been shown using electromyography to be directly related to the activation of the deep neck flexors62 in asymptomatic controls. While it is not possible to directly palpate the deep neck flexors, it is possible to palpate the superficial flexors muscles, which should be minimally active during this test. One of the key features that clinically identifies deep neck flexor dysfunction is increased superficial flexor muscle during the craniocervical flexion test62, in an attempt to gain range of motion.
There are a number of reports of muscle tightness42,57,60,63 and trigger points64 associated with CGH. Various muscles have been implicated, including upper trapezius, sternocleidomastoid, scalenes, levator scapulae, pectoralis major and minor, and short sub-occipital extensors. In one study, muscle tightness was found in 35% of CGH subjects compared to only 17% in migraine and 16% for tension-type headache subjects42; in that study, no one muscle predominated. An earlier study found muscle tightness predominated in the upper trapezius muscle57.
Sensorimotor disturbance has been implicated in neck disorders. Clinical measures of sensorimotor disturbance include cervical joint position sense, postural stability, and oculomotor control; these have been described elsewhere65. Dizziness, neck pain, and headache are a common feature of sensorimotor disturbance of the cervical spine66. However, joint position sense or cervical kinaesthesia has been shown to be no different in subjects with either migraine, CGH, or tension-type headache40.
Neural System
The IHS5 recognizes a variety of neural disorders that can cause headache. These can be broadly classified under disorders of the neck and cranial neuralgias and include, among others, occipital neuralgia, neck-tongue syndrome, post-herpetic neuralgia, and trigeminal neuralgia. Classification of neuropathic pain, based on aetiology such as occipital neuralgia, has inherent problems as pathology of a nerve does not always cause pain67,68. In contrast, it has been proposed that neuropathic pain be classified according to a dominance of patho-mechanisms69. Neural tissue-related pain disorders have been classified70 as either 1) peripheral nerve sensitization exhibiting increased nerve trunk mechano-sensitivity; 2) denervation with neurological deficit; or 3) central sensitization showing positive features (paraesthesia, allodynia, dysaethesia, hyperalgesia and stimulus independent pain). In the upper cervical spine, denervation disorders are relatively rare compared to the lower cervical region. In part this is due to the difference in gross anatomy, with no disc and the relatively small nerve root/spinal nerve, with comparatively more free space in the intervertebral foramen, in the upper cervical region.
In our clinical experience, the most prevalent neural disorders causing headache present with peripheral nerve sensitization but normal neurological function. Peripheral nerve sensitization can be assessed according to the principles described by Elvey and Hall71. There should be evidence of pain provocation and limitation of movement during neural tissue provocation tests, which elongate the upper cervical neural structures. In addition, there should be evidence of pain on palpation of the same tissues. An important test in this respect is upper cervical flexion, which elongates the neuromeningeal tissues in the high cervical region72. To distinguish pain responses of peripherally sensitized neural tissue from adjacent joints and muscles, it is important to repeat the test with the arms positioned in abduction, or the lower limbs in straight-leg-raise, to increase the mechanical provocation of, and thereby implicate, the neural tissue.
Although there is some evidence of altered responses to neural tissue provocation tests in subjects with CGH when compared to migraine73, the presence of increased neural tissue mechano-sensitivity in patients with CGH is relatively rare, with the reported incidence between 7 and 10%42. Nevertheless, it is important to identify these patients as they usually respond inadequately to joint mobilization or motor control retraining.
Headache patients with a dominance of peripheral nerve sensitization usually present with a very typical pattern. They tend to adopt an antalgic poker chin posture. Active upper cervical flexion is diminished in range, and in long sitting this movement is more provocative. Similar restriction is demonstrated passively in supine as previously discussed. Finally, palpation of nerve trunks arising from the upper cervical spine is also provocative, for example, the greater occipital nerve, lesser occipital nerve, or third occipital nerve.
