Abstract
Background/Objective:
Children with spinal cord injury (SCI) are at risk for musculoskeletal and cardiovascular complications. Stationary cycling using functional electrical stimulation (FES) or passive motion has been suggested to address these complications. The purpose of this case series is to report the outcomes of a 6-month at-home cycling program for 4 children with SCI.
Methods:
Two children cycled with FES and 2 cycled passively at home for 1 hour, 3 times per week.
Outcome Measures:
Data collected included bone mineral density of the left femoral neck, distal femur, and proximal tibia; quadriceps and hamstring muscle volume; stimulated quadriceps and hamstring muscle strength; a fasting lipid profile; and heart rate and oxygen consumption during incremental upper extremity ergometry testing.
Results:
The 2 children cycling with FES and 1 child cycling passively exhibited improved bone mineral density, muscle volume, stimulated quadriceps strength, and lower resting heart rate. For the second child cycling passively, few changes were realized. Overall, the lipid results were inconsistent, with some positive and some negative changes seen.
Conclusions:
This case series suggests that cycling with or without FES may have positive health benefits and was a practical home exercise option for these children with SCI.
Keywords: Spinal cord injuries, Tetraplegia, Paraplegia, Child, Exercise, Functional electrical stimulation, Cycling, Pediatrics, Bone mineral density
INTRODUCTION
The incidence of pediatric spinal cord injury (SCI) is approximately 1.99 per 100,000 children (1). Because of improvements in health care, life expectancy for these children has increased (2). With age, they face the same health problems experienced by the general population, in addition to the neuromuscular effects of SCI, which can lead to decreased quality of life and significant lifetime medical costs (3).
One means to address the neuromuscular effects of SCI is through exercise, particularly cycling. For adults with SCI, studies have shown that cycling with functional electrical stimulation (FES) increases muscle cross-sectional area (4,5), lean body mass (4,5), voluntary and electrically induced muscle force (4,5), muscle endurance (6,7), and the body's utilization of oxygen (energy expenditure) (8–11) and causes improvements in heart rate, stroke volume, and cardiac output during exercise and at rest (12). Bone mineral density (BMD) has been shown to increase following a program of FES cycling in some studies (13–15), but not in others (16,17). In addition, FES cycling with more than 1,500 revolutions per week has also led to reductions in spasticity (18,19). Less research has focused on the benefits of passive cycling in individuals with SCI. Improvements have been reported in spasticity (20), cardiac output and stroke volume (21), and muscle atrophy (22) after a program of passive cycling.
The aforementioned studies have focused on cycling for adults with SCI, with no information on the potential benefits for children. This is the first study to examine the outcomes of these interventions in children with SCI. We report the results after 6 months of home cycling with FES or passively for 4 children with SCI.
METHODS
The children in this case series were selected from those participating in a randomized controlled trial (RCT) comparing the 2 cycling modes to a noncycling electrical stimulation program. A nonintervention control group was not included, and the larger RCT was registered with the clinicaltrials.gov database. The 4 children presented in this case series were selected as a convenience sample to provide a representative sample of the children in the RCT. One child with tetraplegia and one child with paraplegia were selected for each intervention. The children were also matched as closely as possible by age.
Parents and children signed informed consent and assent forms, respectively, which were approved by the governing institutional review board. Inclusion criteria were at least 12 months post injury, cervical or thoracic level SCI with an American Spinal Injury Association (ASIA) A or ASIA B classification, age 5 to 13 years, and innervated lower extremity muscles. Exclusion criteria included chronic steroid treatment, history of seizures, cardiac disease, lower limb stress fractures, uncontrolled autonomic dysreflexia, heterotopic ossification, and hip dislocation.
