Abstract
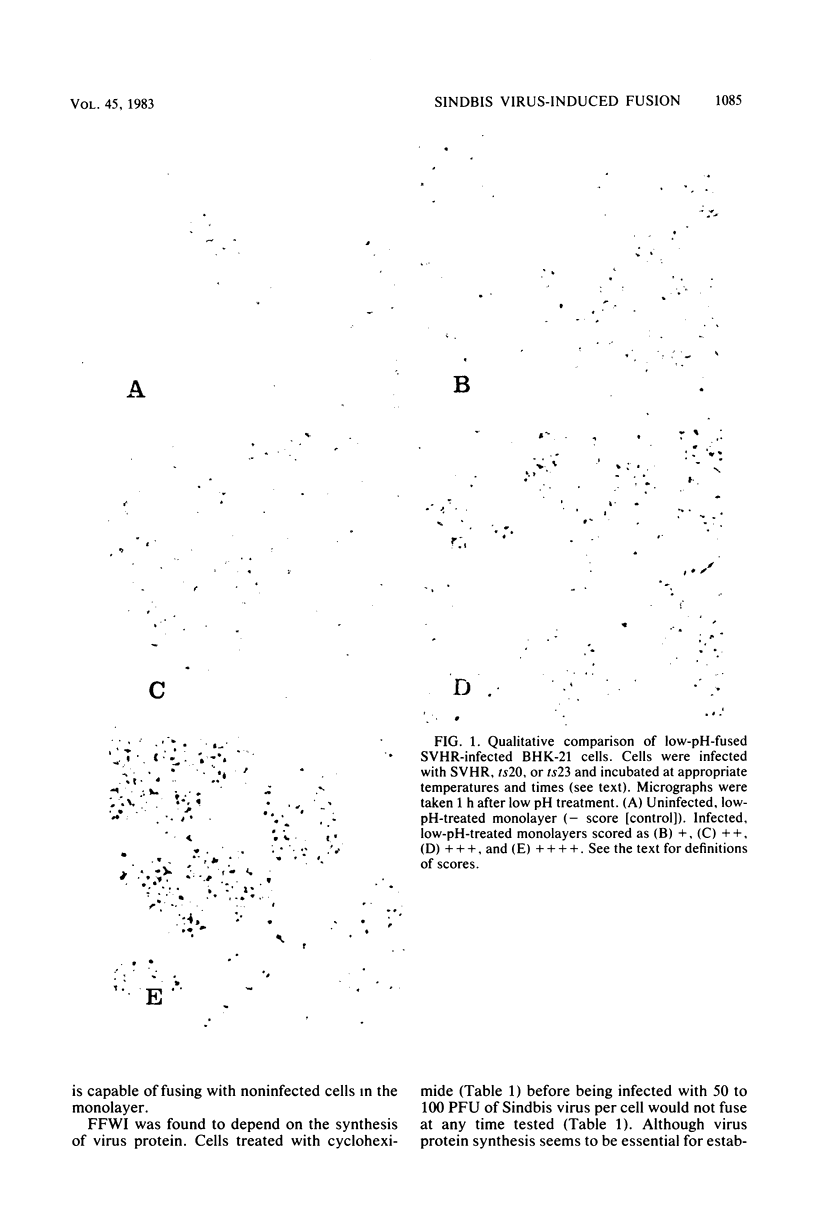
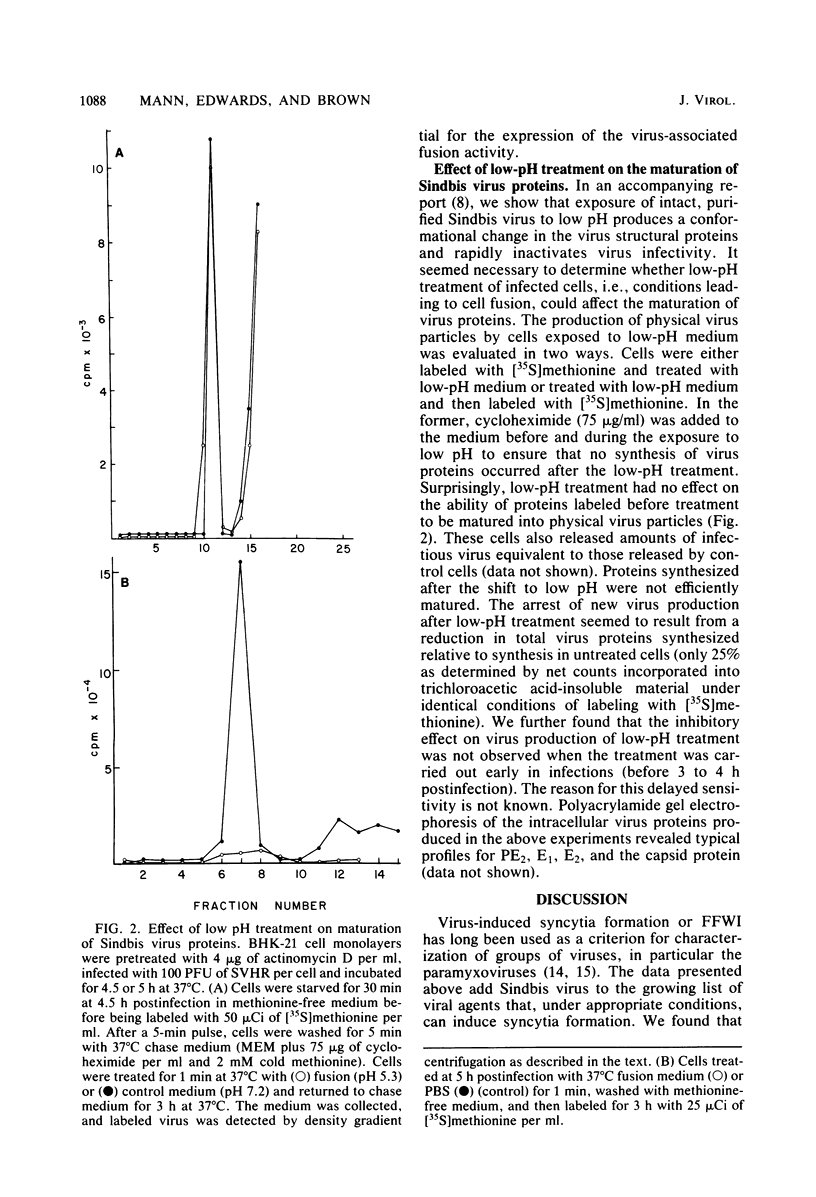
The process of cell fusion mediated by Sindbis virus membrane proteins synthesized after infection was examined. At the times after infection at which virus proteins were detectable on the cell surface, Sindbis virus-infected BHK-21 cells were found to express a fusion function after brief treatment at acid pH. In studies employing wild-type virus and temperature-sensitive mutants and testing drug or protease inhibition of virus production, we made the following observations on Sindbis virus-mediated fusion from within. (i) Fusion requires the synthesis of virus glycoproteins and their transport to the cell surface. (ii) Modification of the cell plasma membrane by polypeptides PE2 and E1 alone is not sufficient for expression of the fusion function. (iii) The proteolytic conversion of plasma membrane-associated PE2 to E2 is not essential for fusion. (iv) Glycosylation of virus plasma membrane proteins is essential for fusion. (v) The lesions of Sindbis virus temperature-sensitive mutants do not affect their ability to fuse cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. H., Brown D. T. Inhibition of Sindbis virus maturation after treatment of infected cells with trypsin. J Virol. 1982 Feb;41(2):692–702. doi: 10.1128/jvi.41.2.692-702.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha M., Schlesinger M. J. Defects in RNA+ temperature-sensitive mutants of Sindbis virus and evidence for a complex of PE2-E1 viral glycoproteins. Virology. 1976 Oct 15;74(2):441–449. doi: 10.1016/0042-6822(76)90350-0. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Smith J. F. Morphology of BHK-21 Cells Infected with Sindbis Virus Temperature-Sensitive Mutants in Complementation Groups D and E. J Virol. 1975 May;15(5):1262–1266. doi: 10.1128/jvi.15.5.1262-1266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs K., Mann E., Edwards J., Brown D. T. Effects of chloroquine and cytochalasin B on the infection of cells by Sindbis virus and vesicular stomatitis virus. J Virol. 1981 Mar;37(3):1060–1065. doi: 10.1128/jvi.37.3.1060-1065.