In this article, we have outlined various aspects of examination that will assist clinicians in identifying headache patients with disorders that are likely to respond to manual therapy intervention. While the individual items of assessment may be of importance, the time-honored approach is to consider the whole examination rather than individual components. This has been supported by a recent study that sought to identify which aspects of the physical examination distinguished subjects with CGH from migraine and tension-type headache. Analysis revealed that collectively, restricted neck movement, in association with evidence on manual examination of upper cervical joint dysfunction and impairment in the deep neck flexors identified by the craniocervical flexion test, had 100% sensitivity and 94% specificity to identify CGH40. While these three features have been shown to be important in identifying CGH, another study has shown no clear pattern of predictors from variables in subjects' demographics and headache history, which might identify those who achieve a significant reduction in headache following manual therapy intervention74.
Conclusion
Headache is a very common complaint, arising from a variety of different causes, not all of which are amenable to manual therapy intervention. The key to identifying appropriate patients is to interpret information from all aspects of the examination including the subjective history. This article has outlined the now considerable evidence underpinning the identification of patients with CGH.
Contributor Information
Toby Hall, School of Physiotherapy, Curtin University of Technology, Bentley, Western Australia..
Kathy Briffa, School of Physiotherapy, Curtin University of Technology, Bentley, Western Australia..
Diana Hopper, Associate Professor, School of Physiotherapy, Curtin University, Perth, Australia..
REFERENCES
- 1.Stovner L, Hagen K, Jensen R, et al. The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 2.Diener I. The impact of cervicogenic headache on patients attending a private physiotherapy practice in Cape Town. S Afr J Physiother. 2001;57:35–39. [Google Scholar]
- 3.Lipton RB, Stewart WF. The epidemiology of migraine. Eur Neurol. 1994;34(Suppl 2):6–11. doi: 10.1159/000119525. [DOI] [PubMed] [Google Scholar]
- 4.Hu H, Markson L, Lipton R, Stewart W, Berger M. Burden of migraine in the United States: Disability and economic costs. Arch Intern Med. 1999;1:813–818. doi: 10.1001/archinte.159.8.813. [DOI] [PubMed] [Google Scholar]
- 5.International Headache Society The International Classification of Headache Disorders. 2nd ed. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 6.Fishbain DA, Cutler R, Cole B, Rosomoff HL, Rosomoff RS. International Headache Society headache diagnostic patterns in pain facility patients. Clin J Pain. 2001;17:78–93. doi: 10.1097/00002508-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Haldeman S, Dagenais S. Cervicogenic headaches: A critical review. Spine J. 2001;1:31–46. doi: 10.1016/s1529-9430(01)00024-9. [DOI] [PubMed] [Google Scholar]
- 8.Sjaastad O, Bakketeig LS. Prevalence of cervicogenic headache: Vaga study of headache epidemiology. Acta Neurol Scand. 2008;117:170–183. doi: 10.1111/j.1600-0404.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 9.van Suijlekom HA, Lame I, Stomp-van den Berg SG, Kessels AG, Weber WE. Quality of life of patients with cervicogenic headache: A comparison with control subjects and patients with migraine or tension-type headache. Headache. 2003;43:1034–1041. doi: 10.1046/j.1526-4610.2003.03204.x. [DOI] [PubMed] [Google Scholar]
