Abstract
Background/Objective:
The objective was to evaluate the effectiveness of neurotoxin treatments of urinary incontinence (UI) in individuals with spinal cord injury (SCI) or multiple sclerosis (MS).
Methods:
Studies were included if published in English, presented randomized adults with SCI or MS, and reported UI outcomes.
Results:
Ten trials randomizing 288 subjects with SCI (43%), MS (52%), or other spinal conditions (5%) and UI refractory to oral antimuscarinics were included. The overall mean age was 41 years, and 46% were women. Study durations ranged from 1 to 18 months. Treatments included botulinum toxin-A (BTX-A, 2 trials) and 2 vanilloid compounds, capsaicin (6 trials) and resiniferatoxin (4 trials). BTX-A was superior to placebo and resiniferatoxin in reducing daily UI episodes, mainly in individuals with SCI, although significant reductions vs placebo were not evident throughout the study duration. There were 1.1 fewer daily UI episodes in the BTX-A 200 unit group vs 0.1 fewer for the placebo group at the final week 24 assessment. Capsaicin was generally superior to placebo. The weighted difference between capsaicin and placebo in a pooled analysis of 2 trials enrolling subjects with either paraplegia or tetraplegia (n = 32) was −3.8 daily UI episodes [95% CI −4.7 to −2.9] after 30 days. Capsaicin was comparable to resiniferatoxin. Pelvic pain and facial flushing were associated with capsaicin.
Conclusion:
Neurotoxins may improve refractive UI in adults with SCI or MS, although trial results were inconsistent. Trials were small in size and relatively short in duration. Further studies are needed to determine the efficacy and tolerability of long-term application.
Keywords: Spinal cord injuries, Multiple sclerosis, Urinary incontinence, Neurogenic bladder, Detrusor overactivity, Botulinum toxin, Capsaicin, Resiniferatoxin, Systematic review
INTRODUCTION
Urinary incontinence (UI) is a common and troublesome problem in adults with spinal cord conditions. Patients with multiple sclerosis (MS) and spinal cord injuries (SCI) make up a substantial proportion of these adults. Neurogenic lower urinary tract dysfunction will affect nearly all individuals with SCI and from 50 to 90% of those with MS at some point during the course of the disease (1,2). UI associated with these neurological disorders is predominately caused by neurogenic detrusor overactivity (NDO), which causes involuntary bladder contractions during the filling phase, leading to urinary leakage. Resulting incontinence episodes can have a significant negative impact on quality of life (3). Clean intermittent self-catheterization is often used to empty the bladder in patients with NDO but has limitations due to pain, inconvenience, and risk of infectious/bleeding complications. Oral antimuscarinic agents have been administered for controlling UI episodes, which typically occur between catheterizations. However, long-term compliance may be difficult due to adverse effects and the need for frequent administration. Antimuscarinics agents are used primarily to improve overactive bladder symptoms through blockade of muscarinic receptors in the detrusor muscle, although the exact mechanism of action remains controversial (4).
Several different neurotoxins have been evaluated for treatment of patients with NDO, including botulinum toxin type A (BTX-A) and vanilloid compounds (capsaicin and resiniferatoxin) (5–7). BTX type A, when intramuscularly injected into the detrusor, may improve NDO by selectively blocking acetylcholine-mediating detrusor muscle contraction (5). The reversible reduction in neuronal activity and muscle weakness inhibits muscle contraction that leads to urge incontinence, lasting several months (5,6). If the appropriate dosage is administered, there may be a minimal risk of systemic adverse effects (6). BTX is commercially available and may be used to treat other urologic conditions, including detrusor-sphincter dyssynergia, pelvic floor spasticity, prostatic pain, and prostatitis (6,7).
To date, use of vanilloid compounds has been largely investigational. Intravesically instilled capsaicin initially excites and then desensitizes C fiber afferent bladder neurons that may be responsible for signals that trigger detrusor overactivity, leading to inhibition of reflex bladder emptying (8). These selective effects are reversible and may last several months (8). However, the use of capsaicin has been associated with acute pain and burning sensation during instillation (9). RTX is a sensory antagonist like capsaicin but approximately 1,000 times more potent. Although pharmacologically similar to capsaicin, RTX is reported to have fewer painful adverse effects (8,9). Because these agents have the potential to improve a common and disabling condition, we conducted a systematic review of evidence from randomized trials to evaluate the efficacy and safety of neurotoxins for UI in adults with SCI or MS.
