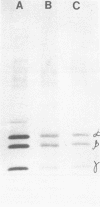
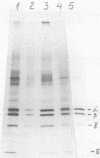
Abstract
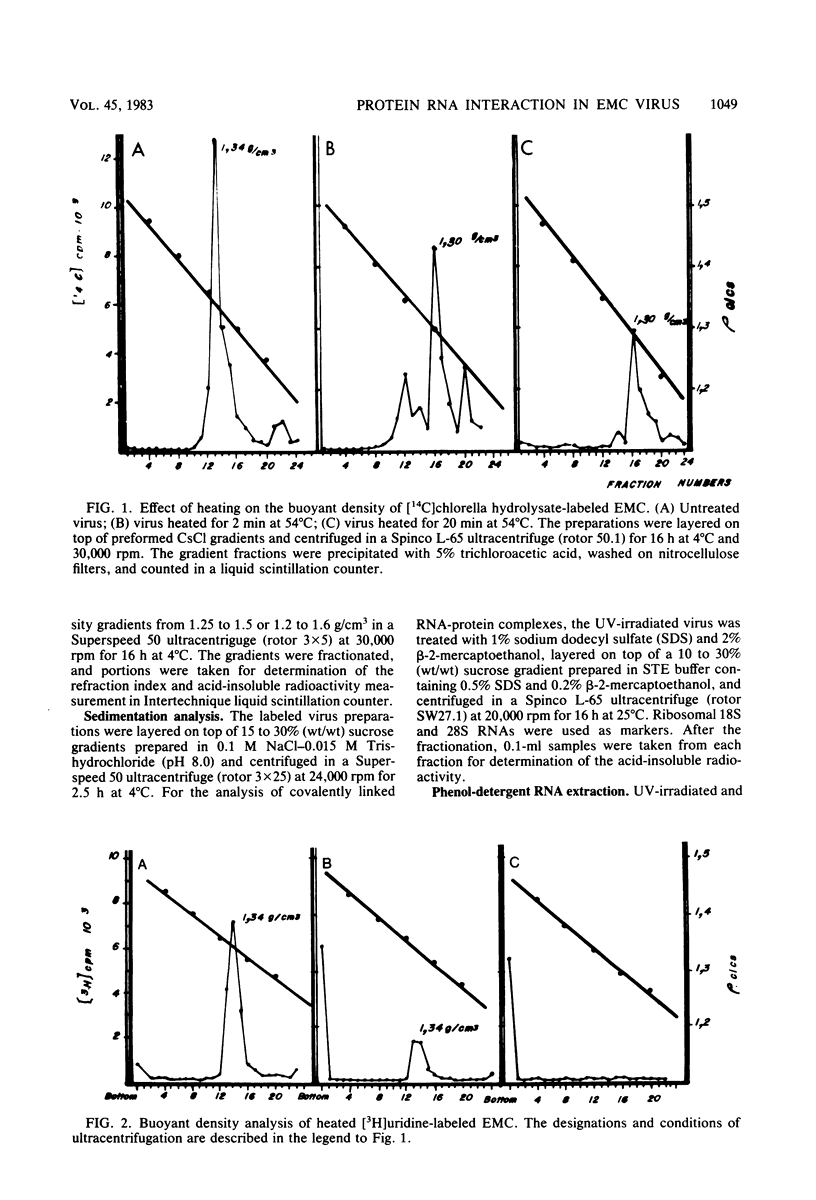
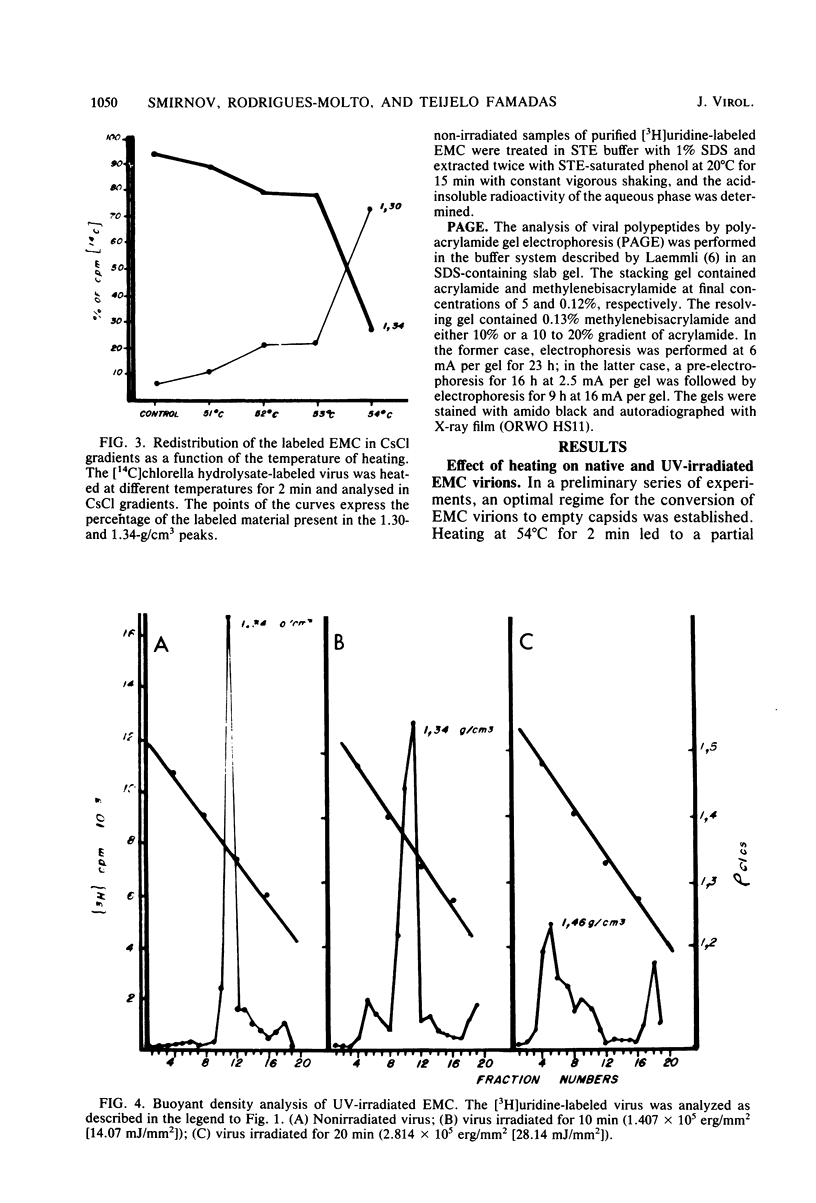
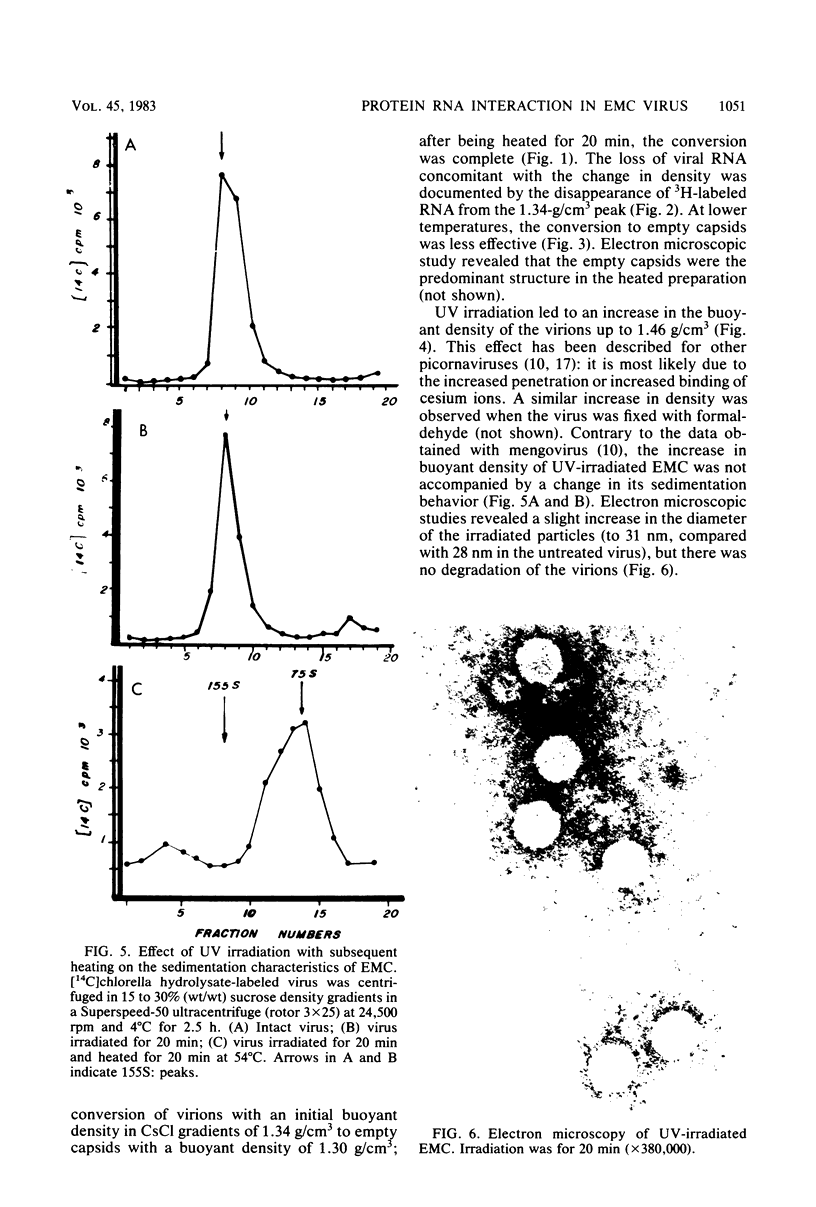
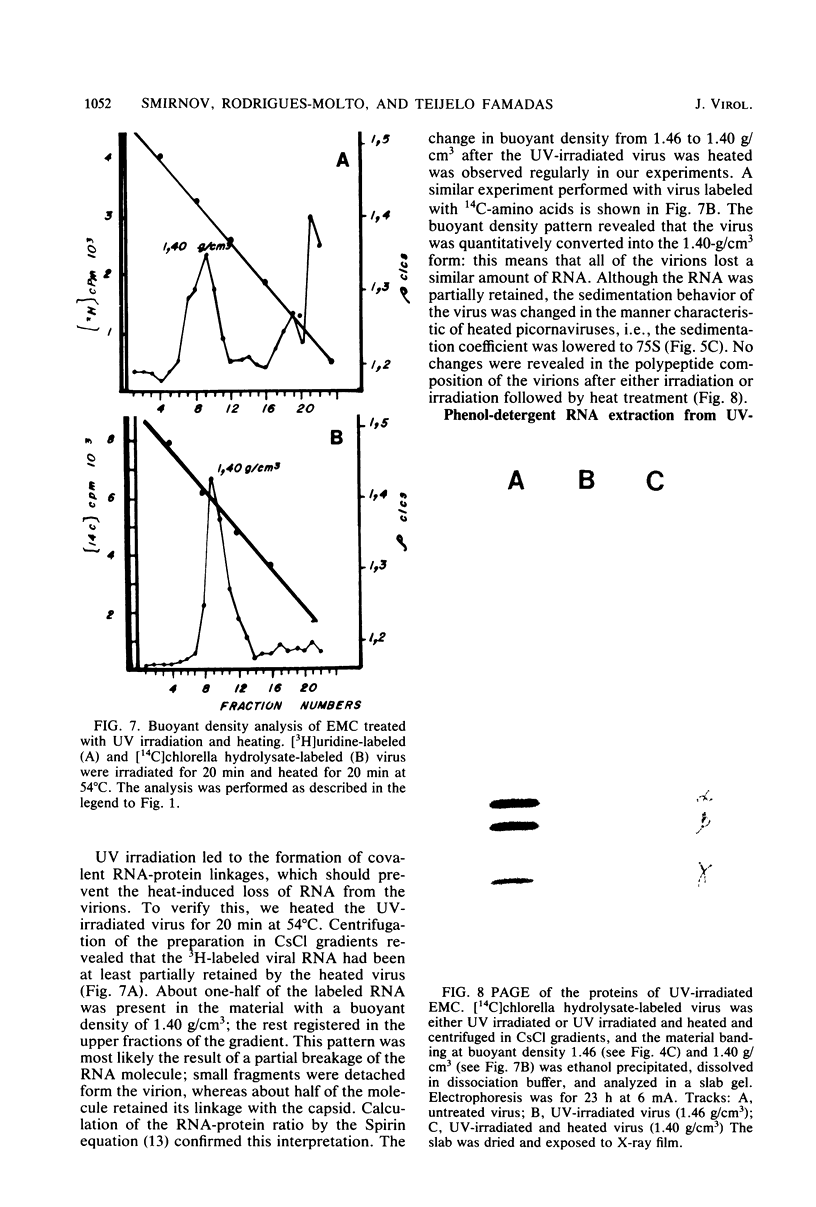
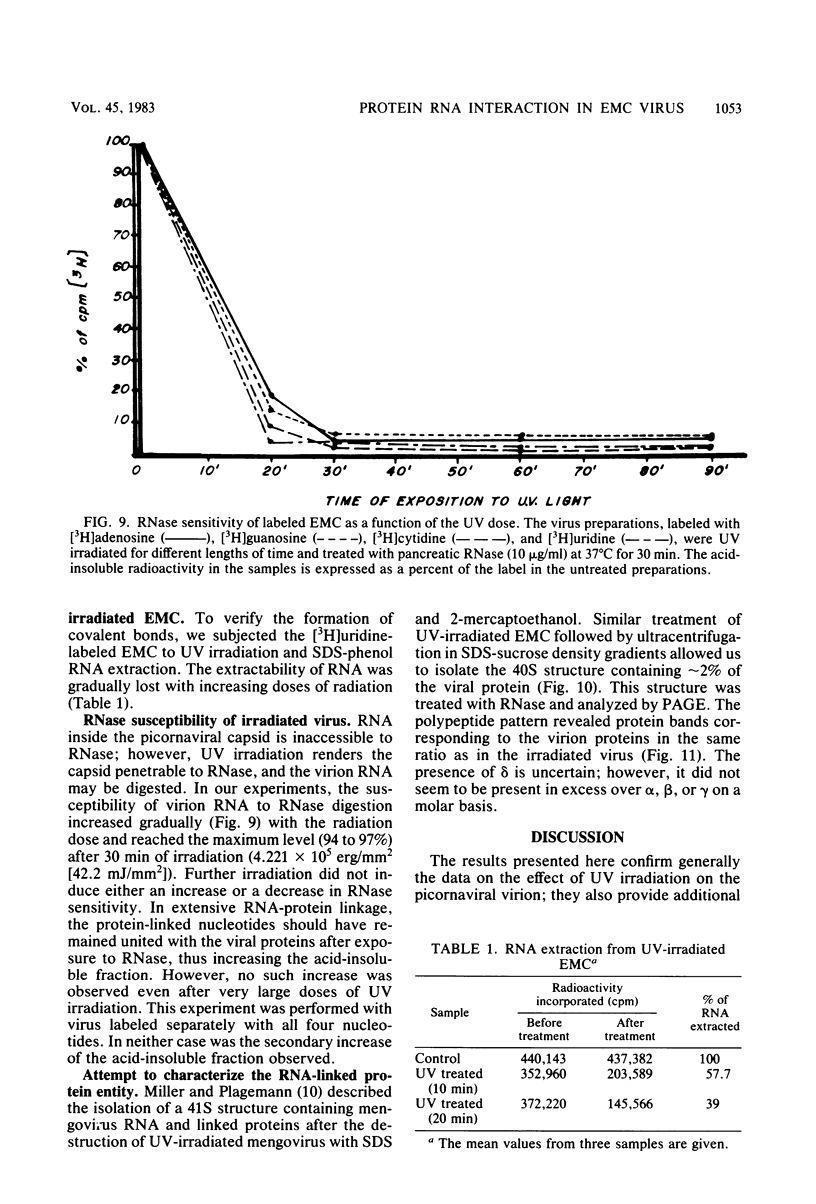
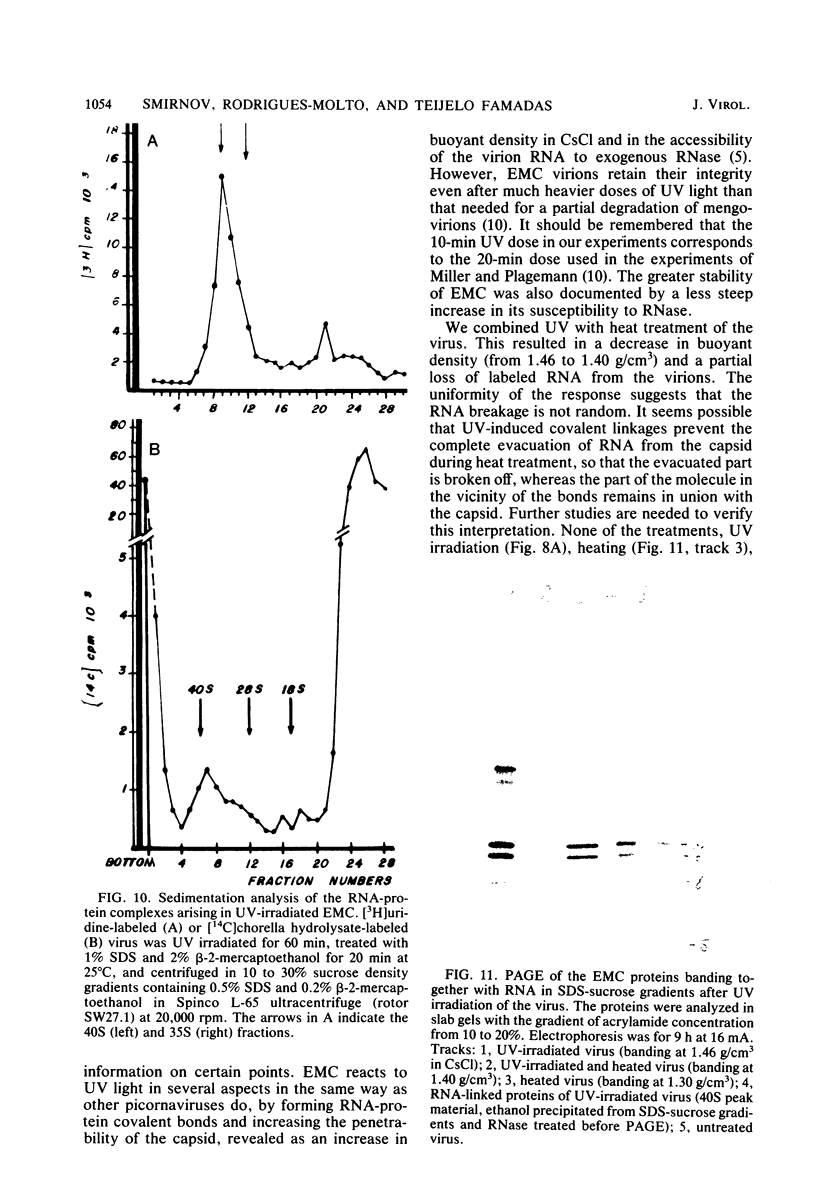
UV irradiation of encephalomyocarditis virus led to an increase in the buoyant density of the virus in CsCl gradients from 1.34 to 1.46 g/cm3. Heat treatment of the irradiated virus (20 min at 54 degrees C) reduced the density to 1.40 g/cm3 and led to the loss of approximately 55% of the labeled RNA from the virions. The non-irradiated virions were converted by such heating into empty capsids. Irradiation also resulted in an increase in the accessibility of RNA inside the virions to the action of pancreatic