Abstract
The R- and K-gingipain proteases of Porphyromonas gingivalis are involved in proteolysis of haemoglobin from which the defensive dimeric haem pigment is formed. Whilst oxyhaemoglobin is refractory towards K-gingipain, methaemoglobin is rapidly degraded. Ligation of methaemoglobin with N3−, which effectively blocks haem dissociation from the protein, prevented haemoglobin breakdown. Haem-free globin was rapidly degraded by K-gingipain. These data emphasise the need for haemoglobin oxidation which encourages haem dissociation and makes the haem-free globin susceptible to proteolytic attack.
Keywords: gingipains, haem, haemoglobin, periodontal disease, Porphyromonas, protease
Porphyromonas gingivalis is considered to be one of the major causal organisms associated with adult periodontitis (Lamont and Jenkinson, 1998). It elaborates a number of pathogenic factors including the arginine-(R)- and lysine-(K)-specific gingipain proteases, which are thought to contribute to periodontal disease progression. Gingi-pains not only directly degrade components of connective tissue but are also able to significantly interfere with host defense mechanisms and with regulated inflammatory and reparative processes in periodontal tissues (Potempa et al., 2000).
It has been recently shown (Smalley et al., 2004, 2007) that the HRgpA and Kgp act in concert to degrade oxyhaemoglobin for the release of iron protoporphyrin IX and production of the µ-oxo dimeric haem-containing black pigment. Whilst R-specific gingipains show little degradative activity towards haemoglobin, they can attack the oxyhaemoglobin molecule forming methaemoglobin (metHb; HbFeq+OH), the oxidised form of the haem-protein (Smalley et al., 2007). Although the individual α- and β-globin subunits contain numerous lysine residues, oxyhaemoglobin remains refractory to Kgp alone, resulting in the formation of a haemoglobin haemachrome which is relatively stable towards further Kgp attack. We have also demonstrated that metHb produced from oxyhaemoglobin by treatment with nitrite (Smalley et al., 2007) or with potassium ferricyanide (J.W. Smalley, unpublished data), is also readily attacked by Kgp. Thus, the effect of HRgpA is to ‘prime’ haemoglobin molecules, oxidising them to the metHb form to facilitate complete degradation by Kgp. This also has the effect of rendering haemoglobin iron protoporphyrin IX molecules into the Fe(III) state [Fe(III)PPIX], so that upon proteolytic release from the haem protein, it can react to give the µ-oxo dimer, [Fe(III)PPIX]2O, according to the following equation:
| (1) |
Although oxyHb and metHb differ imperceptibly from a structural point of view, these molecules differ in other physicochemical parameters. These include the inability of the metHb form to carry O2, and stability of the haemglobin linkage. It has been known for many years that whilst the haemoglobin holoprotein is relatively stable, loss of the haem moiety to yield apohaemoglobin is accompanied by increased protease susceptibility (Antonini and Brunori, 1971a). This would offer an explanation for the ability of Kgp to more easily degrade metHb whether it is generated by pre-treatment of oxyHb with HRgpA or by nitrite (Smalley et al., 2007). Accordingly, in this study we have examined further the effect of Kgp on apohaemoglobin and with azide liganded methaemoglobin (azidometHb), in which the haem-globin linkage is stabilised. We show here that whilst the azide liganded metHb species is less susceptible to attack, the apoprotein is rendered completely susceptible to breakdown by Kgp.
