Abstract
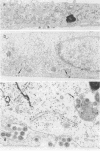
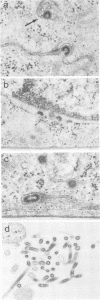
We have investigated the maturation sites of avian and mammalian C-type retroviruses in polarized epithelial cells. Examination of thin sections of Madin Darby canine kidney cells infected with RD114 or avian reticuloendotheliosis virus revealed that these viruses mature from the basolateral membrane domains. Similar results were obtained with a continuous line of mouse mammary epithelial cells infected with Friend, Moloney, Rauscher, or Kirsten murine leukemia viruses, or Friend virus-related or Moloney virus-related mink cell focus-forming viruses. Immunofluorescence observations indicate that viral glycoproteins are inserted only at the basolateral membranes in these cells. Because of the availability of DNA and protein sequence data, and of molecularly cloned viruses, these virus systems offer advantages for molecular studies on directional transport of plasma membrane glycoproteins.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Bassin R. H., Weaver C. Comparison of murine sarcoma viruses in nonproducer and S + L - -transformed cells. J Virol. 1972 Apr;9(4):701–704. doi: 10.1128/jvi.9.4.701-704.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso F. V., Compans R. W. Differential effect of monensin on enveloped viruses that form at distinct plasma membrane domains. J Cell Biol. 1981 Jun;89(3):700–705. doi: 10.1083/jcb.89.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANG F. B. The development of Newcastle disease virus in cells of the chorio-allantoic membrane as studied by thin section. Bull Johns Hopkins Hosp. 1953 Apr;92(4):309–329. [PubMed] [Google Scholar]
- Blok J., Air G. M., Laver W. G., Ward C. W., Lilley G. G., Woods E. F., Roxburgh C. M., Inglis A. S. Studies on the size, chemical composition, and partial sequence of the neuraminidase (NA) from type A influenza viruses show that the N-terminal region of the NA is not processed and serves to anchor the NA in the viral membrane. Virology. 1982 May;119(1):109–121. doi: 10.1016/0042-6822(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Montelaro R. C., Frank H., Schäfer W. Assembly of type C oncornaviruses: a model. Science. 1978 Jan 13;199(4325):183–186. doi: 10.1126/science.202022. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Robbins E. S., Dolan W. J., Rotunno C. A., Sabatini D. D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978 Jun;77(3):853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C. H., Knudsen K. A., Dorio R. J., Buck C. A. Manipulation of cell-cell and cell-substratum interactions in mouse mammary tumor epithelial cells using broad spectrum antisera. J Cell Biol. 1981 May;89(2):173–184. doi: 10.1083/jcb.89.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. H., Dresler S., Kabat D. Synthesis and glycosylation of polyprotein precursors to the internal core proteins of Friend murine leukemia virus. J Virol. 1977 Dec;24(3):865–874. doi: 10.1128/jvi.24.3.865-874.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Kawai K., Asano S., Nakao M. Protein components of two different regions of an intestinal epithelial cell membrane. Regional singularities. Biochim Biophys Acta. 1973 Apr 25;307(1):141–151. doi: 10.1016/0005-2736(73)90032-1. [DOI] [PubMed] [Google Scholar]
- Green R. F., Meiss H. K., Rodriguez-Boulan E. Glycosylation does not determine segregation of viral envelope proteins in the plasma membrane of epithelial cells. J Cell Biol. 1981 May;89(2):230–239. doi: 10.1083/jcb.89.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvard D. Apical membrane aminopeptidase appears at site of cell-cell contact in cultured kidney epithelial cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4132–4136. doi: 10.1073/pnas.77.7.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., McMillan P. N., Bolognesi D. P. An ultrastructural study of C-type virion assembly in mouse cells. Cancer Res. 1974 Dec;34(12):3303–3310. [PubMed] [Google Scholar]
- MURPHY J. S., BANG F. B. Observations with the electron microscope on cells of the chick chorio-allantoic membrane infected with influenza virus. J Exp Med. 1952 Mar;95(3):259–268. doi: 10.1084/jem.95.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooren H. W., Prins F. A., Herbrink P., Warnaar S. O. Electron microscopic studies on the role of the envelope antigens of R-MuLV-ts29 in budding. Virology. 1981 Aug;113(1):254–262. doi: 10.1016/0042-6822(81)90152-5. [DOI] [PubMed] [Google Scholar]
- Pinter A., deHarven E. Protein composition of a defective murine sarcoma virus particle possessing the enveloped type-A morphology. Virology. 1979 Nov;99(1):103–110. doi: 10.1016/0042-6822(79)90041-2. [DOI] [PubMed] [Google Scholar]
- Richardson J. C., Simmons N. L. Demonstration of protein asymmetries in the plasma membrane of cultured renal (MDCK) epithelial cells by lactoperoxidase-mediated iodination. FEBS Lett. 1979 Sep 15;105(2):201–204. doi: 10.1016/0014-5793(79)80611-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Pendergast M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell. 1980 May;20(1):45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Roth M. G., Compans R. W. Antibody-resistant spread of vesicular stomatitis virus infection in cell lines of epithelial origin. J Virol. 1980 Aug;35(2):547–550. doi: 10.1128/jvi.35.2.547-550.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Fitzpatrick J. P., Compans R. W. Polarity of influenza and vesicular stomatitis virus maturation in MDCK cells: lack of a requirement for glycosylation of viral glycoproteins. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6430–6434. doi: 10.1073/pnas.76.12.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta M., Murray M. J., Webb M. C., Kabat D. A murine leukemia virus mutant with a temperature-sensitive defect in membrane glycoprotein synthesis. Cell. 1979 Jan;16(1):77–88. doi: 10.1016/0092-8674(79)90189-2. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Oroszlan S. Murine leukemia virus gag polyproteins: the peptide chain unique to Pr80 is located at the amino terminus. Virology. 1978 Dec;91(2):481–486. doi: 10.1016/0042-6822(78)90395-1. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Rabin E. H., Oroszlan S. Post-translational modification of Rauscher leukemia virus precursor polyproteins encoded by the gag gene. J Virol. 1979 Apr;30(1):255–266. doi: 10.1128/jvi.30.1.255-266.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas R. V., Melsen L. R., Compans R. W. Effects of monensin on morphogenesis and infectivity of Friend murine leukemia virus. J Virol. 1982 Jun;42(3):1067–1075. doi: 10.1128/jvi.42.3.1067-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohrer R., Hunter E. Inhibition of Rous sarcoma virus replication by 2-deoxyglucose and tunicamycin: identification of an unglycosylated env gene product. J Virol. 1979 Nov;32(2):412–419. doi: 10.1128/jvi.32.2.412-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Giuli C., Kawai S., Dales S., Hanafusa H. Absence of surface projections of some noninfectious forms of RSV. Virology. 1975 Jul;66(1):253–260. doi: 10.1016/0042-6822(75)90195-6. [DOI] [PubMed] [Google Scholar]