METHODS
Literature Search
A MEDLINE search from 1966 through August 2007 combined an optimally sensitive Cochrane Collaboration search strategy with the following MeSH headings (and subheadings): “urinary incontinence,” “spinal cord injury,” “neurogenic bladder,” “multiple sclerosis,” “botulinum toxins,” “capsaicin,” “vanilloid agents,” and “resiniferatoxin” (10). In addition, the Cochrane Library, the Cochrane Urinary Incontinence Review Group specialized registry, and reference lists of identified trials and reviews were searched. Studies were restricted to the English language.
Because BTX is commercially available and increasingly used as a therapeutic option for NDO, we supplemented efficacy and adverse events data provided by the randomized studies. MEDLINE from 2000 through August 2007 was searched for nonrandomized BTX studies or reviews, combining search terms “botulinum toxins” and “neurogenic detrusor overactivity.” Vanilloid compound therapy has been mainly investigational, and study inclusions were limited to randomized control trials.
Inclusion Criteria
Studies were included if they enrolled adults with UI associated with NDO; reported clinical outcomes (eg, number returning to continence based on questionnaire or voiding diary, leakage episodes, pad tests); randomly assigned participants to treatment or control (placebo, active control, or usual care/no treatment); and were published in English. Nonrandomized studies of BTX were selected and extracted based on the same criteria for randomized trials, except they did not require random assignment or a control group.
Data Extraction and Study Appraisal
Study and demographic characteristics, enrollment criteria, efficacy outcomes, quality of life data, adverse effects, and number and reasons for dropout were extracted. Methodological study quality, the quality of concealment of treatment allocation for randomization, was determined based on the scale developed by Schultz (1 = poorest quality, 2 = unclear, 3 = best quality) (11). We assessed whether subjects, investigators, or outcomes assessors were blinded to the treatment, if intention-to-treat analysis was used, and the percent lost to follow up or withdrawn from study protocol.
Statistical Methods
If applicable, clinical outcomes and adverse event data were pooled and analyzed in Cochrane Collaboration Review Manager (RevMan 4.2) software (12). Weighted mean differences, the difference between treatment and control pooled means at endpoint, along with 95% CI, were calculated for continuous variables. Relative risks and their 95% CI were calculated for categorical outcomes. A fixed-effects model was used if there was no evidence of heterogeneity between the studies, based on the chi-square test for heterogeneity and the I2 test (13,14).
RESULTS
Description of Studies
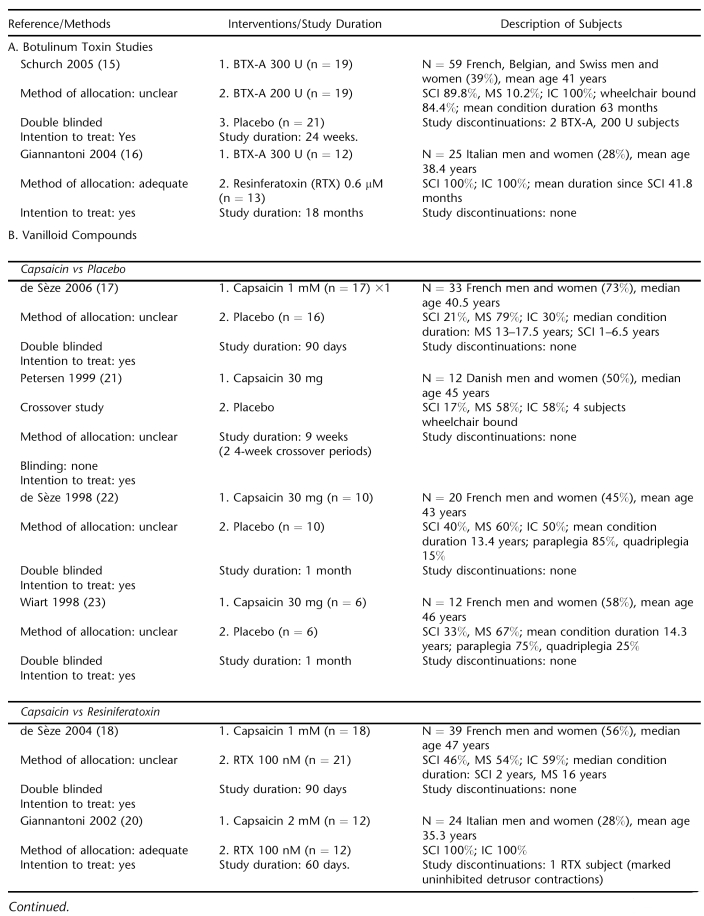