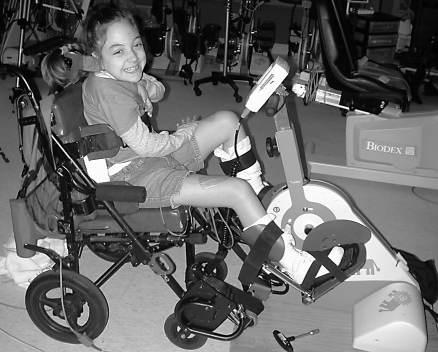
Children cycled at home with parental assistance for 1 hour, 3 times per week for 6 months. If more than 6 sessions were missed over the 6 months, subjects added 1 session per week until the targeted number of overall sessions was reached. Subjects using FES cycled at 50 rpm using an RT300-P FES cycle (Restorative-Therapies, Inc., Baltimore, MD) while seated in their wheelchairs (Figure 1). The cycle provided bilateral cyclical stimulation to the quadriceps, hamstring, and gluteal muscles using the largest surface electrodes (Axelgaard Manufacturing Co, Inc, Fallbrook, CA) appropriate for the child's leg. Stimulation pulse duration (150 μs) and frequency (33 Hz) were fixed, and current amplitude (maximum of 140 mA) increased automatically to generate sufficient force to maintain the cadence. Children cycling passively used the RT100 (Restorative-Therapies, Inc., Baltimore, MD) motorized cycle, which passively moved the legs at 50 rpm.
Figure 1. A child with a spinal cord injury using the functional electrical stimulation cycle.
To standardize cycling mechanics across children, adjustments were made to accommodate children's sizes and wheelchair configurations. For example, crank arm length was shortened to accommodate smaller legs, and the calf support could be manipulated to prevent it from hitting the wheelchair. The cycle's height was raised with a custom-made wooden platform so the children's feet would reach the pedals.
Prior to exercising, children participated in lower extremity muscle stretches. Children were permitted to continue their previously established activities including standing and walking with braces but were not permitted to participate in non–study-related lower extremity repetitive motion tasks or electrically stimulated exercise. It was decided not to alter the prestudy exercise routine as declines from baseline values might have occurred if these activities were stopped. Throughout the study, 3 children (1 FES, 2 passive) participated in standing activities and 1 child (passive) participated in upper extremity strengthening exercises. None of these activities were new to the children prior to study participation.
Outcome Measures
Musculoskeletal and cardiovascular/respiratory measures were collected. Musculoskeletal measures included bone mineral density (BMD) of the left femoral neck, distal femur, and proximal tibia; left quadriceps muscle volume using magnetic resonance imaging (MRI); electrically stimulated strength of the left quadriceps; and spasticity of the quadriceps and hamstrings muscles. Cardiovascular and respiratory measures included a fasting lipid profile and an incremental upper extremity (UE) ergometry test to assess heart rate (HR) and oxygen consumption (VO2/kg). The investigators performing testing were blinded to the measures of BMD, muscle volume, and lipid levels, but not to the other measures.
Musculoskeletal Measures.
To assess BMD, dual-energy x-ray absorptiometry (DEXA) (Hologic, Inc., Bedford, MA) was used with the child in the supine position. The femoral neck was analyzed using the standard hip algorithm, and a modified lumbar spine algorithm (23) was employed for the distal femur and proximal tibia. Measurement error has been determined to be as high as 20% for assessing BMD using DEXA (24).
Magnetic resonance imaging was performed for the left thigh (most proximal to most distal quadriceps femoris) in 0.7-cm slices using a 1.5-tesla magnet (GE Medical Systems, Waukesha, WI) and a torso coil. Chemically selective fat suppression enhanced definition between muscles. The MRI data were processed using a custom program in Interactive Data Language (Version 6.2) (ITT Visual Information Solutions, Boulder, CO) (25). The head of the femur was used as the reference starting point, and the rectus femoris, vastus lateralis, vastus medialis, and vastus intermedius muscles were outlined separately to determine cross-sectional area. Muscle volume was calculated by multiplying the cross-sectional area of each muscle by 0.7 cm (26). Measurement error for this technique has been reported to range from 6% to 13% (26).
To assess stimulated isometric quadriceps strength, the children were seated with the hip and knee flexed at 80° and 60°, respectively, on an isokinetic dynamometer (Chattex Corp, Chattanooga, TN). The dynamometer's axis was aligned with the knee joint's axis, and the distal attachment was made approximately 3 cm above the lateral malleolus. Surface electrodes were used, and maximum stimulation levels were determined by increasing the current until the force output plateaued. After a 3-minute rest, 3 trials were performed with 2-minute rest periods between trials, and the highest value was selected. Measurement error for this technique is unknown.
