Abstract
Myelopathy is a form of neurological disease caused by compression of the spinal cord. Upper and lower quarter screens are commonly used in identifying myelopathy, although most of the screen components demonstrate poor or unstudied diagnostic value. The purpose of this case report is to describe the diagnostic process in detecting syringomyelia, an intramedullary lesion that may cause myelopathy. The patient was a 47-year-old female with a thoracic syrinx that was discovered by spinal magnetic resonance imaging (MRI) following a complicated and delayed clinical diagnostic course. Following surgical intervention and a two-week inpatient rehabilitation stay, the patient was discharged using a rolling walker for ambulation and was performing most transfers with modified independence. A complicating pattern of signs and symptoms combined with a diagnostic process guided by poorly studied screen components demonstrates the diagnostic dilemma associated with identifying the cause of myelopathy within the thoracic spine. This also indicates the need for further investigation of individual and clustered components of the neurological screen to improve the ability to identify patients in need of complete imaging studies in a more timely fashion.
Keywords: Intramedullary Lesion, Myelopathy, Spinal Cord, Syrinx
Myelopathy is a pathological disease process that is derived from compression or ischemia of the spinal cord1,2,3. Diagnosis of myelopathy is difficult for a number of reasons, including the variable natural history of the disease process, inconsistencies in pattern of presentation, and poorly described clinical tests with low or marginal quality4. Recognition of the typical pattern of findings indicative of myelopathy is critical in the physical therapy setting, as this condition warrants referral for further exploration of the underlying cause.
Myelopathy may be a result of numerous different lesions, including a variety of uncommon intramedullary lesions, extramedullary intradural lesions, and extradural lesions (Table 1)1,3. Syringomyelia is an intramedullary lesion in which a fluid-filled cyst, also known as a syrinx, forms within the spinal cord1,5,6,7,8,9,10,11. The pathophysiology of syringomyelia is widely debated although it is generally accepted that fluid pressure imbalance leads to the flow of fluid into the syrinx12,13. Chiari I malformation, displacement of the cerebellar tonsils through the foramen magnum, or trauma are most commonly the cause of syrinx formation. Early symptoms include headache1,10, altered pain and temperature sensation7,10,14, and parasthesia10,15. If fluid continues to enlarge the cyst, the syrinx has the potential to compress or destroy the affected portions of the spinal cord.
TABLE 1.
| Intramedullary | Extramedullary, Intradural | Extradural |
|---|---|---|
| Syringomyelia, hydromyelia | Meningioma | Herniated disk |
| Intramedullary tumor (e.g., ependymoma, astrocytoma) | Neurof broma | Spinal stenosis, spondylosis, osteophyte |
| Inf ammation (e.g., abscess, myelitis) | Metastasis (e.g., leptomeningeal seeding or hematogenous) | Ligamentum f avum thickening, intraspinal ligament ossif cation |
| Multiple sclerosis | Meningioma | |
| Neurogenic tumor (e.g., neurof broma) | ||
| Metastasis | ||
| Vertebral neoplasm with intraspinal extension | ||
Surgical intervention is typically recommended to treat this condition, although a successful procedure often is limited to providing minimal neurological improvement or simply halting the deterioration6,15,16,17,18. Because delay of progression of symptoms is the primary outcome of surgery, duration of symptoms6,18 and pre-operative level of function15,16 significantly influence the functional outcome of each surgical case. This further illustrates the need for prompt recognition of myelopathy and appropriate referral and intervention for the underlying condition.
Patient interview and medical history are the first examination components that may indicate spinal cord disease. Subjective complaints may include symptoms associated with upper motor neuron lesions such as changes in bowel or bladder control1,19, progressive extremity weakness1,19,20,21, difficulty walking19, and clumsiness1. Myelopathy is most common in the cervical spine22,23 with consequential distribution of numbness throughout the arms and upper trunk10,14 and difficulty with fine motor coordination of the hands10,24. History of trauma1,7,8,10,25,26, tumor1,27,28, or degenerative diseases such as rheumatoid arthritis29,30 or cervical spondylosis25,31 may also raise concern of myelopathy.