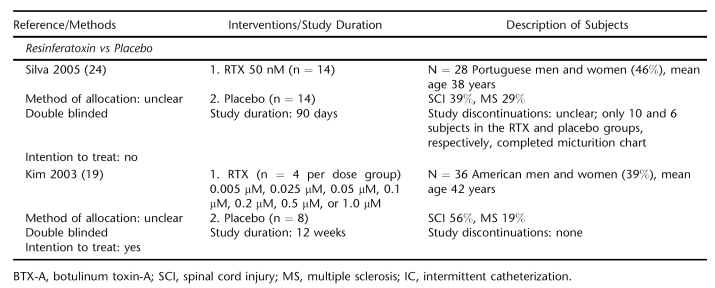
Ten trials were identified, 8 with mixed SCI and MS populations (15,17–19,21–24) and 2 investigating UI treatments with exclusively SCI subjects (15,19) (Table 1). Treatments evaluated included BTX (2 trials) (15,16), capsaicin (6 trials) (17,18,20–23), and RTX (3 trials) (16,19,24). Seven studies were placebo controlled (15,17,19,21–24) and double blinded (15,17–19,22–24). One trial was a crossover study (22). The quality of concealment of treatment allocation for randomization was adequate in 2 trials (16, 20). Intention-to-treat analysis was reported for 8 trials (15–20,22,23). Study durations ranged from 4 weeks to up to 18 months, with 6 studies of at least 90 days' duration.
Table 1.
Description of Studies
Table 1.
Continued
Demographic and Baseline Characteristics
A total of 288 subjects with NDO refractory to oral antimuscarinic therapy were randomized (Table 1). Mean age was 41 years (6 trials reporting), and 46% were women. There were 3 patients who discontinued study participation (1%). Only 1 trial reported racial data, and nearly all participants were white (15). The majority of subjects enrolled were adults with MS (52%) followed by SCI (43%), with the remainder having other spinal conditions. Men were nearly three-quarters of participants (71%) in the SCI trials. Mean duration of disease was 4.6 years for population studies of SCI or predominately SCI populations (15,16,20). Mean duration of condition of mixed population studies was 13.7 years (22,23). Median duration of condition ranged from 13 to 17.5 years and 1 to 6.5 years for MS and SCI subjects in 2 trials, respectively (17,18). Mean daily UI episodes ranged from approximately 2 to 5.5.
Botulinum Toxin Studies
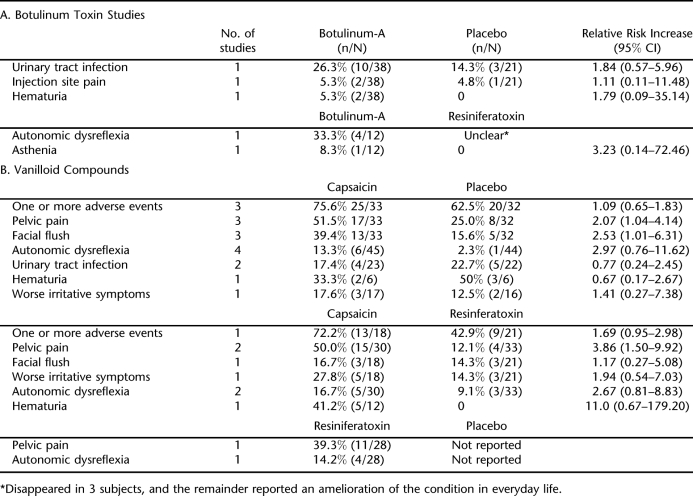
BTX-A, in 200 or 300 units (U) administered intramuscularly into the detrusor muscle at baseline, was compared with placebo in 59 mostly SCI subjects over 24 weeks (15). BTX-A decreased daily UI episodes compared with placebo, but the reductions were only statistically significant at the week 2 and 6 for BTX-A 300 U and only the final assessment at 24 weeks for BTX-A 200 (−1.1 episodes compared with −0.1 for the placebo group). There were 24 subjects in the combined BTX groups reporting no incontinence episodes for at least 1, 1-week post-treatment period compared with 5 subjects in the placebo group (Table 2). Adverse effects occurring in more than 1 subject are shown in Table 3. There were 10 incidences of urinary tract infection in the BTX treatment group compared with 3 in the placebo group, despite all subjects' receiving prophylactic antibiotics. Among BTX subjects, there were 2 episodes each of pain at the injection site and hematuria. No systemic effects or cases autonomic dysreflexia were observed, and all serum samples were negative for antibodies to BTX at baseline and at week 24.