Quadriceps and hamstring spasticity was assessed using the Ashworth Scale, which was performed with children seated on the isokinetic dynamometer. The examiner (same for all testing) quickly moved each leg 3 times into extension and flexion. All 3 scores were recorded per leg, and the mode was then calculated for each muscle group. Intrarater reliability of the Ashworth Scale for muscles around the knee has been shown to range from 0.27 to 0.54 (Kappa) and 0.44 to 0.66 (Kendall's τ-b) in individuals post stroke (27).
Cardiovascular Measures.
The fasting lipid profile included cholesterol, high-density lipoproteins (HDL), low density lipoproteins (LDL), and triglycerides. Children fasted overnight for at least 10 hours prior to the blood draw, which is consistent with other studies (28). Measurement error has been reported to be 6.1 mg/dL for cholesterol, 2.3 mg/dL for HDL, 7.4 mg/dL for LDL, and 18.2 mg/dL for triglycerides in a sample of 19 children (29).
During the upper extremity ergometry test, HR and VO2/kg data were collected using a breath-by-breath technique with a SensorMedics VMax29 metabolic cart (Viasys Healthcare, Yorba Linda, CA). The children wore a small airtight facemask (Hans Rudolph, Inc, Kansas City, MO) over the mouth and nose that held the flow sensor that measured the volume of oxygen (VO2 in mL) per kilogram of body weight (VO2/kg). VO2/kg was measured under 4 consecutive conditions: (a) sitting quietly for 5 minutes (baseline), (b) UE cycling at 10 W at a self-selected cadence for 1 minute (warm-up), (c) UE cycling at 10 W with increases of 10 W every minute until self-determined fatigue, and (d) sitting quietly for 3 minutes (recovery). Resting HR was averaged from baseline data, and peak HR and peak VO2/kg were calculated by determining the highest 15-second average value. Due to potential power output differences between baseline and 6 months, peak HR was compared at the highest resistance level that was achieved at both data collection points. Measurement error for this test is unknown for children with SCI.
Parent Reports and Program Adherence
Throughout the 6-month period, biweekly phone calls were made to the parents to assess program adherence and to identify any problems. Parents were asked whether the child had exercised 3 times in each of the previous 2 weeks and also were asked whether they noticed any changes in their child during that same time period. The answers to these questions were recorded on a data sheet. If the child had not exercised enough during the 2 weeks, the parents were asked why and were instructed to resume the regular schedule as soon as possible.
CASES
FES Cycling
Participant 1.
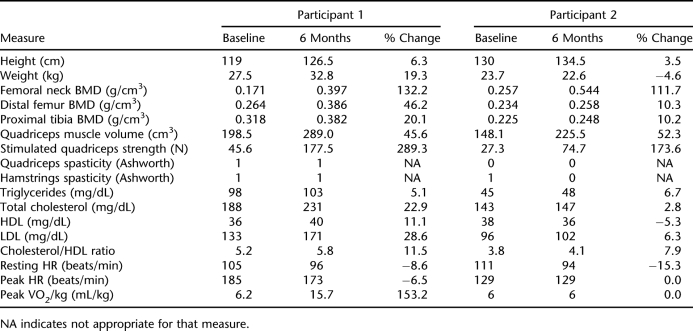
Participant 1 was a 7-year-old white girl who sustained a T4-T6 ASIA A SCI in a motor vehicle crash at the age of 2 years. The maximum stimulation current amplitudes were set at 115 mA (quadriceps), 130 mA (hamstrings), and 100 mA (gluteals). Gluteal stimulation was set lower as higher amplitudes led to a dysreflexic response. Participant 1 had a 92.5% adherence rate and experienced improvements in BMD at the femoral neck, distal femur, and proximal tibia; quadriceps muscle volume; stimulated strength of the quadriceps muscles; HDL cholesterol; resting HR; peak VO2/kg; and peak HR (Table 1). However, cholesterol, LDL, and triglyceride levels and the cholesterol/HDL ratio increased as compared to baseline. There were no changes in her Ashworth scores, but her parents reported decreased spasticity and looser muscles.
Table 1.
Results for Children Participating in FES Cycling
Participant 2.