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock N. J. Review article initial stages in infection with animal viruses. J Gen Virol. 1982 Mar;59(Pt 1):1–22. doi: 10.1099/0022-1317-59-1-1. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Edwards J., Mann E., Brown D. T. Conformational changes in Sindbis virus envelope proteins accompanying exposure to low pH. J Virol. 1983 Mar;45(3):1090–1097. doi: 10.1128/jvi.45.3.1090-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin C., Brown D. T. Intracellular distribution of Sindbis virus membrane proteins in BHK-21 cells infected with wild-type virus and maturation-defective mutants. J Virol. 1980 Dec;36(3):775–786. doi: 10.1128/jvi.36.3.775-786.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. J., Waite M. R., Bose H. R. Cleavage of a viral envelope precursor during the morphogenesis of Sindbis virus. J Virol. 1974 Apr;13(4):809–817. doi: 10.1128/jvi.13.4.809-817.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poste G. Mechanisms of virus-induced cell fusion. Int Rev Cytol. 1972;33:157–252. doi: 10.1016/s0074-7696(08)61451-5. [DOI] [PubMed] [Google Scholar]
- Renz D., Brown D. T. Characteristics of Sindbis virus temperature-sensitive mutants in cultured BHK-21 and Aedes albopictus (Mosquito) cells. J Virol. 1976 Sep;19(3):775–781. doi: 10.1128/jvi.19.3.775-781.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheefers H., Scheefers-Borchel U., Edwards J., Brown D. T. Distribution of virus structural proteins and protein-protein interactions in plasma membrane of baby hamster kidney cells infected with Sindbis or vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7277–7281. doi: 10.1073/pnas.77.12.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. F., Brown D. T. Envelopments of Sindbis virus: synthesis and organization of proteins in cells infected with wild type and maturation-defective mutants. J Virol. 1977 Jun;22(3):662–678. doi: 10.1128/jvi.22.3.662-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vänänen P., Käriäinen L. Fusion and haemolysis of erythrocytes caused by three togaviruses: Semliki Forest, Sindbis and rubella. J Gen Virol. 1980 Feb;46(2):467–475. doi: 10.1099/0022-1317-46-2-467. [DOI] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]