Table 2.
Effect of Treatment on the Number of Daily Urinary Incontinence Episodes
Table 3.
Reported Adverse Effects
Table 2.
Continued
Effectiveness of BTX-A 300 U was compared with intravesically instilled RTX 0.6 μM in a trial enrolling 25 SCI patients (16). Mean follow up was 14.2 ± 3.9 months for the BTX-A group and 14.8 ± 3.9 months for the RTX group. The mean numbers of treatments per patient were 2.1 ± 0.7 and 8.6 ± 1.9 for the BTX and RTX groups, respectively. Mean time between 2 consecutive injections was 6.8 ±1.5 months for the BTX group compared with 51.6 ± 8.2 days for the RTX group. Treatment with BTX-A significantly reduced daily UI episodes compared with RTX but only at the 12- and 18-month points. Nine subjects in the BTX group had achieved and maintained continence at 12 months, and 6 of the 9 maintained continence at 18 months. Five subjects treated with RTX achieved and maintained continence during the follow up. No local or systemic adverse effects were reported for RTX. Episodes of autonomic dysreflexia were brief and disappeared during urodynamic testing in 4 BTX- and 3 RTX-treated subjects. One BTX-treated subject reported mild asthenia shortly after the first treatment, lasting 10 days.
Vanilloid Compounds
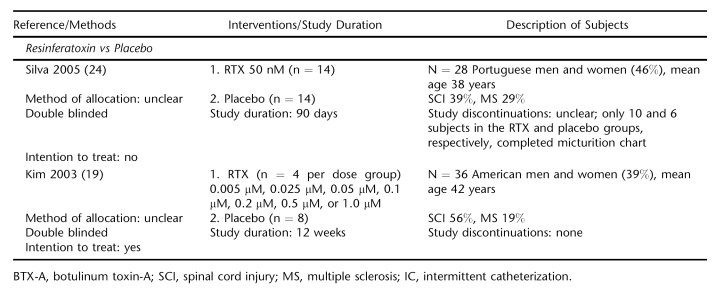
Capsaicin vs Placebo.
Four placebo-controlled trials randomizing predominately MS subjects (n = 77) evaluated intravesically instilled capsaicin in 30-mg or 1-mM doses (17,21–23) (Table 2). Capsaicin significantly reduced daily UI episodes within 30 days after initiation of treatment compared with placebo in 3 studies (17,22,23). A pooled analysis of 2 trials (n = 32) enrolling patients with paraplegia or quadriplegia estimated that the weighted mean difference in the mean of number of leakages/24 hours between capsaicin and placebo was −3.8 episodes [95% CI −4.7 to −2.9] at the 30-day point (22,23). Subjective satisfaction was reported for all 16 subjects in the capsaicin groups compared with 1 subject each in the placebo groups (22,23). Mean number of pads used per day at the end of study in 1 trial was 4 for the capsaicin group compared with 10 for the placebo group (P = 0.02) (22).
Subjects treated with capsaicin reported significantly greater incidences of pelvic pain/ burning and flushing (17,22,23). More than 50% of the subjects reported pelvic pain compared with 25% of placebo-treated subjects (relative risk 2.07 [95% CI 1.04 to 4.14], 3 trials reporting). The ethanol solvent has been presumed as a major factor for the irritability of capsaicin (22). However, the trial evaluating the tolerability of an alternative formulation of capsaicin in glucidic acid still observed a significantly greater incidence of pelvic pain compared with placebo, 59% vs 12.5%, P = 0.01 (17). Other adverse effects occurring at the time of, or shortly after, instillation were generally comparable in the 2 groups. Incidences of autonomic dysreflexia were more frequent in subjects receiving capsaicin, with none leading to study withdrawal (17,21–23).