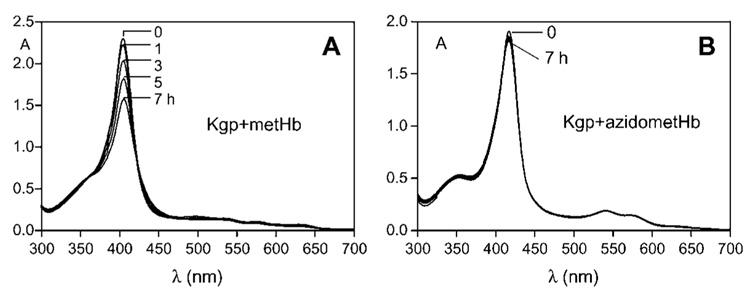
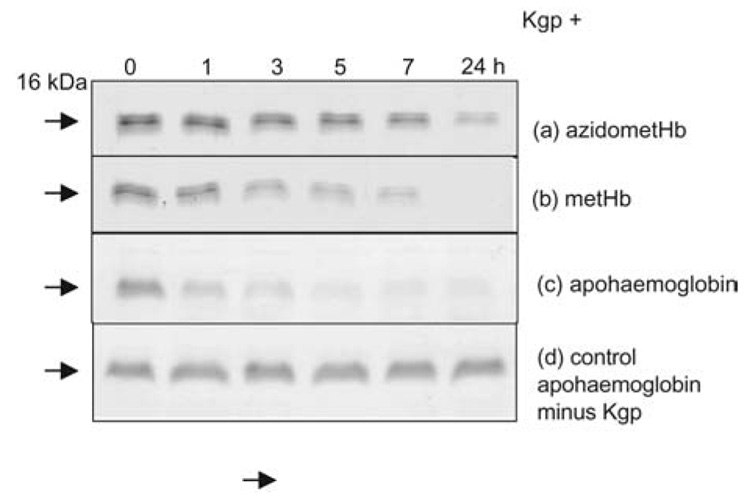
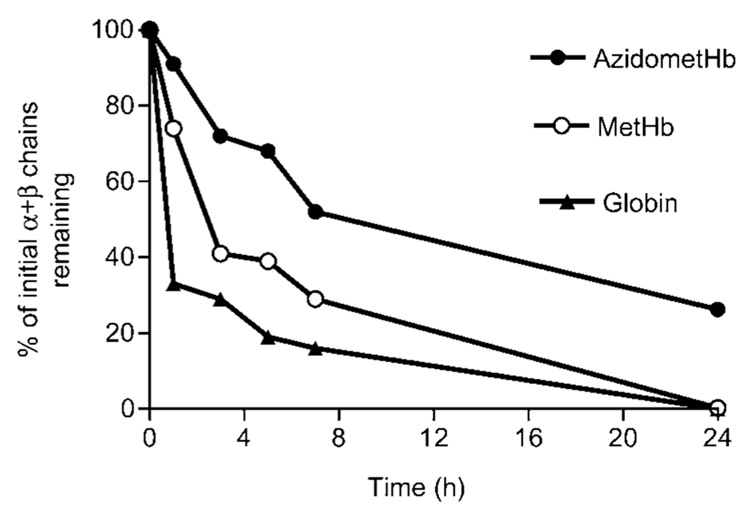
Incubation of metHb with Kgp resulted in the gradual decrease in the Soret band intensity (Figure 1A) indicating disruption of the haemprotein and loss of haem from the molecule. Over this time period there was little change to the intensity of the control metHb Soret band (data not shown). Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) of the Kgp-metHb incubation mixture showed that the loss of Soret absorbance was matched by a progressive digestion of the α- and β-globin chains (Figure 2A). In contrast, in the presence of Kgp, the degree of Soret band loss of the azidometHb was minimal over the first 7 h incubation (Figure 1B), compared to the control. Densitometry confirmed that there was minimal breakdown of the α- and β-chains of azidometHb compared to metHb (Figure 3). However, when apohaemoglobin was incubated with Kgp there was a much more rapid breakdown of the α-and β-globin chains (Figure 3), with approximately 70% of the total protein degraded over the first hour compared to approximately 25% for methaemoglobin. Under these enzyme assay conditions, Kgp showed a loss of only 1% and 8% of initial activity after 7 and 24 h, respectively, as assessed using the synthetic substrate substrate Nα- acetyl-lysine-p-nitroanilide (data not presented). In this context, the longevity of Kgp is biologically relevant for P. gingivalis since its haem pigmentation develops over several days and Hb is continuously supplied by lysing erythrocytes at the infected periodontitis sites.
Figure 1.
UV-visible absorption spectra of horse methaemoglobin (A) and azidomethaemoglobin (B) recorded during incubation with Kgp at 37°C.
Methaemoglobin was prepared by incubation of oxyhaemoglobin with NaNO2 as previously described (Smalley et al., 2007). Azidomethaemoglobin was prepared as described by Antonini and Brunori (1971b). Kgp was isolated and purified from the culture medium of P. gingivalis strain HG66 (Pike et al., 1994). Haemoglobin and enzyme concentrations were 4 and 0.4 µM, respectively. The buffer was 0.14 M NaCl, 0.1 M Tris-HCl, pH 7.5. Spectra were recorded in a 1-cm path length cuvette.
Figure 2.
SDS-PAGE of azidomethaemoglobin (A), methaemoglobin (B) and apohaemoglobin (C) during incubation with Kgp. (D) Control apohaemoglobin minus Kgp. Incubation conditions as in Figure 1. Haem-free apohaemoglobin was prepared by the acid-acetone method of Ascoli et al. (1981). Enzyme and substrate concentrations were 0.4 and 4 µM, respectively. SDS-PAGE was carried out on 15% acrylamide gels after sample solubilisation at 100°C for 5 min under reducing conditions as previously described (Smalley et al., 2007). And, 5 µg of protein was loaded per track.
Figure 3.
Loss of intact globin chains of azidomethaemoglobin, methaemoglobin and haem-free globin chains after incubation with Kgp.
The α- and β-chains of each haemoglobin species, separated by SDS-PAGE as in Figure 2, were scanned densitometrically after staining with Coomassie blue and integrated using UVi- Band software ••• Name of manufacturer, city, country. ••• Incubation conditions and protein substrate and enzyme concentrations were the same as described for Figure 2.
