Abstract
Background/Objective:
To compare the t-scores of proximal femur and lumbar spine of patients with spinal cord injury (SCI) with different levels of weight bearing.
Methods:
Cross-sectional study comparing 3 groups of patients with SCI: patients with daily standing times of more than 1 hour, patients with daily standing times of less than 1 hour, and nonstanding patients. Seventy-one patients with chronic SCI were recruited. They were assigned to 1 of 3 groups according to their reported daily standing time. The bone density of lumbar and proximal femoral regions was measured with dual-energy x-ray absorptiometry.
Results:
The 3 groups were similar in terms of demographics and clinical variables. No significant difference was found among the mean t-scores of lumbar and proximal femoral regions of the groups. However, the patients in the group that stood more than 1 hour daily had a slight tendency to have higher t-scores than those in the control group.
Conclusions:
There was no significant difference among the 3 groups. However, standing might be partially helpful in protecting the bone density in SCI by opposing the effects of immobilization.
Keywords: Spinal cord injuries, Standing, Bone mineral density, Osteoporosis, Dual energy x-ray absorptiometry, Immobilization
INTRODUCTION
Bone mineral loss is a well-known complication of spinal cord injury (SCI). Although it has been more than 60 years since bone mineral loss was first reported in the literature (1), its mechanism is still unknown, and preventive methods are still controversial. It is generally accepted that osteoporosis after SCI is the result of immobilization and can be categorized with the other types of loss of weight-bearing like prolonged bed rest and space flight (2,3). SCI may not only cause bone loss, but may also alter bone structure and microstructure. A new steady-state level between bone resorption and formation is believed to be reestablished approximately 1 to 2 years after SCI (4–7); however, de Bruin et al (8) claim that no steady state exists in bone metabolism of patients with SCI.
Some authors argue that neurologic damage and hormonal alterations due to the SCI per se may be the causal factors in the loss of bone density rather than immobilization alone (1,6,7,9). The reason for their argument was the different nature of calcium excretion and response to physical activity in those patients. Due to its unique nature this loss of bone density was called “neurogenic osteoporosis” by BeDell et al (10).
The basic activities that provide weight bearing are standing and walking. There are not enough data in the literature about the effect of either activity and the effect of daily ambulation time on bone density in individuals with SCI. The purpose of our study was to compare the bone density loss in patients with chronic SCI who perform therapeutic standing with that of their nonstanding counterparts and to investigate the association between bone density and the average daily standing time.
METHODS
Patients aged 18 to 46 years who were at least 1 year post-SCI were included in the study. The patients who applied to our rehabilitation center (meeting the above criteria) were consecutively asked to participate. The study protocol was explained to the patients, and their informed consent was obtained. Patients having certain pathologies (eg, hyperthyroidism, hyperparathyroidism, Paget's disease, lower-extremity fractures) or taking medications (eg, bisphosphonates, corticosteroids, phenytoin) liable to modify bone metabolism were excluded. A total of 92 patients were enrolled for the study. To prevent confusion that might have originated from the effect of muscle strength, 21 motor-incomplete (American Spinal Injury Association grades C, D, and E) cases were excluded as well.
Participants were asked to estimate their average daily standing time since their injury. They were asked not to consider the first 6 weeks after the injury. Standing was defined as therapeutic standing with a standing frame, standing wheelchair, or crutches and braces. Not standing was defined as staying in a bed, chair, or wheelchair all day. Standing patients formed 2 different groups according to their average daily standing time: Group A was composed of patients standing at least 1 hour or more daily, and the patients reporting a daily standing time of less than 1 hour formed group B. Nonstanding patients were assigned to the control group (group C). There were 20 patients in group A, 11 in group B, and 40 in group C.
Bone mineral density (BMD) was measured by a Lunar DPX-MD dual-energy x-ray absorptiometer (Lunar Radiation Corporation, Madison, WI). Sites measured were trochanters, Ward's triangles, the femoral necks of both hips, and the L2 to L4 spine. The hip with lower total bone density was taken into consideration. The in vivo coefficients of variation in our laboratory are: lumbar spine BMD, 1.27%; femoral neck BMD, 1.64%; Ward's triangle BMD, 1.38%; and trochanter BMD, 1.76%. The technician who performed the BMD measurements was blinded to patients' assignments to the groups. The t-scores of measured sites were calculated by using the reference values provided by the densitometry manufacturer. The t-score is preferred as the primary outcome to actual BMD values because of the definition of osteoporosis by the World Health Organization (11). The differences among the 3 groups for the t-scores of measured sites were analyzed by using analysis of variance (SPSS for Windows, v11.5 SPSS Inc., Chicago, IL). The differences for demographic and clinical variables were tested with chi-square and analysis of variance.