Radiculopathy, a pattern of symptoms due to nerve root irritation2, and myelopathy have some similar clinical features; therefore, care must be taken to distinguish between the two. An evaluation process that incorporates an upper or lower quarter screen can assist with this distinction2,32. Traditional upper and lower quarter screens feature a combination of sensibility, myotome, and neurological tests and may involve tests that quickly target functional activities. Physical exam findings indicative of myelopathy may include numbness19,21,24, weakness19,21,24, spasticity17,20,22,24, and abnormal gait4,10,17,20,22,24. Neurological tests, including Hoffmann's sign, the Finger escape sign, and the Babinski's reflex as well as the tests listed in Table 2, may also lend to suspicion of serious neurological disease, such as multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and cervical spondylotic myelopathy.
TABLE 2.
| Neurological Finding | Description | Sensitivity |
|---|---|---|
| Absent abdominal reflexes | Contraction of the abdominal wall during stimulation of the skin (typically near the naval) | Not studied |
| Babinski's sign | Upgoing great toe sign during stroking of plantar aspect of the foot | 0-80% |
| Clonus | A series of involuntary muscular contractions during sudden stretching of the muscle that continue for >3 or 4 beats | Not studied |
| Crossed adductor reflex | Contraction of the adductors of the thigh and internal rotation of the limb during tapping of the suprapatellar region or the sole of the foot | Not studied |
| Finger escape sign | Digits 3 and 4 will flex and abduct when the patient attempts to hold fingers in extension and adduction | 55% |
| Grip and release test | Repetitive gripping and releasing of the hand for up to 20 attempts | Not studied |
| Hoffmann's sign | Thumb flexion or flexion of third and fourth digits during clicking or flicking the distal middle finger | 28–94% |
| Increased deep tendon reflexes (DTR) | Hyper-reflexia of traditional deep tendon reflex testing | Not studied |
| Inverted supinator sign | Finger flexion or elbow extension during tapping of brachioradialis reflex testing | Not studied |
| Lhermitte's sign | Electrical sensations during active cervical flexion | 3–17% |
| Jaw jerk | Tapping of the jaw just below the bottom lip. A positive response is exaggerated closing during tapping | Not studied |
Nonetheless, the screening tests alone are not sufficient to provide a definitive diagnosis of myelopathy4. Diagnostic values have only been calculated for a portion of the tests and measures comprising the screens. Of those studied, none have high sensitivity23, which is critical when attempting to rule out a serious condition like myelopathy. Diagnostic values of magnetic resonance imaging (MRI) are higher for detecting a variety of lesions that may cause myelopathy21,23, demonstrating a beneficial contribution to making the appropriate diagnosis. However, tools such as the MRI must be used judiciously secondary to costs and must be targeted at the appropriate tissue to identify the abnormality.
The purpose of this case report is to describe the diagnostic process in detecting syringomyelia. The diagnostic dilemma faced by medical professionals when attempting to identify myelopathy without sensitive screening tools is evident in this case. Furthermore, it demonstrates the utility of sensitive diagnostic imaging in making the difficult diagnosis of myelopathy caused by thoracic syringomyelia.
Case Description
Subject
The patient was a 47-year-old female with an extensive medical history that included general malaise, severe bronchial asthma, pleural effusion, increasing hearing loss, tinnitus, daily bi-frontal headaches, Epstein-Barr, post-traumatic stress disorder (PTSD), and post-partum depression. Prior to this hospitalization, the patient worked within the medical profession and lived in a two-story home with her husband and young child.
Pre-Admission Patient History
Hospital admission was precipitated by a puzzling combination of signs and symptoms, providing difficulty for the medical team to discern a clear cause of her functional decline. Twelve days prior to admission, she was seen by a neurologist for a second opinion regarding her symptoms. At that time, the patient reported extreme fatigue and feeling “very, very sick,” she had been unable to practice at her medical specialty for approximately 10 weeks. Upon reflection, she recalled trouble ambulating in darkness, feeling unsteady, and walking with a widened base of support for approximately six months. Also reported were memory difficulties, problems completing crossword puzzles, general clumsiness, and occasional nighttime incontinence, which awakened her from sleep. Neurological findings of concern from the first visit included 4+ reflexes in all extremities; bilateral positive Hoffmann's; an abnormal (4+) jaw jerk; one beat of ankle clonus bilaterally; loss of temperature perception on the left posterior lower leg; difficulty with finger-to-nose, heel-to-shin, and rapid alternating movements; positive Romberg; and observed hesitancy with ambulation. Motor strength, though not graded, was noted to be uniform throughout all extremities.