- 10.Buzzi M, Moskowitz M. The pathophysiology of migraine: Year 2005. J Headache Pain. 2005;6:105–111. doi: 10.1007/s10194-005-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aurora SK, Barrodale PM, Tipton RL, Khodavirdi A. Brainstem dysfunction in chronic migraine as evidenced by neurophysiological and positron emission tomography studies. Headache. 2007;47:996–1003. doi: 10.1111/j.1526-4610.2007.00853.x. discussion 1004–1007. [DOI] [PubMed] [Google Scholar]
- 12.Bogduk N. Headache and the neck. In: Goadsby P, Silberstein S, editors. Headache. Melbourne, Australia: Butterworth-Heinemann; 1997. [Google Scholar]
- 13.Edmeads J. Disorders of the neck: Cervicogenic headache. In: Silberstein SD, Lipton RB, Dalessio DJ, editors. Wolff's Headache and Other Head Pain. Oxford, UK: Oxford University Press; 2001. [Google Scholar]
- 14.Edeling J. Migraine and other chronic headaches: Preliminary report on experimental physical treatment. S Afr J Physiother. 1974;30:2–3. [Google Scholar]
- 15.Jull G, Trott P, Potter H, et al. A randomized controlled trial of exercise and manipulative therapy for cervicogenic headache. Spine. 2002;27:1835–1843. doi: 10.1097/00007632-200209010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Bronfort G, Nilsson N, Haas M, et al. Non-invasive physical treatments for chronic/recurrent headache. Cochrane Database Syst Rev. 2004;(3) doi: 10.1002/14651858.CD001878.pub2. CD001878. [DOI] [PubMed] [Google Scholar]
- 17.Biondi DM. Physical treatments for headache: A structured review. Headache. 2005;45:738–746. doi: 10.1111/j.1526-4610.2005.05141.x. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-de-Las-Penas C, Alonso-Blanco C, Cuadrado ML, Miangolarra JC, Barriga FJ, Pareja JA. Are manual therapies effective in reducing pain from tension-type headache? A systematic review. Clin J Pain. 2006;22:278–285. doi: 10.1097/01.ajp.0000173017.64741.86. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-des-las-Penas C, Alonos-Blanco C, San-Roman J, Miangolarra-Page J. Methodological quality or randomized controlled trials of spinal manipulation and mobilisation in tension-type headache, migraine, and cervicogenic headache. J Orthop Sports Phys Ther. 2006;36:160–169. doi: 10.2519/jospt.2006.36.3.160. [DOI] [PubMed] [Google Scholar]
- 20.Ogince M, Hall T, Robinson K. The diagnostic validity of the cervical flexion-rotation test in C1/2-related cervicogenic headache. Man Ther. 2007;12:256–262. doi: 10.1016/j.math.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Sjaastad O, Bovim G. Cervicogenic headache: The differentiation from common migraine. An overview. Funct Neurol. 1991;6:93–100. [PubMed] [Google Scholar]
- 22.Pfaffenrath V, Kaube H. Diagnostics of cervicogenic headache. Funct Neurol. 1990;5:159–164. [PubMed] [Google Scholar]
- 23.Sjaastad O, Fredriksen TA, Pfaffenrath V. Cervicogenic headache: Diagnostic criteria. The Cervicogenic Headache International Study Group. Headache. 1998;38:442–445. doi: 10.1046/j.1526-4610.1998.3806442.x. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson GG, Gaston J. Cervical headache. J Orthop Sports Phys Ther. 2001;31:184–193. doi: 10.2519/jospt.2001.31.4.184. [DOI] [PubMed] [Google Scholar]
- 25.Landtblom AM, Fridriksson S, Boivie J, Hillman J, Johansson G, Johansson I. Sudden onset headache: A prospective study of features, incidence and causes. Cephalalgia. 2002;22:354–360. doi: 10.1046/j.1468-2982.2002.00368.x. [DOI] [PubMed] [Google Scholar]