RESULTS
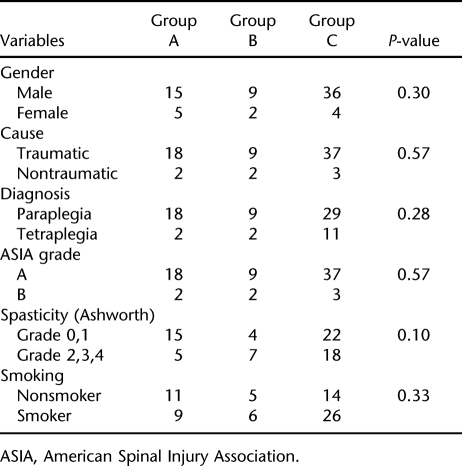
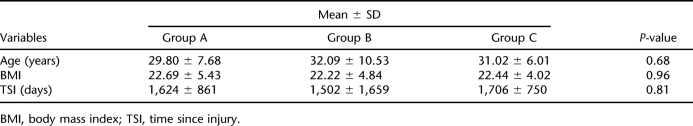
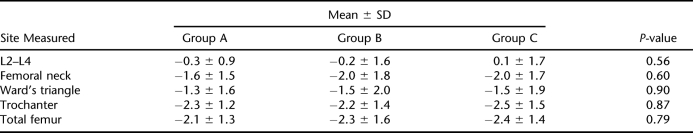
The L2 to L4 tests of 5 patients (3 from group A, 2 from group C) were not taken into consideration for analysis because of the effect of osteosynthesis. Similarly, the femoral region values of 4 patients (all from group C) were not used for analysis because of bilateral heterotopic ossification. The groups were not significantly different in terms of clinical and demographic variables (eg, gender, cause, diagnosis, American Spinal Injury Association grade, spasticity, smoking status, age, body mass index, and time since injury) (Tables 1 and 2). The excluded values did not produce significant differences, either. Mean t-score of the L2 to L4 region of the control group was slightly increased (0.1 ± 1.7), whereas groups A and B showed a slight decrease (−0.3 ± 0.9 and −0.2 ± 1.6, respectively). The differences among the 3 groups were not statistically significant (P = 0.56). The region t-scores were markedly decreased for all the groups (−2.3 to −1.3 for group A, −2.3 to −1.5 for group B, and −2.5 to −1.5 for group C). Similarly, the differences were not statistically significant (P-value for femoral neck, 0.60; for Ward's triangle, 0.90; for trochanter, 0.87; and for total femur, 0.79) (Table 3). Because an analysis of variance revealed no significant results, further analysis with post hoc tests was not considered necessary.
Table 1.
Comparison of the Groups for Dichotomous Demographic and Clinical Variables
Table 2.
Comparison of the Groups for Interval Demographic and Clinical Variables
Table 3.
Comparison of t-Scores of Three Groups
DISCUSSION
Osteoporosis after SCI is associated with increased risk of fracture as expected. The risk is reported to be 1 to 20% in different articles (10,12,13). Various methods of treatment including standing, physical activity, functional electric stimulation, and medications are being used to prevent or reverse the bone mineral loss. Nevertheless, it is not clear whether these methods are helpful in patients with neurogenic osteoporosis. Studies with functional electric stimulation reported conflicting results. Mohr et al (14), Hangartner et al (15), and Bloomfield et al (16) reported significant increases in BMD with functional electric stimulation, whereas other studies found no significant BMD changes (4,17–19). Shields et al (4) demonstrated that compressive loads of 1 to 2 times body weight induced by muscle contractions partially prevented the loss of BMD after SCI. A few studies investigated the effect of exercise or standing on bone density. Jones et al (2) reported an increase in bone density of upper limbs and a decrease in other regions in physically active patients with SCI compared with active, able-bodied persons. Kunkel et al (20) found no significant BMD increase in their patients who stood for two 45-minute sessions daily with a standing frame. They followed their single group of 6 patients for 6 months. Goemaere et al (21) reported an increase in bone density of the femoral shaft but not of the hip region in their patients with SCI who were standing for therapeutic purposes. A recent study demonstrated that body weight–supported treadmill training did not prevent bone loss in patients with SCI (22).
Therapeutic standing is traditionally advised to patients with chronic SCI and is usually incorporated in their rehabilitation programs in order to prevent osteoporosis, besides other purposes. To date, it has not been proven to be effective. Although previous studies reported that standing was not effective in preventing osteoporosis in SCI patients, it was not clear whether different standing times were associated with differences in bone density loss. To our knowledge, this is the first study comparing SCI patients who do not perform therapeutic standing with patients having different standing times.