Participant 2 was a 9-year-old white girl who sustained a C7 ASIA A SCI in a motor vehicle crash at the age of 4 years. During the study, the maximum stimulation current amplitudes were set at 60 mA (quadriceps), 50 mA (hamstrings), and 10 mA (gluteals), as higher levels caused autonomic dysreflexia. Participant 2 had a 100% adherence rate and experienced improvements in BMD at the femoral neck, distal femur, and proximal tibia; quadriceps muscle volume; stimulated quadriceps muscle strength; and hamstring muscle spasticity (Table 1). However, cholesterol, LDL, HDL, and triglyceride levels and the cholesterol/HDL ratio worsened as compared to baseline. Her parents reported bigger, firmer muscles; decreased bowel program completion times; increased appetite; and increased spasticity that did not require medical intervention.
Passive Cycling
Participant 3.
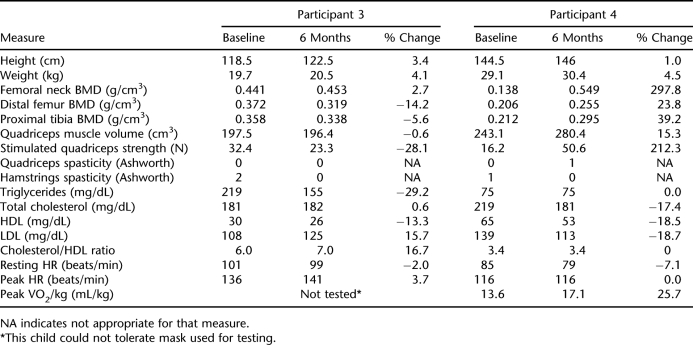
Participant 3 was a 7-year-old Hispanic boy who sustained a T3 ASIA A SCI in a motor vehicle crash at the age of 3 years. He was taking spasticity-reducing medication, which remained unchanged during the study. Participant 3 had a 100% adherence rate and realized improvements in femoral neck BMD, hamstring spasticity, and triglyceride levels (Table 2). Distal femur and proximal tibia BMD and stimulated quadriceps strength were lower as compared to baseline, and LDL levels and the cholesterol/HDL ratio were elevated. His parents reported decreased bowel accidents and new sensation in his knees and stomach.
Table 2.
Results for Children Participating in Passive Cycling
Participant 4.
Participant 4 was an 11-year-old white boy who sustained a C7 ASIA A SCI in a motor vehicle crash at the age of 3 years. Participant 4 had a 100% adherence rate and experienced improvements in BMD at the femoral neck, distal femur, and proximal tibia; quadriceps muscle volume; stimulated quadriceps strength; hamstring spasticity; cholesterol; LDL cholesterol; resting HR; and peak VO2/kg (Table 2). HDL cholesterol decreased as compared to baseline but the cholesterol/HDL ratio was unchanged. His parents reported decreased spasticity, looser muscles, increased energy, decreased lower extremity swelling, and increased appetite.
DISCUSSION
The results suggest that cycling can be performed at home with parental assistance. Reported adherence to the exercise program was greater than 90%, indicating that families saw exercise as important and were able to incorporate exercise into their busy lives. For a study examining longer-term effects of cycling, it was important for the participants to cycle at home to remove potential barriers to participation. With an at-home program, children and parents had the flexibility to exercise at times convenient for them, potentially resulting in improved adherence and outcomes.
Both children cycling with FES showed BMD improvements. A systematic review examining BMD changes following exercise for children with typical development found BMD gains of 0.3% to 5.5% depending on pubertal status (30). In our study, the BMD changes for the 2 children cycling with FES greatly exceeded these values, indicating a substantial improvement. These children also showed comparable or greater increases than those reported for adults with SCI cycling with FES who had knee BMD improvements of approximately 10% (14,31), with no effects at the femoral neck (14,31).