- 26.Bigal ME, Lipton RB. The differential diagnosis of chronic daily headaches: An algorithm-based approach. J Headache Pain. 2007;8 doi: 10.1007/s10194-007-0418-3. DOI 10.1007/s10194–007–0418–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirchmann M. Migraine with aura: New understanding from clinical epidemiologic studies. Curr Opin Neurol. 2006;19:286–293. doi: 10.1097/01.wco.0000227040.16071.a9. [DOI] [PubMed] [Google Scholar]
- 28.Vincent MB, Luna RA. Cervicogenic headache: A comparison with migraine and tension-type headache. Cephalalgia. 1999;19(Suppl 25):11–16. doi: 10.1177/0333102499019s2503. [DOI] [PubMed] [Google Scholar]
- 29.Sjaastad O, Fredriksen TA, Pfaffenrath V. Cervicogenic headache: Diagnostic criteria. Headache. 1990;30:725–726. doi: 10.1111/j.1526-4610.1990.hed3011725.x. [DOI] [PubMed] [Google Scholar]
- 30.Antonaci F, Ghirmai S, Bono G, Sandrini G, Nappi G. Cervicogenic headache: Evaluation of the original diagnostic criteria. Cephalalgia. 2001;21:573–583. doi: 10.1046/j.0333-1024.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- 31.Vincent M. Validation of criteria for cervicogenic headache. Funct Neurol. 1998;13:74–75. [PubMed] [Google Scholar]
- 32.Antonaci F, Bono G, Chimento P. Diagnosing cervicogenic headache. J Headache Pain. 2006;7:145–148. doi: 10.1007/s10194-006-0277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jansen J, Sjaastad O. Cervicogenic headache: Smith/Robinson approach in bilateral cases. Funct Neurol. 2006;21:205–210. [PubMed] [Google Scholar]
- 34.Sjaastad O, Bakketeig LS. Migraine without aura: Comparison with cervicogenic headache. Vaga study of headache epidemiology. Acta Neurol Scand. 2007;PMID:18031560. doi: 10.1111/j.1600-0404.2007.00966.x. [DOI] [PubMed] [Google Scholar]
- 35.Amiri M, Jull G, Bullock-Saxton J, Darnell R, Lander C. Cervical musculoskeletal impairment in frequent intermittent headache. Part 2: Subjects with concurrent headache types. Cephalalgia. 2007;27:891–898. doi: 10.1111/j.1468-2982.2007.01346.x. [DOI] [PubMed] [Google Scholar]
- 36.Blau J, MacGregor E. Migraine and the neck. Headache. 1994;34:88–90. doi: 10.1111/j.1526-4610.1994.hed3402088.x. [DOI] [PubMed] [Google Scholar]
- 37.Henry P, Dartigues J, Puymirat C, Peytour P, Lucas J. The association cervicalgia-headaches: An epidemiologic study. Cephalalgia. 1987;7(Suppl 6):189–190. [Google Scholar]
- 38.Nilsson N. The prevalence of cervicogenic headache in a random population sample of 20–59-year-olds. Spine. 1995;20:1884–1888. doi: 10.1097/00007632-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Bogduk N. Cervicogenic headache: Anatomical basis and pathophysiological mechanisms. Journal of Current Pain Headache Report. 2001;5:382–386. doi: 10.1007/s11916-001-0029-7. [DOI] [PubMed] [Google Scholar]
- 40.Jull G, Amiri M, Bullock-Saxton J, Darnell R, Lander C. Cervical musculoskeletal impairment in frequent intermittent headache. Part 1: Subjects with single headaches. Cephalalgia. 2007;27:793–802. doi: 10.1111/j.1468-2982.2007.01345.x. [DOI] [PubMed] [Google Scholar]
- 41.Sjaastad O, Fredriksen T, Pareja J, Stolt-Nielsen A, Vincent M. Co-existence of cervicogenic headache and migraine without aura. Funct Neurol. 1999;14:209–218. [PubMed] [Google Scholar]
- 42.Zito G, Jull G, Story I. Clinical tests of musculoskeletal dysfunction in the diagnosis of cervicogenic headache. Man Ther. 2006;11:118–129. doi: 10.1016/j.math.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Zwart JA. Neck mobility in different headache disorders. Headache. 1997;37:6–11. doi: 10.1046/j.1526-4610.1997.3701006.x. [DOI] [PubMed] [Google Scholar]