As was shown in most studies, there was a marked bone density loss in the proximal femoral region in all groups. No significant difference was found among the t-scores of the 3 groups. This is consistent with the findings of Kunkel et al (20), Goemaere et al (21), and Giangregorio et al (22). In their prospective study, Kunkel et al (20) measured the bone density of their standing patients at 3-month intervals. They did not detect any improvement in bone density over time. In their study with single-photon absorptiometry, Goemaere et al (21) reported no difference between the proximal femoral density of standing and nonstanding patients. However, they reported relatively better-preserved densities in patients standing with braces than in those using a standing frame or standing wheelchair. They concluded that this result could be due to mechanical loading. In their recent study, Giangregorio et al (22) indicated that the level of mechanical strain on the bone imposed by the body weight–supported treadmill training was not sufficient to prevent bone loss. In our study, the mean t-score of group A was slightly higher than those of the other two groups, and the mean t-score of group B was higher than that of group C for femoral neck and total femoral measurements. In general, the mean t-scores of the patients standing more than 1 hour daily were higher for all the measured sites in the proximal femur than those of the patients who were not standing. This finding may indicate that standing has a small effect on bone density in patients with chronic SCI. Standing opposes the loss of bone mineral content caused by immobilization. However, immobilization is only partially responsible for osteoporosis in SCI. Other factors like neurologic and hormonal changes have been proposed, but no scientific evidence has been presented in the literature. Prospectively designed, controlled studies are needed to further examine the extent of the effect of weight bearing on bone density in patients with SCI.
Mean t-scores of all the groups were normal (around 0) for lumbar region measurements. This is consistent with most of the published articles (2,3,5,20,23,24). Preserved lumbar bone density is usually attributed to weight bearing during sitting or wheelchair activities. On the other hand, Liu et al (25) measured the lumbar bone density of their patients with both dual-energy x-ray absorptiometry and single-energy quantitative computed tomography and found a mean lumbar z-score of −2.4 with quantitative computed tomography, whereas the mean z-score of the same patients with dual-energy x-ray absorptiometry was 1.3. They argued that preserved lumbar bone density is the result of falsely elevated dual-energy x-ray absorptiometry measurements due to osteosynthesis, heterotopic bone formation, and neuropathic and osteoarthritic changes occurring after SCI. This theory may also explain why standing does not produce a considerable effect on proximal femoral density, whereas sitting seems to protect or even increase lumbar bone density.
The limitations of our study were its retrospective design and adherence to self-reported standing times. Self-reporting can be unreliable in some ways (it may not reflect true standing times); however, it can also be an advantage (the patients can give us information about their whole lives, not only about the time they spend for a certain intervention). Studies with prospective design and a predetermined standing schedule also including daily standing times with longer duration should provide more reliable information about the benefits of standing.
CONCLUSION
In conclusion, different daily standing times of less than 1 hour or more than 1 hour for a mean period of 4.2 years did not produce a significant effect on bone density in patients with chronic SCI. However, a slight increase in proximal femur bone density was found in favor of longer-standing group. Lumbar bone density was found to be preserved regardless of standing. Whether this is the effect of prolonged sitting in the wheelchair or it is falsely measured by dual-energy x-ray absorptiometry should be elucidated with further research. Osteoporosis after SCI may be the result of combined effects of immobilization and other factors that are not clear today. Therapeutic standing aims to reduce immobilization, but apparently has no effect on other factors. Therefore, it has a limited effect on proximal femoral bone density. Whether this effect is clinically important should be investigated by future studies.
REFERENCES
- Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D. Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil. 1999;80:243–251. doi: 10.1016/s0003-9993(99)90133-8. [DOI] [PubMed] [Google Scholar]