For children cycling passively, femoral neck BMD improved, with Participant 4 having a large change (297.8%) and Participant 3 having a small change (2.7%) consistent with that expected from exercise (30). Similarly, Participant 4 experienced large gains in knee BMD while Participant 3 did not. This may represent a ceiling effect for the influence of passive cycling on BMD. Participant 4, the child with large gains, had 31% to 60% lower baseline values compared to the other child cycling passively. Perhaps passive cycling provided a musculoskeletal load that was influential with smaller BMD values. Greater loads, such as those provided by FES, may be needed to further increase BMD. For example, at baseline, Participant 1 (FES cycling) had distal femur and proximal tibia BMD values that were within 70% and 88%, respectively, of Participant 3′s values (passive cycling). However, Participant 1 realized distal femur and proximal tibia BMD increases of 46% and 20%, respectively, while Participant 3 experienced no changes. Further study is needed to determine the mechanism for increased BMD with passive cycling. It also needs to be determined whether there are significant increases in BMD with FES cycling and whether these increases are significantly different than those obtained through passive cycling.
For children without disability, normative values are available for femoral neck BMD based on age and sex (32), but not for BMD around the knee. While the femoral neck normative values can be used for comparison, these comparisons should be made cautiously as they were performed on a different scanner than used in this study. Using these normative values, the children cycling with FES improved from 3.3 to 1.4 (Participant 1) and 2.8 to 0.8 (Participant 2) standard deviations below the mean, and the subjects cycling passively improved from 1.8 to 1.7 (Participant 3) and 3.8 to 1.4 (Participant 4) standard deviations below the mean. In adults, each unit of standard deviation decrement at the femoral neck as compared to age-matched controls increases the risk of fracture in the lower extremity by 2.8 (33).
There were large increases in quadriceps muscle volume and stimulated strength for children cycling with FES. In adults with SCI, improvements in muscle cross-sectional area with FES cycling of approximately 38% in the quadriceps muscles have been reported (4,5). Another study reported significant increases in stimulated quadriceps strength following 3 months of FES cycling in adults with SCI (16), although they did not report the magnitude of this increase. Clinically, increasing muscle mass may be very important, as decreased muscle mass is related to insulin resistance in adults with SCI (34) and can decrease the metabolic rate and increase the risk of metabolic disease (35). In a small sample of adults with tetraplegia, a decrease in insulin-mediated glucose utilization showed a direct linear relationship with a decrease in muscle mass (36).
One child cycling passively experienced large increases in stimulated quadriceps strength but only a modest increase in muscle volume. The large increase in strength may be due to spasms that occurred during baseline testing, which may have underestimated the muscles' potential. During testing, the force would increase and then decrease simultaneously with a visible flexor spasm. A topical anesthetic was applied to decrease reflex activity due to cutaneous stimulation, and the child was retested; however, the problem continued. At follow-up, flexor spasms did not occur, which may be related to self-reports of decreased spasticity. The other child cycling passively showed no gains in muscle volume or strength.
There was no apparent correlation between Ashworth scores and self-reported changes in spasticity. This is not surprising since the Ashworth Scale measures spasticity at one moment during the day at a specific joint position, while the participants' reports are their recollection of how spasticity affects their everyday function. For example, Participant 3 (passive) reported no change in spasticity, but a 2-point drop was noted for the hamstring muscle using the Ashworth Scale. In addition, Participant 1 (FES) reported decreased spasticity, but no change was noted in Ashworth scores.
Data suggest that both children cycling with FES experienced exercise effects on the cardiorespiratory system, including a decline in resting HR for both children and a decrease in peak HR for the child with paraplegia, indicating that the heart did not have to work as hard during exercise. The child with tetraplegia experienced no change in peak HR. This finding is consistent with the literature, as adults with tetraplegia have a blunted HR response to exercise due to alterations in sympathetic drive (37), which likely is also true for children with tetraplegia. The child with paraplegia also experienced a 153% increase in peak VO2/kg, which likely cannot be explained by her other activities, which only included passive standing in a standing frame. A study examining VO2 max changes following a 12-week aerobic exercise program for 10- to 12-year-old children with typical development showed average VO2 max increases of 6.5% (38), indicating that the child in our study made a significant gain. For the children cycling passively, 1 child's resting HR declined. Peak VO2/kg also increased, which was unanticipated. However, an exercise response may have occurred, as his parents reported fatigue after passive cycling and improved overall energy level throughout the study.