- 44.Dumas JP, Arsenault AB, Boudreau G, et al. Physical impairments in cervicogenic headache: Traumatic vs. non-traumatic onset. Cephalalgia. 2001;21:884–893. doi: 10.1046/j.1468-2982.2001.00264.x. [DOI] [PubMed] [Google Scholar]
- 45.Hall T, Robinson K. The flexion-rotation test and active cervical mobility: A comparative measurement study in cervicogenic headache. Man Ther. 2004;9:197–202. doi: 10.1016/j.math.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Sandmark H, Nisell R. Validity of five common manual neck pain provocation tests. Scand J Rehabil Med. 1995;27:131–136. [PubMed] [Google Scholar]
- 47.Jull G, Bogduk N, Marsland A. The accuracy of manual diagnosis for cervical zygapophyseal joint pain syndromes. Med J Aust. 1988;148:233–236. doi: 10.5694/j.1326-5377.1988.tb99431.x. [DOI] [PubMed] [Google Scholar]
- 48.Jull G, Zito G, Trott P, Potter H, Shirley D, Richardson C. Inter-examiner reliability to detect painful upper cervical joint dysfunction. Aust J Physiother. 1997;43:125–129. doi: 10.1016/s0004-9514(14)60406-2. [DOI] [PubMed] [Google Scholar]
- 49.Maitland G, Hengeveld E, Banks K, English K. Maitland's Vertebral Manipulation. 6th ed. London, UK: Butterworth Heinemann; 2001. [Google Scholar]
- 50.Stochkendahl M, Christensen H, Hartvigsen J, et al. Manual examination of the spine: A systematic review of reproducibility. J Manipulative & Physiol Ther. 2006;29:475–485. doi: 10.1016/j.jmpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Dvorak J, Herdmann J, Janssen B, Theiler R, Grob D. Motor-evoked potentials in patients with cervical spine disorders. Spine. 1990;15:1013–1016. doi: 10.1097/00007632-199015100-00006. [DOI] [PubMed] [Google Scholar]
- 52.Amiri M, Jull G, Bullock-Saxton J. Measuring range of active cervical rotation in a position of full head flexion using the 3D Fastrak measurement system: An intra-tester reliability study. Man Ther. 2003;8:176–179. doi: 10.1016/s1356-689x(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 53.Dvorak J, Antinnes JA, Panjabi M, Loustalot D, Bonomo M. Age- and gender-related normal motion of the cervical spine. Spine. 1992;17(Suppl 10):393–398. doi: 10.1097/00007632-199210001-00009. [DOI] [PubMed] [Google Scholar]
- 54.Hall T, Robinson K, Fujinawa O, Kiyokazu A. The influence of examiner experience on interpretation of the cervical flexion-rotation test 15th International World Confederation for Physical Therapy. Vancouver; 2007.
- 55.Hall T, Robinson K, Fujinawa O, Kiyokazu A. Inter-tester reliability and diagnostic validity of the cervical flexion-rotation test in cervicogenic headache. J Manipulative & Physiol Ther. 2007 doi: 10.1016/j.jmpt.2008.03.012. Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 56.Smith K, Hall T, Robinson K. The influence of age, gender, lifestyle factors and sub-clinical neck pain on cervical range of motion. Man Ther. 2007 doi: 10.1016/j.math.2007.07.005. DOI:10.1016/j.math.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Jull G, Barrett C, Magee R, Ho P. Further clinical clarification of the muscle dysfunction in cervical headache. Cephalalgia. 1999;19:179–185. doi: 10.1046/j.1468-2982.1999.1903179.x. [DOI] [PubMed] [Google Scholar]
- 58.Watson DH, Trott PH. Cervical headache: An investigation of natural head posture and upper cervical flexor muscle performance. Cephalalgia. 1993;13:272–284. doi: 10.1046/j.1468-2982.1993.1304272.x. discussion 232. [DOI] [PubMed] [Google Scholar]