Capsaicin vs Resiniferatoxin.
Two trials totaling 63 subjects compared capsaicin with intravesically instilled RTX (18,20). One trial of mixed MS and SCI patients found no difference between treatments, although RTX appeared more effective in the long term (18). Both agents decreased daily UI episodes within 30 days after instillation. By day 90, the median number of episodes for the RTX group was 1 (range 0–7) compared with 4 (0–22) for the capsaicin group. In a trial of 24 patients with SCI, RTX significantly reduced daily urinary leakages compared with pretreatment, but capsaicin did not (20). Five of 10 subjects in the RTX group and 9 of 10 in the capsaicin group were using pads at days 30 and 60 after instillation.
Comparable to the placebo trials, incidence of pelvic pain was significantly greater in the capsaicin group, 50% vs 12% for the RTX group (relative risk 3.86 [95% CI 1.50 to 9.92], 2 trials reporting) (Table 3). No local or systemic adverse effects were reported for the RTX group vs capsaicin. One RTX subject developed marked uninhibited detrusor contractions during instillation and was withdrawn (20).
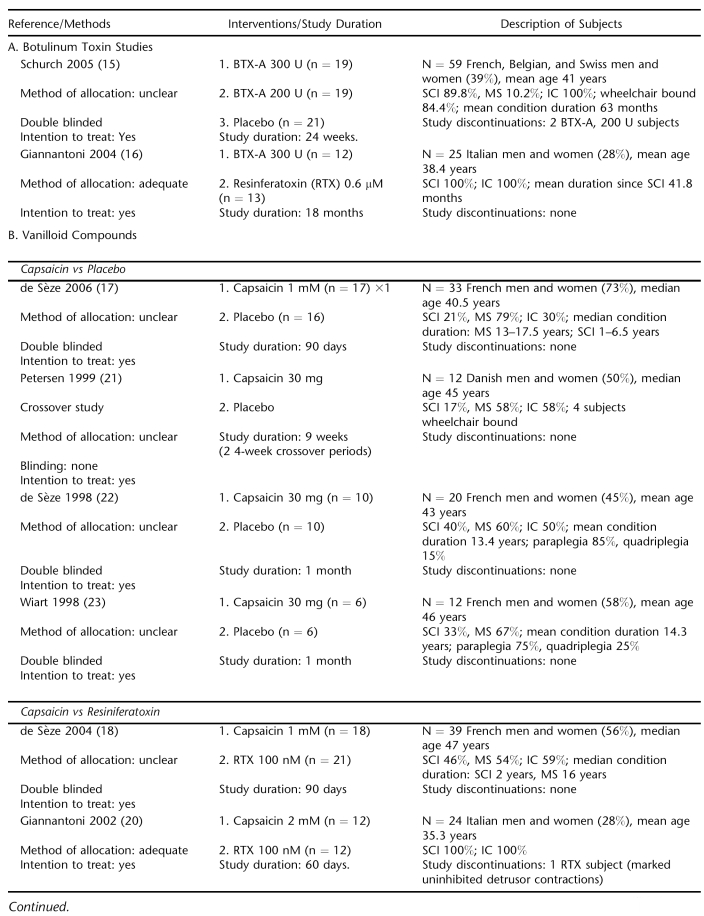
Resiniferatoxin vs Placebo.
Two trials totaling 63 subjects compared intravesically instilled RTX with placebo (19,24). Mean UI decreased significantly after RTX treatment but not placebo in a trial of 28 mixed MS and SCI subjects (24). Due to the trial's small sample size, the differences between groups were not significant. No adverse events were reported for either group.
A small dose escalation trial found none of the 7 tested doses of RTX statistically improved the number of mean daily UI episodes compared with placebo over the 12 weeks (19). There were only 4 subjects per dose group. The most common adverse effect in RTX-treated subjects were pelvic pain, which occurred in 39%, followed by autonomic dysreflexia (14%) (Table 3). No complications were reported for placebo.