A meta-analysis of lipid profiles post aerobic exercise in typically developing children and adolescents (5–19 years) reported changes only in triglyceride levels (average change 12%). The confidence intervals for lipid changes were found to be −4.4 to 3.3 mg/dL for cholesterol, −4.8 to 1.9 mg/dL for HDL, −4.3 to 6.7 mg/dL for LDL, and −22.8 to 0.8 mg/dL for triglycerides (39). Using these confidence intervals, 1 child cycling with FES had positive HDL changes (4-point increase), 1 child cycling passively had decreased cholesterol (38-point decrease) and LDL levels (26-point decrease), and 1 child cycling passively had decreased triglyceride levels (64-point decrease). In addition, 3 of the 4 children showed an increase (negative change) in their cholesterol/HDL ratios. When taking measurement error into account, the only positive changes in lipids were experienced by subjects in the passive cycling group. However, the American Academy of Pediatrics reports that in children and adolescents, even a small reduction in total cholesterol and LDL levels in children and adolescents could have a large impact on decreasing the incidence of coronary heart disease, if these lower values are carried over into adulthood (40).
Due to the overall inconsistent changes in lipid profiles seen, a different approach may be needed to improve lipid levels in children with SCI. Diet was not addressed in this study, and an intervention combining diet and exercise may be more effective in altering lipid profiles as compared to exercise alone.
There are several limitations of this study. First, a control group not receiving intervention was not included in the RCT; therefore, the results may have been influenced by the developmental stage and growth of the children in the study. It is unknown how these impacted this study. Due to this, normative values for children without disability and data from adults with SCI following exercise programs were used to assist in the understanding of the results. The physical development of children with SCI for the measures tested is not known and warrants further investigation. A second limitation is that the parents provided the information on program adherence through biweekly phone calls, and their reports of changes in their children were subjective. There did not appear to be a relationship between reported adherence and satisfaction with the results of the program. Typical reasons for not exercising were family and school obligations. Another limitation is that measurement error is not known for all tests performed in this study, as some of these techniques have not been previously used for children with SCI. Future work should address the issues of reliability and measurement error for children with SCI. Finally, this report is a case series, so the data represent a small sample of children. Larger studies are needed to determine the effects of FES and passive cycling for children with SCI.
CONCLUSIONS
For the 2 children who cycled with FES for 6 months, improvements in BMD, muscle volume, muscle strength, and resting HR were realized. Improvements of similar magnitude were realized for 1 of the children participating in passive cycling. These results provide preliminary data on the outcomes of FES and passive cycling for children with SCI. Stationary cycling appeared to be a home exercise option for these children.
Acknowledgments
This study was funded by Shriners Hospitals for Children, Grant 8540.
REFERENCES
- Vitale MG, Goss JM, Matsumoto MA, Roye DP. Epidemiology of pediatric spinal cord injury in the United States. J Pediatr Orthop. 2006;26:745–749. doi: 10.1097/01.bpo.0000235400.49536.83. [DOI] [PubMed] [Google Scholar]
- Annual Report for the Model Spinal Cord Injury Care Systems. National Spinal Cord Injury Statistical Center. 2006. Available at: http://www.uab.edu/images/spinalcord/pdffiles/facts05.pdf. Accessed December 20, 2006.