RNase. An increase in the UV dose did not enlarge the fraction of RNA molecules covalently linked to protein; this was revealed by the lack of any secondary increase in the apparent RNase resistance of the labeled RNA in the irradiated virions. Destruction of the irradiated virus with sodium dodecyl sulfate and 2-mercaptoethanol allowed the isolation of a 40S structure containing viral RNA and RNA-linked proteins. The latter comprised no more than 2.5% of the whole protein content of the virion. Polyacrylamide gel electrophoretic analysis of the RNase-treated 40S structure revealed at least three viral structural proteins in the same ratio as was present in the intact virions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breindl M. The structure of heated poliovirus particles. J Gen Virol. 1971 Jun;11(3):147–156. doi: 10.1099/0022-1317-11-3-147. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J., Harris W. J. Heterogeneity of apparently empty poliovirus particles stained with phosphotungstic acid after heating of the virus at "low" temperature. Virology. 1967 Apr;31(4):715–719. doi: 10.1016/0042-6822(67)90200-0. [DOI] [PubMed] [Google Scholar]
- Hasegawa A., Inouye S. Antigenicity and polypeptide composition of native and heated echovirus type 7 procapsids. J Gen Virol. 1979 Jan;42(1):119–125. doi: 10.1099/0022-1317-42-1-119. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Katagiri S., Fukuda M., Fukushi K., Watanabe Y. Kinetic studies on the thermal degradation of purified poliovirus. Biken J. 1965 Sep;8(3):143–153. [PubMed] [Google Scholar]
- Katagiri S., Hinuma Y., Ishida N. Biophysical properties of poliovirus particles irradiated with ultraviolet light. Virology. 1967 Jun;32(2):337–343. doi: 10.1016/0042-6822(67)90282-6. [DOI] [PubMed] [Google Scholar]