Neurological findings at the initial consultation with the neurologist were not indicative of a particular pathology, although suspicion was raised of possible degenerative disease such as MS or ALS secondary to the abnormal jaw jerk findings. The worrisome findings prompted further examination of medical history and lab/radiology results, collaboration with colleagues, and follow-up testing. Subsequent review and testing results were within normal limits for the following: MRI of the brain to detect the presence of changes consistent with MS; visual and upper-extremity evoked potentials also used to identify changes consistent with MS; computed tomography (CT) of the chest, abdomen, and pelvis; lumbar puncture to discover any cerebrospinal fluid pressure changes or inflammation of the brain or spinal cord; and Vitamin E, Vitamin B12, and folate as deficiencies, which may sometimes cause symptoms similar to MS. Results of the electroencephalogram (EEG), which is used to detect changes in the brain's electrical activity, were “abnormal secondary to mild background slowing”; this finding was attributed to the patient's use of antidepressant medications.
Declining clinical presentation just 12 days later prompted further action. Motor strength in bilateral upper extremities and the right lower extremity was graded 5/5 throughout. The left quadriceps and all distal musculature were graded 4-/5 throughout–a substantial change from the previous visit. Cog-wheeling–a regular, jerky pattern of resistance to passive movement–was also evident in bilateral upper extremities and was worse on the right side. All reflexes remained at 4+, and crossed adductor reflex was seen in bilateral lower extremities. Hoffmann's sign remained positive bilaterally but subjectively appeared much more compelling on the right. The patient complained of the inability to stand independently even with a widened base of support, a finding that was confirmed during the examination. Based on these signs and symptoms, the patient was admitted to the hospital for comprehensive investigation of the cause of her declining neurological status.
Pre-Surgical Care
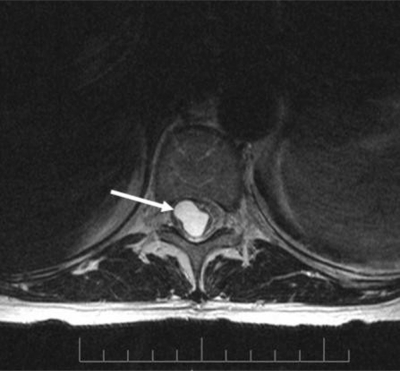
Based on initial suspicion of a lesion at or above the cervical region, a series of brain and spinal MRIs were ordered following admission. The brain and cervical MRIs were again within normal limits; however, the spinal MRI revealed a 2.5 centimeter (cm) mass. Per radiology report, the mass was located at T11 and was described as “a large syrinx … centrally located … [that] appears to herniate somewhat intralaterally to the right”. Figures 1 and 2 demonstrate this abnormal finding.
Figure 1.
Cross-section magnetic resonance image with arrow depicting the syrinx.
Figure 2.
Sagittal plane magnetic resonance image with arrow depicting the syrinx.
Surgical Intervention
The MRI reading was confirmed and a neurosurgery consult was immediately provided. Surgical intervention was scheduled in an effort to prevent further neurological decline and to stabilize current symptoms. Per operative report, laminectomies of T10 and T11 and partial laminectomy of T12 were performed. The spinal cord was opened at the thinnest portion and minimally expanded (8mm) to allow complete drainage of the syrinx.
Post-Surgical Acute Care
Following recovery, physical therapy was requested on post-operative Day One. However, difficulties with pain management, patient fatigue, and scheduling delayed treatment until post-operative Day Six. The following functional deficits were noted upon evaluation: 1) decreased bilateral lower extremity strength–left weaker than right, and 2) significantly decreased endurance. The patient was able to ambulate 40 feet with moderate assistance and a rolling walker. The therapist observed significant gait deviations and subsequent compensations including increased upper-extremity weight-bearing on a rolling walker, widened base of support, decreased weight shift to the left, circumduction of the left lower extremity, decreased left hip abduction, decreased left hip/knee flexion, decreased heel strike bilaterally, and decreased toe-off bilaterally. Frequent rests were required and the patient reported extreme fatigue following the session. The patient was also seen by occupational therapy following surgery; both therapies recommended continuation of acute inpatient rehabilitation.