- Vogel LC, Klaas SJ, Lubicky JP, Anderson CJ. Long-term outcomes and life satisfaction of adults who had pediatric spinal cord injuries. Arch Phys Med Rehabil. 1998;79:1496–1503. doi: 10.1016/s0003-9993(98)90409-9. [DOI] [PubMed] [Google Scholar]
- Scremin AM, Kurta L, Gentili A, et al. Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch Phys Med Rehabil. 1999;80:1531–1536. doi: 10.1016/s0003-9993(99)90326-x. [DOI] [PubMed] [Google Scholar]
- Hjeltnes N, Aksnes AK, Birkeland KI, et al. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol. 1997;273:R1072–R1079. doi: 10.1152/ajpregu.1997.273.3.R1072. [DOI] [PubMed] [Google Scholar]
- Chilibeck PD, Jeon J, Weiss C, Bell G, Burnham R. Histochemical changes in muscle of individuals with spinal cord injury following functional electrical stimulated exercise training. Spinal Cord. 1999;37:264–268. doi: 10.1038/sj.sc.3100785. [DOI] [PubMed] [Google Scholar]
- Mohr T, Andersen JL, Biering-Sorensen F, et al. Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord. 1997;35:1–16. doi: 10.1038/sj.sc.3100343. [DOI] [PubMed] [Google Scholar]
- Bhambhani Y, Tuchak C, Burnham R, Jeon J, Maikala R. Quadriceps muscle deoxygenation during functional electrical stimulation in adults with spinal cord injury. Spinal Cord. 2000;38:630–638. doi: 10.1038/sj.sc.3101079. [DOI] [PubMed] [Google Scholar]
- Raymond J, Davis GM, Climstein M, Sutton JR. Cardiorespiratory responses to arm cranking and electrical stimulation leg cycling in people with paraplegia. Med Sci Sports Exerc. 1999;31:822–828. doi: 10.1097/00005768-199906000-00010. [DOI] [PubMed] [Google Scholar]
- Hooker SP, Scremin AM, Mutton DL, Kunkel CF, Cagle G. Peak and submaximal physiologic responses following electrical stimulation leg cycle ergometer training. J Rehabil Res Dev. 1995;32:361–366. [PubMed] [Google Scholar]
- Hooker SP, Figoni SF, Rodgers MM, et al. Physiologic effects of electrical stimulation leg cycle exercise training in spinal cord injured persons. Arch Phys Med Rehabil. 1992;73:470–476. [PubMed] [Google Scholar]
- Faghri PD, Glaser RM, Figoni SF. Functional electrical stimulation leg cycle ergometer exercise: training effects on cardiorespiratory responses of spinal cord injured subjects at rest and during submaximal exercise. Arch Phys Med Rehabil. 1992;73:1085–1093. [PubMed] [Google Scholar]
- Belanger M, Stein RB, Wheeler GD, Gordon T, Leduc B. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals. Arch Phys Med Rehabil. 2000;81:1090–1098. doi: 10.1053/apmr.2000.7170. [DOI] [PubMed] [Google Scholar]
- Chen SC, Lai CH, Chan WP, et al. Increases in bone mineral density after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil Rehabil. 2005;27:1337–1341. doi: 10.1080/09638280500164032. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Becker D, Sadowsky CL, Jane JA, Sr, Conturo TE, Schultz LM. Late recovery following spinal cord injury: case report and review of the literature. J Neurosurg. 2002;97:252–265. doi: 10.3171/spi.2002.97.2.0252. [DOI] [PubMed] [Google Scholar]
- Sloan KE, Bremner LA, Byrne J, Day RE, Scull ER. Musculoskeletal effects of an electrical stimulation induced cycling programme in the spinal injured. Paraplegia. 1994;32:407–415. doi: 10.1038/sc.1994.67. [DOI] [PubMed] [Google Scholar]
- BeDell KK, Scremin AM, Perell KL, Kunkel CF. Effects of functional electrical stimulation–induced lower extremity cycling on bone density of spinal cord–injured patients. Am J Phys Med Rehabil. 1996;75:29–34. doi: 10.1097/00002060-199601000-00008. [DOI] [PubMed] [Google Scholar]
- Skold C, Lonn L, Harms-Ringdahl K, et al. Effects of functional electrical stimulation training for six months on body composition and spasticity in motor complete tetraplegic spinal cord–injured individuals. J Rehabil Med. 2002;34:25–32. doi: 10.1080/165019702317242677. [DOI] [PubMed] [Google Scholar]
- Jacobs PL, Nash MS. Modes, benefits and risks of voluntary and electrically induced exercise in persons with spinal cord injury. J Spinal Cord Med. 2001;24:10–18. doi: 10.1080/10790268.2001.11753549. [DOI] [PubMed] [Google Scholar]