Botulinum Toxin Studies, Evidence from Nonrandomized Studies
Due to the paucity of randomized trials, additional nonrandomized reports were identified nonsystematically for further assessment of efficacy and adverse effects. The quality of evidence from these studies is considered inferior to the randomized trials. In general, these studies had small sample sizes, did not have good statistical power, and were susceptible to recruitment bias (25). An early study by Schurch assessed BTX 200 to 400 units in 19 SCI subjects over 36 weeks (5). At the 6-week assessment period, complete restoration of continence was reported for 17 of the subjects. A recent review by Patel et al evaluated data on more than 600 adult subjects (n = 32 studies, ranging from 1 to 200 subjects) with NDO (25). Significant reduction in incontinent episodes was seen in several case series, even in studies with relatively small numbers. Duration of treatment benefit ranged from 3 to 14 months. Two recent studies assessed BTX therapy for refractory NDO in SCI subjects (26,27). In the study by Patki, continence was restored in 18 of the 26 subjects who were incontinent prior to BTX-A injection over a mean follow up of 7 months (26). The small study (n = 15) conducted in Singapore reported a significant reduction in mean number of leakages per day from baseline at 26 weeks post injection, 3.75 to 1.50 (P = 0.01) (27). By 39 weeks, the mean number of daily leakages was no longer significant (1.75). Effects of repeated injections were investigated in 66 subjects with severe NDO in order to evaluate possible drug resistance (28). Increased resistance was not observed, and the intervals between subsequent injections, on average 9 to 11 months, were not statistically significant. Significant improvements were noted in clinical parameters, but there were 12 treatment failures indicated by lack of effect.
Reported adverse effects of BTX include general weakness, dysphagia, diplopia, and blurred vision (6). Severe generalized muscle weakness was reported for 2 subjects with NDO in 1 study (29). Four subjects (6%) in the repeated injection study reported transient muscular weakness in the trunk and/or extremities (28). The review by Patel noted that adverse effects were mild and short lived in case series assessed (26). No adverse effects or injection-related complications were reported in a retrospective analysis European study of 231 subjects (30).
DISCUSSION
This is the first systematic review evaluating evidence from randomized trials of neurotoxin treatments for UI for subjects with MS or SCI. To date, there is not sufficient evidence to support the effectiveness of BTX and vanilloid compounds. Published results are limited by relatively few studies, small sample size, short study duration, inconsistent dosing, and variation in outcomes assessed and consistency in findings. Results suggest BTX may be an effective treatment option, but significant reductions were only evident at selected follow-up intervals in the placebo-controlled study. Nonrandomized studies lend support to the use of BTX, but the quality of evidence is limited. Vanilloid compound trials did not demonstrate consistent therapeutic effects. Capsaicin was generally superior to placebo in the short term, particularly among subjects with paraplegia or quadriplegia. However, this assessment was based on 2 small trials totaling 32 subjects (22,23). Treatment with capsaicin is also limited due to distressing adverse effects, such as pelvic pain and burning, even when diluted with a nonethanol solvent (17). There were no significant differences in effectiveness between capsaicin and RTX.
Potential benefits of BTX therapy include requiring fewer treatments on average with longer intervals between treatments compared with RTX therapy. BTX does not have the adverse effects of oral antimuscarinics, nor does it requires daily administration. However, long-term effectiveness remains unclear because the trials were limited by the short-term study durations, with only 1 trial duration up to 18 months. Drug tolerance, although not reported in any of the studies assessed, could possibly increase with repeated treatment (6). However, 1 nonrandomized trial of 66 subjects with severe NDO found no indication of increased drug resistance after repeated injections (28). In contrast to the vanilloid compounds, administration of BTX requires either sedation or anesthesia for the patient and a skilled endoscopist to ensure the injections are properly placed (14). This likely limits its widespread application. It is also unknown if the evaluated therapies can reduce intermittent self-catheterization in subjects requiring catheterization. The mutagenic and carcinogenic effects of vanilloid compounds, particularly RTX, on the bladder are indeterminate to date. Future trials should be initiated to assess long-term efficacy, tolerability, duration of effectiveness, optimal dosage, and/or whether tolerance develops.
The quality of the included studies was limited by several factors. The studies were small, with no trial enrolling more than 59 subjects. Trials were also short term in duration, generally 3 months or less with only 2 that were 24 weeks or longer (15,16). Treatment allocation for randomization was adequate for only 2 studies (16,20). Most of the capsaicin trials using an alcohol solvent reported double blinding, but the burning sensation precluded blinding to treatment in at least 1 trial (19). Many trials were not adequately powered due to the small sample sizes to detect clinically important differences in the outcomes assessed. In addition, data were reported in an inconsistent fashion that did not allow overall pooling, and outcomes assessed varied across trials and information from validated UI questionnaires was rarely provided.