Inpatient Rehabilitation
The patient was admitted to inpatient rehabilitation on post-operative Day Seven and evaluated by physical therapy the next day. She presented with the same strength, mobility, and endurance deficits noted during her acute care evaluation. Functional abilities were also quantified by assigning functional independence measure (FIM) scores for necessary transfers and mobility. The FIM, commonly used in rehabilitation settings to quantify patient progress, is an 18-item tool that measures a person's disability in terms of burden of care. Each item is rated on a scale ranging from 1 (total assistance) to 7 (complete independence). A zero indicates a task not completed or tested. Scores are summed to obtain a total motor score (13 items for a score ranging from 0 to 91) and total cognitive score (five items for a score ranging from 0 to 35)33. A change score of 11 points has been associated with clinically meaningful changes in stroke patients33. These scores are reported in Table 3 along with discharge goals, which were set at the time of evaluation.
TABLE 3.
Functional Independence Scores: Inpatient rehabilitation.
| Function | Admission Status | Discharge Goal | Comments | Discharge Status | |
|---|---|---|---|---|---|
| Transfers: | Bed/WC | 4 | 6 | 6 | |
| Car | 0 | 6 | 6 | ||
| Floor | 0 | 6 | 6 | ||
| Supine →Sit | 7 | 7 | 7 | ||
| Sit →Stand | 4 | 6 | 6 | ||
| W/C Management: | Uneven Surfaces | 0 | 6 | Pt. will be ambulatory | 6 |
| Even Surfaces | 2 | 6 | Pt. will be ambulatory | 6 | |
| Ambulation—Even Surfaces: | Assistance | 2 (with rolling walker) | 6 (with rolling walker) | 6 (with rolling walker) | |
| Distance | 100′ | >/=150′ | 400′ | ||
| Ambulation— Uneven Surfaces: | Assistance | 0 | 6 | 6 | |
| Distance | 0′ | >/=150′ | 150′ | ||
| Stairs: | Assistance | 2 | 2 | 4 | |
| # of Stairs | 4 | 4 | 18 | ||
Each function is rated on a scale ranging from 1 (total assistance) to 7 (complete independence). A total change score of 11 points has been associated with clinically meaningful changes in stroke patients33.
The patient participated in physical therapy for 45–150 minutes daily for 12 of the 14 days she remained in the facility. Intervention details are documented in Table 4 to the extent available in the medical chart. During this stay, the patient also worked with occupational and recreational therapies. She was discharged following a two-week stay with her husband offering supervision at all times initially. Durable medical equipment issued upon discharge is detailed in Table 4.
TABLE 4.
Physical therapy interventions: Inpatient rehabilitation.