- Kakebeeke TH, Lechner HE, Knapp PA. The effect of passive cycling movements on spasticity after spinal cord injury: preliminary results. Spinal Cord. 2005;43:483–488. doi: 10.1038/sj.sc.3101747. [DOI] [PubMed] [Google Scholar]
- Muraki S, Ehara Y, Yamasaki M. Cardiovascular responses at the onset of passive leg cycle exercise in paraplegics with spinal cord injury. Eur J Appl Physiol. 2000;81:271–274. doi: 10.1007/s004210050042. [DOI] [PubMed] [Google Scholar]
- Willoughby DS, Priest JW, Jennings RA. Myosin heavy chain isoform and ubiquitin protease mRNA expression after passive leg cycling in persons with spinal cord injury. Arch Phys Med Rehabil. 2000;81:157–163. doi: 10.1016/s0003-9993(00)90134-5. [DOI] [PubMed] [Google Scholar]
- Shields RK, Schlechte J, Dudley-Javoroski S, et al. Bone mineral density after spinal cord injury: a reliable method for knee measurement. Arch Phys Med Rehabil. 2005;86:1969–1973. doi: 10.1016/j.apmr.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotin HH, Sievanen H, Grashuis JL, Kuiper JW, Jarvinen TL. Inaccuracies inherent in patient-specific dual-energy X-ray absorptiometry bone mineral density measurements: comprehensive phantom-based evaluation. J Bone Miner Res. 2001;16:417–426. doi: 10.1359/jbmr.2001.16.2.417. [DOI] [PubMed] [Google Scholar]
- Stackhouse SK, Binder-Macleod SA, Stackhouse CA, McCarthy JJ, Prosser LA, Lee SC. Neuromuscular electrical stimulation versus volitional isometric strength training in children with spastic diplegic cerebral palsy: a preliminary study. Neurorehabil Neural Repair. 2007;21:475–485. doi: 10.1177/1545968306298932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MA, Walter GA, Gulish H, et al. Volumetric measurement of human calf muscle from magnetic resonance imaging. MAGMA. 1997;5:93–98. doi: 10.1007/BF02592238. [DOI] [PubMed] [Google Scholar]
- Biering-Sorensen F, Nielsen JB, Klinge K. Spasticity-assessment: a review. Spinal Cord. 2006;44:708–722. doi: 10.1038/sj.sc.3101928. [DOI] [PubMed] [Google Scholar]
- Cholesterol and atherosclerosis in children. American Heart Association. 2006. Available at: http://www.americanheart.org/presenter.jhtml?identifier=4499. Accessed October 31, 2007.
- Tolfrey K, Jones AM, Campbell IG. Lipid-lipoproteins in children: an exercise dose-response study. Med Sci Sports Exerc. 2004;36:418–427. doi: 10.1249/01.mss.0000117132.70711.2b. [DOI] [PubMed] [Google Scholar]
- Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone. 2007;40:14–27. doi: 10.1016/j.bone.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Mohr T, Podenphant J, Biering-Sorensen F, et al. Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int. 1997;61:22–25. doi: 10.1007/s002239900286. [DOI] [PubMed] [Google Scholar]
- Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2–20-year-old population. Bone. 1995;16:393S–399S. doi: 10.1016/8756-3282(95)00082-o. [DOI] [PubMed] [Google Scholar]
- Lazo MG, Shirazi P, Sam M, Giobbi-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39:208–214. doi: 10.1038/sj.sc.3101139. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am. 2000;11:109–140. [PubMed] [Google Scholar]
- Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 2006;29:489–500. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksnes AK, Hjeltnes N, Wahlstrom EO, et al. Intact glucose transport in morphologically altered denervated skeletal muscle from quadriplegic patients. Am J Physiol. 1996;271:E593–E600. doi: 10.1152/ajpendo.1996.271.3.E593. [DOI] [PubMed] [Google Scholar]
- Jacobs PL, Nash MS. Exercise recommendations for individuals with spinal cord injury. Sports Med. 2004;34:727–751. doi: 10.2165/00007256-200434110-00003. [DOI] [PubMed] [Google Scholar]
- Rowland TW, Boyajian A. Aerobic response to endurance exercise training in children. Pediatrics. 1995;96:654–658. [PubMed] [Google Scholar]
- Kelley GA, Kelley KS. Aerobic exercise and lipids and lipoproteins in children and adolescents: a meta-analysis of randomized controlled trials. Atherosclerosis. 2007;191:447–453. doi: 10.1016/j.atherosclerosis.2006.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics. Committee on Nutrition. Cholesterol in childhood. Pediatrics. 1998;101:141–147. [PubMed] [Google Scholar]