CONCLUSION
Randomized trials of neurotoxins for treating subjects with SCI or MS UI refractive to oral medications are limited in number, small in size, and of relatively short duration. The ideal patient, dose, and interval for these therapies remain unclear. Limited evidence suggests that BTX-A may be an effective treatment option, although the effects of long-term application remain unclear. The results of the investigational vanilloid compound trials were inconsistent, but capsaicin was generally superior to placebo, especially among individuals with paraplegia or quadriplegia. Acute painful adverse effects associated with capsaicin limit its applicability. Because UI associated with SCI or MS is a common, problematic, and difficult-to-treat condition, further studies are needed to evaluate BTX and vanilloid compounds. Future studies should be randomized trials of longer duration with a larger number of subjects to provide sufficient power to evaluate effectiveness and harms.
Acknowledgments
The authors acknowledge Indulis Rutks for performing literature searches and retrieving articles.
Footnotes
The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs.
This project was supported by NIDDK R01 DK063300-01A2, the Department of Veterans Affairs Health Services Research and Development Service, and the Minneapolis VA Center for Chronic Disease Outcomes Research.
REFERENCES
- Burns AS, Rivas DA, Ditunno JF. The management of neurogenic bladder and sexual dysfunction after spinal cord injury. Spine. 2001;16:S129–S136. doi: 10.1097/00007632-200112151-00022. [DOI] [PubMed] [Google Scholar]
- Fingerman J, Finkelstein D. The overactive bladder in multiple sclerosis. J Am Osteopath Assoc. 2000;100:59–63. [PubMed] [Google Scholar]
- Jackson S. The patient with an overactive bladder: symptoms and quality-of-life issues. Urology. 1997;50:18–22. doi: 10.1016/s0090-4295(97)00580-3. [DOI] [PubMed] [Google Scholar]
- Chapple C, Khullar V, Gabriel Z, et al. The effects of antimuscarinic treatments in overactive bladder: a systematic review and meta-analysis. Eur Urol. 2005;48:5–26. doi: 10.1016/j.eururo.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Schurch B, Stohrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000;164:692–697. doi: 10.1097/00005392-200009010-00018. [DOI] [PubMed] [Google Scholar]
- Leippold T, Reitz A, Schurch B. Botulinum toxin as a new therapy option for voiding disorders: current state of the art. Eur Urol. 2003;44:165–174. doi: 10.1016/s0302-2838(03)00250-1. [DOI] [PubMed] [Google Scholar]
- Sahai A, Khan M, Fowler CJ, Dasgupta P. Botulinum toxin for the treatment of lower urinary tract symptoms: a review. Neurourol Urodyn. 2005;24:2–12. doi: 10.1002/nau.20090. [DOI] [PubMed] [Google Scholar]
- Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J Urol. 1999;162:3–11. doi: 10.1097/00005392-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Lazzeri M, Spinelli M, Beneforti P, Zanollo A, Turini D. Intravesical resiniferatoxin for the treatment of detrusor hyperreflexia refractory to capsaicin in patients with chronic spinal cord diseases. Scand J Urol Nephrol. 1998;32:331–334. doi: 10.1080/003655998750015287. [DOI] [PubMed] [Google Scholar]
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ. 1994(309):1286–1291. [DOI] [PMC free article] [PubMed]
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- Review Manager [computer program]: Version 4.1 for Windows. Oxford, England: The Cochrane Collaboration; 2001. [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurch B, de Seze M, Denys P, et al. Botulinum toxin type-a is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200. doi: 10.1097/01.ju.0000162035.73977.1c. [DOI] [PubMed] [Google Scholar]
- Giannantoni A, Di Stasi SM, Stephen RL, Bini V, Costantini E, Porena M. Intravesical resiniferatoxin versus botulinum-A toxin injections for neurogenic detrusor overactivity: a prospective randomized study. J Urol. 2004;172:240–243. doi: 10.1097/01.ju.0000132152.53532.5d. [DOI] [PubMed] [Google Scholar]