| Interventions | Comments | |
|---|---|---|
| Admission | None performed | Patient admitted to inpatient rehabilitation |
| Day 1 | Initial evaluation; transfer training, ambulation 50′ × 2 with rolling walker and contact guard assist | |
| Day 2 | Transfer training; ambulation 100′ × 4 with rolling walker and contact guard assist; long-arc quad × 15 bilaterally with 6 lbs; clamshell × 15 bilaterally; mini-squats × 15 in parallel bars | |
| Day 3 | Transfer training; wheelchair mobility × 50′; ambulation 100′ with rolling walker and contact guard assist; NuStep × 10 min | Decreased treatment time due to patient complaints of dizziness during treatment session |
| Day 4 | Transfer training; ambulation 100′ with rolling walker, contact guard assist, and verbal cues; ambulation 20′ with minimum assist to facilitate neuromuscular re-education of bilateral lower extremities to increase dorsiflexion bilaterally, increase heel strike bilaterally, and control hip rotation (lef > right) | Increased gait quality with multimodal cuing (verbal, tactile) |
| Day 5 | Transfer training; ambulation 125′ with minimum assist to facilitate neuromuscular re-education for bilateral hip flexors; transcutaneous electric nerve stimulation (TENS) trial to lumbar region; stretching of bilateral hamstrings and hip rotators | Pain prior to TENS: 6/10 |
| Pain following TENS application: 3/10 | ||
| Day 6 | Transfer training; ambulation 60′ × 2 with stand-by assist and verbal cues to increase gait quality; hamstring stretch; gastrocnemius stretch; piriformis stretch; active dorsiflexion × 15; active eversion × 15; short-arc quad × 15; hip abduction/adduction × 15; all performed bilaterally | Significantly decreased endurance noted, evidenced by increase in gait deviations throughout treatment session |
| Day 7 | None performed | Patient refused treatment, citing pain and fatigue |
| Day 8 | Transfer training; wheelchair mobility × 50′; ambulation 150′ with rolling walker, contact guard assist, and verbal cues; hamstring stretch; gastrocnemius stretch; short arc quad × 20; hip abduction/adduction × 20; all performed bilaterally | |
| Day 9 | Transfer training; ambulation 150′ with rolling walker, contact guard assist, and verbal cues; patient education on energy conservation and safety | Improved ambulation pattern but continued to fatigue quickly |
| Day 10 | Transfer training; ambulation 300′ × 2; NuStep × 10 min; clamshell × 20; long arc quad × 20; active dorsifiexion × 20; all performed bilaterally | |
| Day 11 | None performed | |
| Day 12 | Transfer training; ambulation 500′ × 1, 100′ × 2 with rolling walker and contact guard assist; parallel bars–backwards walking, side-stepping, braiding; hip abduction/adduction × 15; heel slides × 15; bridging × 10 | Patient with improved endurance to treatment session |
| Day 13 | Transfer training; ambulation 250′ × 2 with rolling walker and stand-by assist; NuStep × 10 min; UBE × 7 min; hip abduction/adduction × 20; heel slides × 20; bridging × 20 | |
| Day 14 | Discharge evaluation; transfer training; ambulation 400′ (level surface) with rolling walker; ambulation 150′ (uneven surface) with rolling walker; stair training × 18 with minimum assist; patient education regarding home exercises program | Patient discharged home with rolling walker, rental wheelchair, bedside commode, and referral to outpatient physical therapy clinic |
Outcomes
The patient's functional gains are incorporated into Table 3, demonstrating that all physical therapy goals were met at the time of discharge. Overall, slow steady functional gains were noted throughout the period of inpatient rehabilitation. While the patient was ambulatory at the time of initial evaluation, decreased strength, decreased endurance, and gait deviations made this a very cumbersome task. Initially, she was only able to walk short distances with significant gait deviations. Endurance gradually improved allowing the patient to exceed the level surface ambulation goal by 250 feet. As endurance improved, the therapist had more opportunities to address specific gait deviations prior to fatigue. The patient was able to progress from significant use of her upper extremities during ambulation to moderate reliance upon the assistive device. Specific references to gait deviations present at the time of discharge were not noted in the medical record. Based on progress notes, it can be inferred that the patient continued with a widened base of support, decreased gait speed, and continued need for an assistive device. At discharge, the therapist also noted that progression to a cane was indicated, but the patient expressed discomfort and fear of this progression. For this reason, she continued use of a rolling walker for ambulation. Following discharge, outpatient physical therapy was recommended and scheduled in order to achieve maximum functional return.
Discussion
This case report describes the dilemma faced by physicians in diagnosing myelopathy caused by thoracic syringomyelia, and illustrates the need for necessary diagnostic procedures to occur in a timely fashion. With the drive to achieve direct access, physical therapists may be the first health care professionals to examine patients presenting with symptoms similar to those in this case. It can be the duty of physical therapists to rule out a sinister pathology such as myelopathy as the cause of patient symptoms or to identify red flags requiring immediate referral to a physician, with whom sophisticated diagnostic equipment is available.