- 59.Jull G, Niere K. The cervical spine and headache. In: Boyling G, Jull G, editors. Grieve's Modern Manual Therapy. London, UK: Churchill Livingstone; 2004. [Google Scholar]
- 60.Treleaven J, Jull G, Atkinson L. Cervical musculoskeletal dysfunction in post-concussional headache. Cephalalgia. 1994;14:273–279. doi: 10.1046/j.1468-2982.1994.1404273.x. [DOI] [PubMed] [Google Scholar]
- 61.Falla D. Unravelling the complexity of muscle impairment in chronic neck pain. Man Ther. 2004;9:125–133. doi: 10.1016/j.math.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Falla DL, Jull GA, Hodges PW. Patients with neck pain demonstrate reduced electromyographic activity of the deep cervical flexor muscles during performance of the craniocervical flexion test. Spine. 2004;29:2108–2114. doi: 10.1097/01.brs.0000141170.89317.0e. [DOI] [PubMed] [Google Scholar]
- 63.McDonnell M, Sahrmann S, Van Dillen L. A specific exercise program and modification of postural alignment for the treatment of cervicogenic headache: A case report. J Orthop Sports Phys Ther. 2005;35:3–15. doi: 10.2519/jospt.2005.35.1.3. [DOI] [PubMed] [Google Scholar]
- 64.Roth JK, Roth RS, Weintraub JR, Simons DG. Cervicogenic headache caused by myofascial trigger points in the sternocleidomastoid: A case report. Cephalalgia. 2007;27:375–380. doi: 10.1111/j.1468-2982.2007.01296.x. [DOI] [PubMed] [Google Scholar]
- 65.Treleaven J. Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control. Man Ther. 2007 doi: 10.1016/j.math.2007.06.003. DOI:10.1016/j.math.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 66.Reid S, Rivett D, Callister R, Katekar M. Sustained natural apophyseal glides (SNAGS) are an effective treatment for cervicogenic dizziness. Man Ther. 2007 doi: 10.1016/j.math.2007.03.006. DOI:10.1016/j.math.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 67.Beattie P, Meyers S, Stratford P, Millard R, Hollenberg G. Associations between patient report of symptoms and anatomic impairment visible on lumbar magnetic resonance imaging. Spine. 2001;25:819–828. doi: 10.1097/00007632-200004010-00010. [DOI] [PubMed] [Google Scholar]
- 68.Boden S, Davis D, Dina T, Patronas N, Wiesel S. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects: A prospective investigation. Journal of Bone and Joint Surgery. 1990;72:403–408. [PubMed] [Google Scholar]
- 69.Backonja MM. Defining neuropathic pain. Anesth Analg. 2003;97:785–790. doi: 10.1213/01.ANE.0000062826.70846.8D. [DOI] [PubMed] [Google Scholar]
- 70.Schafer A, Hall TM, Briffa K. Classification of low back-related leg pain: A proposed pathomechanism-based approach. Man Ther. 2007 doi: 10.1016/j.math.2007.10.003. DOI:10.1016/j.math.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Elvey R, Hall T. Neural tissue evaluation and treatment. In: Donatelli R, editor. Physical Therapy of the Shoulder. New York: Churchill Livingstone; 1997. [Google Scholar]
- 72.Hall TM, Elvey RL. Management of mechano-sensitivity of the nervous system in spinal pain syndromes. In: Boyling G, Jull G, editors. Grieve's Modern Manual Therapy. London, UK: Churchill Livingstone; 2004. [Google Scholar]
- 73.von Piekartz HJ, Schouten S, Aufdemkampe G. Neurodynamic responses in children with migraine or cervicogenic headache versus a control group: A comparative study. Man Ther. 2007;12:153–160. doi: 10.1016/j.math.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Jull GA, Stanton WR. Predictors of responsiveness to physiotherapy management of cervicogenic headache. Cephalalgia. 2005;25:101–108. doi: 10.1111/j.1468-2982.2004.00811.x. [DOI] [PubMed] [Google Scholar]