- Jones LM, Legge M, Goulding A. Intensive exercise may preserve bone mass of the upper limbs in spinal cord injured males but does not retard demineralization of the lower body. Spinal Cord. 2002;40:230–235. doi: 10.1038/sj.sc.3101286. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM, Wang J, Pierson RN, Jr, Schwartz E. Continuous loss of bone during chronic immobilization: a monozygotic twin study. Osteoporos Int. 1999;10:123–127. doi: 10.1007/s001980050206. [DOI] [PubMed] [Google Scholar]
- Shields RK, Dudley-Javoroski S, Frey Law LA. Electrically induced muscle contractions influence bone density decline after spinal cord ınjury. Spine. 2006;31:548–553. doi: 10.1097/01.brs.0000201303.49308.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goktepe AS, Yilmaz B, Alaca R, Yazicioglu K, Mohur H, Gunduz S. Bone density loss after spinal cord injury: elite paraplegic basketball players vs paraplegic sedentary persons. Am J Phys Med Rehabil. 2004;83:279–283. doi: 10.1097/01.phm.0000118036.20170.6c. [DOI] [PubMed] [Google Scholar]
- Jiang SD, Dai LY, Jiang LS. Osteoporosis after spinal cord injury. Osteoporos Int. 2006;17:180–192. doi: 10.1007/s00198-005-2028-8. [DOI] [PubMed] [Google Scholar]
- Maimoun L, Fattal C, Micallef J-P, Peruchon E, Rabischong P. Bone loss in spinal cord–injured patients: from physiopathology to therapy. Spinal Cord. 2006;44:203–210. doi: 10.1038/sj.sc.3101832. [DOI] [PubMed] [Google Scholar]
- de Bruin ED, Vanwanseele B, Dambacher MA, Dietz V, Stussi E.Long-term changes in the tibia and radius bone mineral density following spinal cord injury Spinal Cord. 200543 (2) 96–101. [DOI] [PubMed] [Google Scholar]
- Jiang SD, Jiang LS, Dai LY. Mechanisms of osteoporosis in spinal cord injury. Clin Endocrinol. 2006;65:555–565. doi: 10.1111/j.1365-2265.2006.02683.x. [DOI] [PubMed] [Google Scholar]
- BeDell KK, Scremin AM, Perell KL, Kunkel CF. Effects of functional electrical stimulation–induced lower extremity cycling on bone density of spinal cord–injured patients. Am J Phys Med Rehabil. 1996;75:29–34. doi: 10.1097/00002060-199601000-00008. [DOI] [PubMed] [Google Scholar]
- Kanis JA, Melton LJ, III, Christiansen C, Johnston CC, Khaltaev N.The diagnosis of osteoporosis J Bone Miner Res. 19949 (8) 1137–1141. [DOI] [PubMed] [Google Scholar]
- Ingram RR, Suman RK, Freeman PA. Lower limb fractures in the chronic spinal cord injured patient. Paraplegia. 1989;27:133–139. doi: 10.1038/sc.1989.20. [DOI] [PubMed] [Google Scholar]
- Roberts D, Lee W, Cuneo RC, et al. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab. 1998;83:415–422. doi: 10.1210/jcem.83.2.4581. [DOI] [PubMed] [Google Scholar]
- Mohr T, Podenphant J, Biering-Sorensen F, Galbo H, Thamsborg G, Kjaer M. Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int. 1997;61:22–25. doi: 10.1007/s002239900286. [DOI] [PubMed] [Google Scholar]
- Hangartner TN, Rodgers MM, Glaser RM, Barre PS. Tibial bone density loss in spinal cord injured patients: effects of FES exercise. J Rehabil Res Dev. 1994;31:50–61. [PubMed] [Google Scholar]
- Bloomfield SA, Mysiw WJ, Jackson RD. Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone. 1996;19:61–68. doi: 10.1016/8756-3282(96)00109-3. [DOI] [PubMed] [Google Scholar]
- Rodgers MM, Glaser RM, Figoni SF, et al. Musculoskeletal responses of spinal cord injured individuals to functional neuromuscular stimulation–induced knee extension exercise training. J Rehabil Res Dev. 1991;28:19–26. doi: 10.1682/jrrd.1991.10.0019. [DOI] [PubMed] [Google Scholar]
- Needham-Shropshire BM, Broton JG, Klose KJ, Lebwohl N, Guest RS, Jacobs PL. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: part 3. Lack of effect on bone mineral density. Arch Phys Med Rehabil. 1997;78:799–803. doi: 10.1016/s0003-9993(97)90190-8. [DOI] [PubMed] [Google Scholar]
- Leeds EM, Klose KJ, Ganz W, Serafini A, Green BA. Bone mineral density after bicycle ergometry training. Arch Phys Med Rehabil. 1990;71:207–209. [PubMed] [Google Scholar]
- Kunkel CF, Scremin AM, Eisenberg B, Garcia JF, Roberts S, Martinez S. Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil. 1993;74:73–78. [PubMed] [Google Scholar]
- Goemaere S, Van Laere M, De Neve P, Kaufman JM. Bone mineral status in paraplegic patients who do or do not perform standing. Osteoporos Int. 1994;4:138–143. doi: 10.1007/BF01623058. [DOI] [PubMed] [Google Scholar]
- Giangregorio LM, Hicks AL, Webber CE, et al. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord. 2005;43:649–657. doi: 10.1038/sj.sc.3101774. [DOI] [PubMed] [Google Scholar]
- Wood DE, Dunkerley AL, Tromans AM. Results from bone mineral density scans in twenty-two complete lesion paraplegics. Spinal Cord. 2001;39:145–148. doi: 10.1038/sj.sc.3101125. [DOI] [PubMed] [Google Scholar]
- Leslie WD, Nance PW. Dissociated hip and spine demineralization: a specific finding in spinal cord injury. Arch Phys Med Rehabil. 1993;74:960–964. [PubMed] [Google Scholar]
- Liu CC, Theodorou DJ, Theodorou SJ, et al. Quantitative computed tomography in the evaluation of spinal osteoporosis following spinal cord injury. Osteoporos Int. 2000;11:889–896. doi: 10.1007/s001980070049. [DOI] [PubMed] [Google Scholar]