This particular case was a diagnostic challenge for a number of reasons, the first of which is the lack of appropriate clinical tests for myelopathy23. Physical therapists rely heavily on highly sensitive clinical tests when performing an interview and physical examination. A sensitive test is designed to correctly identify a patient with a particular pathology; a negative test can subsequently rule out that pathology as the cause of signs and symptoms24. A screening tool with high sensitivity allows confidence in ruling out a suspected diagnosis if the test is negative (SnOut). A test exhibits high sensitivity if there are few false negatives when it is applied. A positive screening test would warrant a diagnostic test high in specificity in order to determine if the disease is a true positive or false positive. A positive result from a highly specific test rules in the suspected diagnosis (SpIn). Unfortunately, the sensitivities of nearly all clinical tests for myelopathy used during this patient's course of care were either very low or have not been studied for assessment of accuracy. Two commonly used clinical tests that have been investigated (Babinski and Hoffmann's), have consistently demonstrated low to moderate sensitivities or have only been investigated in a population of patients with a head injury or brain attack. There were problems with both masking (investigators were not blinded to the subjects' condition) and spectrum bias (participants did not have myelopathy, thereby affecting the generalizability to a population with suspected myelopathy) in the study that yielded a sensitivity of 80% for Babinski23 and Hoffmann's23. Interestingly, Hoffmann's sensitivity in a separate study increased from 28% to 58% when the investigators were not masked to other components of the exam23. This indicates that Hoffmann's test may be valuable when used in conjunction with other clinical findings. However, without well-designed studies investigating the diagnostic values, there remains uncertainty in using these tests to diagnose myelopathy. As in this case, the puzzling combination of signs and symptoms was unable to guide a clinician to a specific diagnosis without further work-up.
Additionally, positive findings of isolated tests that guided components of this workup were misleading. While none of the other tests performed are generally used to identify lesion location, an abnormal jaw jerk reflex has been noted in subjects with some pathologies of the brainstem such as MS34,35, one of the differential diagnoses considered for this patient. A positive finding in this case prompted orders for upper-extremity evoked potentials testing, a lumbar puncture, and the review of a previous brain MRI. With no significant findings for these tests, an additional MRI of the brain was completed with the same results, followed by a third unremarkable brain MRI performed after hospital admission. This cumbersome work-up demonstrates the danger and expense of using isolated tests with poor or unstudied diagnostic value to guide the diagnostic process.
Further complicating matters was the unlikely location of the lesion causing the myelopathy. Cervical lesions are most commonly the source of myelopathy22,23, with thoracic and lumbar lesions as less frequent causes. Although the pattern of signs and symptoms did suggest neurological disease, the areas first examined, albeit unsuccessfully, were those that are more commonly associated with myelopathy.
The test that ultimately depicted the cause of myelopathy for this patient was the spinal MRI. With demonstrated sensitivities of 79–95%23, the images successfully identified the lesion within the patient's spinal cord. Unfortunately, there was a significant time lapse between this patient's initial presentation and performance of the spinal MRI, during which time she experienced considerable neurological deterioration. Upon discovery, surgical intervention was performed within 48 hours and was successful in retarding further decline. This indicates that the appropriate MRI used in a more timely fashion might have allowed for earlier surgical intervention, preventing the functional decline she suffered.
Conclusion
With physical therapists serving as a point of access to the medical system, it is necessary to recognize the tools available to assist in making a difficult diagnosis. This case demonstrates the challenge in making a diagnosis without appropriate decision-guiding tests. It clearly shows the importance of using examination in conjunction with highly sensitive diagnostic studies in a timely fashion to discover the cause of myelopathy.
The lack of highly sensitive clinical tests to detect myelopathy also suggests that further investigation of individual and clustered neurological tests is needed to determine a combination that yields higher diagnostic value in the clinical setting. With screening tools that correctly recognize patients in need of further work-up, physical therapists may be of assistance in preventing progressive neurological decline that can occur without timely lesion identification.
Contributor Information
Beverly Rene Hudson, Staff Physical Therapist, University of North Carolina Hospital, Chapel Hill, NC..
Chad Cook, Assistant Professor, Division of Physical Therapy, Duke University, Durham, NC.; Director of Clinical Outcomes Research, Centers for Excellence in Surgical Outcomes, Duke University, Durham, NC.
Adam Goode, Assistant Professor, Division of Physical Therapy, Duke University, Durham, NC..