- de Seze M, Gallien P, Denys P, et al. Intravesical glucidic capsaicin versus glucidic solvent in neurogenic detrusor overactivity: a double blind controlled randomized study. Neurourol Urodyn. 2006;25:752–757. doi: 10.1002/nau.20296. [DOI] [PubMed] [Google Scholar]
- de Seze M, Wiart L, de Seze MP, et al. Intravesical capsaicin versus resiniferatoxin for the treatment of detrusor hyperreflexia in spinal cord injured patients: a double-blind, randomized, controlled study. J Urol. 2004;171:251–255. doi: 10.1097/01.ju.0000100385.93801.d4. [DOI] [PubMed] [Google Scholar]
- Kim JH, Rivas DA, Shenot PJ, et al. Intravesical resiniferatoxin for refractory detrusor hyperreflexia: a multicenter, blinded, randomized, placebo-controlled trial. J Spinal Cord Med. 2003;26:358–363. doi: 10.1080/10790268.2003.11753706. [DOI] [PubMed] [Google Scholar]
- Giannantoni A, Di Stasi SM, Stephen RL, et al. Intravesical capsaicin versus resiniferatoxin in patients with detrusor hyperreflexia: a prospective randomized study. J Urol. 2002;167:1710–1714. [PubMed] [Google Scholar]
- Petersen T, Nielsen JB, Schroder HD. Intravesical capsaicin in patients with detrusor hyper-reflexia: a placebo-controlled cross-over study. Scand J Urol Nephrol. 1999;3:104–110. doi: 10.1080/003655999750016078. [DOI] [PubMed] [Google Scholar]
- de Seze M, Wiart L, Joseph PA, Dosque JP, Mazaux JM, Barat M. Capsaicin and neurogenic detrusor hyperreflexia: a double-blind placebo-controlled study in 20 patients with spinal cord lesions. Neurourol Urodyn. 1998;17:513–523. doi: 10.1002/(sici)1520-6777(1998)17:5<513::aid-nau7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Wiart L, Joseph PA, Petit H, et al. The effects of capsaicin on the neurogenic hyperreflexic detrusor: a double blind placebo controlled study in patients with spinal cord disease. Preliminary results. Spinal Cord. 1998;36:95–99. doi: 10.1038/sj.sc.3100505. [DOI] [PubMed] [Google Scholar]
- Silva C, Silva J, Ribeiro M-J, Avelino A, Cruz F. Urodynamic effect of intravesical resiniferatoxin in patients with neurogenic detrusor overactivity of spinal origin: results of a double-blind randomized placebo-controlled trial. Eur Urol. 2005;48:650–655. doi: 10.1016/j.eururo.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Patel AK, Patterson JM, Chapple CR. Botulinum toxin injections for neurogenic and idiopathic detrusor overactivity: a critical analysis of results. Eur Urol. 2006;50:684–709. doi: 10.1016/j.eururo.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Patki PS, Hamid R, Arumugam K, Shah PJ, Craggs M. Botulinum toxin-type A in the treatment of drug-resistant neurogenic detrusor overactivity secondary to traumatic spinal cord injury. BJU Int. 2006;98:77–82. doi: 10.1111/j.1464-410X.2006.06192.x. [DOI] [PubMed] [Google Scholar]
- Tow AM, Toh KL, Chan SP, Consigliere D. Botulinum toxin type A for refractory neurogenic detrusor overactivity in spinal cord injured patients in Singapore. Ann Acad Med Singapore. 2007;36:11–17. [PubMed] [Google Scholar]
- Grosse J, Kramer G, Stohrer M. Success of repeat detrusor injections of botulinum-a toxin in patients with severe neurogenic detrusor overactivity and incontinence. Eur Urol. 2005;47:653–659. doi: 10.1016/j.eururo.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Wyndaele JJ, Van Dromme SA. Muscular weakness as side effect of botulinum toxin injection for neurogenic detrusor overactivity. Spinal Cord. 2002 ;40:599–600. [DOI] [PubMed]
- Reitz A, Stohrer M, Kramer G, et al. European experience of 200 cases treated with botulinum-A toxin injections into the detrusor muscle for urinary incontinence due to neurogenic detrusor overactivity. Eur Urol. 2004;45:510–515. doi: 10.1016/j.eururo.2003.12.004. [DOI] [PubMed] [Google Scholar]