- LE BOUVIER G. L. The modification of poliovirus antigens by heat and ultraviolet light. Lancet. 1955 Nov 12;269(6898):1013–1016. doi: 10.1016/s0140-6736(55)93435-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGregor S., Mayer H. D. Biophysical studies on rhinovirus and poliovirus. I. Morphology of viral ribonucleoprotein. J Virol. 1968 Feb;2(2):149–154. doi: 10.1128/jvi.2.2.149-154.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor S., Mayor H. D. Internal components released from rhinovirus and poliovirus by heat. J Gen Virol. 1971 Feb;10(2):203–207. doi: 10.1099/0022-1317-10-2-203. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Plagemann P. G. Effect of ultraviolet light on mengovirus: formation of uracil dimers, instability and degradation of capsid, and covalent linkage of protein to viral RNA. J Virol. 1974 Mar;13(3):729–739. doi: 10.1128/jvi.13.3.729-739.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnadi J. C., Wang S. Y. D2O solvent effect on photoproduct compositions of pyrimidines. Tetrahedron Lett. 1969 Jun;(27):2211–2213. doi: 10.1016/s0040-4039(01)88124-7. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., MAYER M. M., ROANE P. R., Jr Immunochemical studies of poliovirus. IV. Alteration of the immunologic specificity of purified poliomyelitis virus by heat and ultraviolet light. J Immunol. 1959 Jan;82(1):19–25. [PubMed] [Google Scholar]
- WEIL M. L., WARREN J., BREESE S. S., Jr, RUSS S. B., JEFFRIES H. Separation of encephalomyocarditis virus from tissue components by means of protamine precipitation and enzymic digestion. J Bacteriol. 1952 Jan;63(1):99–105. doi: 10.1128/jb.63.1.99-105.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetz K., Habermehl K. O. Specific cross-linking of capsid proteins to virus RNA by ultraviolet irradiation of poliovirus. J Gen Virol. 1982 Apr;59(Pt 2):397–401. doi: 10.1099/0022-1317-59-2-397. [DOI] [PubMed] [Google Scholar]
- Wetz K., Habermehl K. O. Topographical studies on poliovirus capsid proteins by chemical modification and cross-linking with bifunctional reagents. J Gen Virol. 1979 Aug;44(2):525–534. doi: 10.1099/0022-1317-44-2-525. [DOI] [PubMed] [Google Scholar]
- Zaslavsky V. G., Kaverin N. V., Smirnov Y. A., Syurina N. V. The difference in buoyant density between viral and normal polyribosomes in krebs II cells. FEBS Lett. 1971 Oct 1;17(2):289–293. doi: 10.1016/0014-5793(71)80166-7. [DOI] [PubMed] [Google Scholar]