REFERENCES
- 1.Patel S, Kettner N, Obourne C. Myelopathy: A report of two cases. J Manipulative Physiol Ther. 2005;28:539–546. doi: 10.1016/j.jmpt.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Cook C. Orthopedic Manual Therapy: An Evidence-Based Approach. Upper Saddle River, NJ: Prentice Hall; 2007. [Google Scholar]
- 3.Reeder M, Bradley W., Jr . Reeder and Felson's Gamuts in Radiology. 3rd ed. New York: Springer-Verlag Talos; 1993. [Google Scholar]
- 4.Cook C, Hegedus E. Orthopedic Physical Examination Tests: An Evidence-Based Approach. Upper Saddle River, NJ: Prentice Hall; 2008. [Google Scholar]
- 5.Wang M, Levi A, Green B. Intradural spinal arachnoid cysts in adults. J Neurosurg. 2003;60:49–55. doi: 10.1016/s0090-3019(03)00149-6. [DOI] [PubMed] [Google Scholar]
- 6.Bogdanov E, Heiss J, Mendelevich E. The post-syrinx syndrome: Stable central myelopathy and collapsed or absent syrinx. J Neurol. 2006;253:707–713. doi: 10.1007/s00415-006-0091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schurch B, Wichmann W, Rossier A. Post-traumatic syringomyelia (cystic myelopathy): A prospective study of 449 patients with spinal cord injury. J Neurol. 1996;60:61–67. doi: 10.1136/jnnp.60.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vannemreddy S, Rowed D, Bharatwal N. Post-traumatic syringomyelia: Predisposing factors. Br J Neurosurg. 2002;16:276–283. doi: 10.1080/02688690220148879. [DOI] [PubMed] [Google Scholar]
- 9.Lee T, Alamede G, Camilo E, Green B. Surgical treatment of post-traumatic myelopathy associated with syringomyelia. Spine. 2001;26:S119–S127. doi: 10.1097/00007632-200112151-00020. [DOI] [PubMed] [Google Scholar]
- 10.Wollman D. Syringomyelia: An uncommon cause of myelopathy in the geriatric population. J Am Geriatr Soc. 2004;52:1033–1034. doi: 10.1111/j.1532-5415.2004.52277_12.x. [DOI] [PubMed] [Google Scholar]
- 11.Brodbelt A, Stoodley M. Syringomyelia and the arachnoid web. Acta Neurochir. 2003;145:707–711. doi: 10.1007/s00701-003-0071-9. [DOI] [PubMed] [Google Scholar]
- 12.Chang H, Nakagawa H. Hypothesis on the pathophysiology of syringomyelia based on simulation of cerebrospinal fluid dynamics. J Neurol Neurosurg Psychiatry. 2003;74:344–347. doi: 10.1136/jnnp.74.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klekamp J. The pathophysiology of syringomyelia: Historical overview and current concept. Acta Neurochir. 2002;144:649–664. doi: 10.1007/s00701-002-0944-3. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty P, Bandyopadhyay D, Mandal S, et al. Unilateral limb hypertrophy and shoulder weakness in a 37-year-old woman. MJA. 2006;184:130–131. doi: 10.5694/j.1326-5377.2006.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 15.Munari B, Silvani A, Porta E, Scuratti A, Lodrini S. Natural history and post-surgical outcome of syringomyelia. Ital J Neurol Sci. 1991;12:575–579. doi: 10.1007/BF02336954. [DOI] [PubMed] [Google Scholar]
- 16.Samii M, Klekamp J. Surgical results of 100 intramedullary tumors in relation to accompanying syringmyelia. J Neurosurg. 1994;35:865–873. doi: 10.1227/00006123-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Helmut W, Heinz-Eugen N, Friedhelm R, Wilhelm G, Lieselotte G. Operative treatment and prognosis of syringomyelia. Neurosurg Rev. 1994;17:37–41. doi: 10.1007/BF00309984. [DOI] [PubMed] [Google Scholar]
- 18.Bindel A, Dunsker S, Tew J. Chiari I malformation: Classification and management. J Neurosurg. 1995;37:1069–1074. doi: 10.1227/00006123-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Shenoi R, Duong T, Brega K, Gaido L. Ossification of the ligamentum flavum causing myelopathy: A case report. Am J Phys Med Rehabil. 1997;76:68–72. doi: 10.1097/00002060-199701000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Kaar G, N'Dow J, Bashir S. Cervical spondylotic myelopathy with syringomyelia. Br J Neurosurg. 1996;10:413–415. doi: 10.1080/02688699647384. [DOI] [PubMed] [Google Scholar]
- 21.Schubeus P, Schorner W, Hosten N, Felix R. Spinal cord cavities: Differential-diagnostic criteria in magnetic resonance imaging. EJR. 1991;12:219–225. doi: 10.1016/0720-048x(91)90076-8. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery D, Brower R. Cervical spondylotic myelopathy: Clinical syndrome and natural history. Orthop Clin North Am. 1992;23:487–493. [PubMed] [Google Scholar]
- 23.Cook C, Hegedus E, Pietrobon R, Goode A. A pragmatic neurological screen for patients with suspected cord compressive myelopathy. Phys Ther. 2007;87:1233–42. doi: 10.2522/ptj.20060150. [DOI] [PubMed] [Google Scholar]
- 24.Sizer P, Jr, Brismee J, Cook C. Medical screening for red flags in the diagnosis and management of musculoskeletal spine pain. Pain Pract. 2007;7:53–71. doi: 10.1111/j.1533-2500.2007.00112.x. [DOI] [PubMed] [Google Scholar]
- 25.Aryan H, Sanchez-Mejia R, Ben-Haim S, Ames C. Successful treatment of cervical myelopathy with minimal morbidity by circumferential decompression and fusion. Eur Spine J. 2007;16:1401–1409. doi: 10.1007/s00586-006-0291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farooq M, Chao T, Bennet M. Paraplegia following post-traumatic thoracic spinal stenosis: A case report. Arch Phys Med Rehabil. 1996;77:84–85. doi: 10.1016/s0003-9993(96)90226-9. [DOI] [PubMed] [Google Scholar]
- 27.Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6:15–24. doi: 10.1016/S1470-2045(04)01709-7. [DOI] [PubMed] [Google Scholar]
- 28.White A, Kwon B, Lindskog D, Friedlaender G, Grauer J. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14:587–598. doi: 10.5435/00124635-200610000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Sunahara N, Matsunaga S, Mori T, Ijiri K, Sakou T. Clinical course of conservatively managed rheumatoid arthritis patients with myelopathy. Spine. 1997;22:2603–2607. doi: 10.1097/00007632-199711150-00004. [DOI] [PubMed] [Google Scholar]
- 30.van Asselt K, Lems W, Bongartz E, et al. Outcome of cervical spine surgery in patients with rheumatoid arthritis. Ann Rheum Dis. 2001;60:448–452. doi: 10.1136/ard.60.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young W. Cervical spondylotic myelopathy: A common cause of spinal cord dysfunction in older persons. Am Fam Physician. 2000;62:1064–1070. 1073. [PubMed] [Google Scholar]
- 32.Dvorak J, Sutter M, Herdmann J. Cervical myelopathy: Clinical and neurophysiological evaluation. Eur Spine J. 2003;12(Supplement 2):S181–S187. doi: 10.1007/s00586-003-0631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace D, Duncan PW, Lai SM. Comparison of the responsiveness of the Barthel Index and the motor component of the Functional Independence Measure in stroke: The impact of using different methods for measuring responsiveness. J Clin Epidemiol. 2002;55:922–928. doi: 10.1016/s0895-4356(02)00410-9. [DOI] [PubMed] [Google Scholar]
- 34.Aramideh M, Ongerboer de Visser B. Brainstem reflexes: Electrodiagnostic techniques, physiology, normative data, and clinical applications. Muscle Nerve. 2002;26:14–30. doi: 10.1002/mus.10120. [DOI] [PubMed] [Google Scholar]
- 35.Sanders E, Ongerboer de Visser B, Barendswaard E, Arts R. Jaw, blink, and corneal reflex latencies in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1985;48:1284–1289. doi: 10.1136/jnnp.48.12.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]