Thus, these present results confirm previous observations (Smalley et al., 2007) that K-gingipain is able to rapidly degrade methaemoglobin compared to oxyhaemoglobin. Previous studies have also shown that the oxidised form of haemoglobin is slightly more susceptible to attack by trypsin (Kimura et al., 1978). It has also been demonstrated that apohaemoglobin is more easily degraded by trypsin, chymotrypsin and papain (Murakami and Murachi, 1978). Structural and conformational differences exist between the oxygenated and deoxygenated haemoglobin species which account for the physiological role of this molecule in oxygen carriage (Perutz, 1970). However, oxyhaemoglobin and methaemoglobin differ imperceptibly in their structures to account for the enhanced susceptibility of the latter towards Kgp, as demonstrated here. It is known that general denaturation, including α-helix unfolding, leads to enhanced protease susceptibility of haemoglobin (Kimura et al., 1975, 1976). In addition, the presence of haem effectively stabilises the globin structure in both haemoglobin and myoglobin (Crumton and Polson, 1965; Kawahara et al., 1965), and loss of haem is followed by loss of helical structure and denaturation. This stability is determined by the affinity of haem for the globin protein (Hargrove and Olson, 1996). Both haemoglobin and myoglobin display extremely high affinities for Fe(II) haem, with dissociation constants in the range of 10−12 to 10−15 m (Hargrove et al., 1996). However, when the haem iron is oxidised to the Fe(III) state, the haem-globin affinity is reduced such that it may be transferred to, and bound by, serum albumin which has an association constant for iron(III) protoporphyrin IX in the region of 8×107 to 2×108 m−1 (Adams and Berman, 1980; Gatoni et al., 1996). Thus, conversion of oxyhaemoglobin into the oxidised species either by nitrite or by pre-treatment with R-gingipain (Smalley et al., 2007) is accompanied by a greater degree of protein breakdown. Blocking the dissociation of ferric haems from methaemoglobin by adding low spin ligands, such as CN− and N3− (Bunn and Jandl, 1968), would thus lead to the predicted reduction in protease susceptibility. This was found to be the case when azidomethaemoglobin was incubated with Kgp. It was shown that the time-dependent loss of Soret band intensity of the azidomethaemoglobin was reflected in the decrease in α- and β-chain breakdown, as shown by SDS-PAGE. This resistance to proteolysis was not as a result of enzyme inhibition since Kgp is not inhibited by NaN3 (J. Potempa, unpublished data), even at 0.4 mm, the concentration of sodium azide used to prepare azidomethaemoglobin. Conversely, when apohaemoglobin was incubated with Kgp, rapid proteolysis of the globin chains was observed.
What is the significance of the current findings in relationship to haem acquisition by P. gingivalis? In moderately deep periodontal pocket sites, mean oxygenation tensions (pO2) of around 30 mm Hg have been observed (Hanioka et al., 2000). In addition, periodontal tissue O2 measurements at such sites reveals haemoglobin oxygen saturation levels in the range of 60–70%, but which can be as high as 80% (Hanioka et al., 2000), indicating that these tissues are perfused with oxygenated blood. Thus, following bleeding, e.g., as a result of micro-ulceration, and haemolysis, periodontal pockets and sub-gingival plaque harbouring P. gingivalis may be exposed to oxygenated haemoglobin. Given the refractory nature of oxyhaemoglobin to Kgp proteolysis (Smalley et al., 2007), these present findings emphasise the need for a prerequisite oxidation step in order for P. gingivalis to wrest haem from the oxyhaemoglobin. In this context, it is noteworthy that P. gingivalis can grow in an oxygenated environment (Diaz and Rogers, 2004).
Methaemoglobin formation, mediated by the action of R-gingipain (Smalley et al., 2007), would serve two important roles. Firstly, it would lower the haem-globin affinity sufficiently to enhance haem dissociation, resulting in collapse of the protein structure, rendering the globin susceptible to proteolytic breakdown and facilitating a fuller release of the iron porphyrin. Secondly, it would poise the iron porphyrin in the Fe(III) state, such that upon proteolytic release, Fe(III)PPIX.OH (haematin) molecules could react together yielding the haem dimer, [Fe(III)PPIX]2O, according to Eq. (1) above (Smalley et al., 2006).
Acknowledgments
This work was supported in part by grant DE09761 (to J.P.) from National Institutes of Health.
References
- Adams PA, Berman MC. Kinetics and mechanism of the interaction between human serum albumin and monomeric haemin. Biochem. J. 1980;191:95–102. doi: 10.1042/bj1910095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini E, Brunori M. Hemoglobin and Myoglobin in their Reactions with Ligands. Amsterdam, Netherlands: North Holland Publishing Company; 1971a. Solution properties of myoglobin and hemoglobin; pp. 98–134. •••Name(s) of editor(s)?•••. [Google Scholar]
- Antonini E, Brunori M. Hemoglobin and Myoglobin in their Reactions with Ligands. Amsterdam, Netherlands: North Holland Publishing Company; 1971b. The derivatives of ferric haemoglobin and myoglobin; pp. 40–54. •••Name(s) of editor(s)?•••. [Google Scholar]
- Ascoli F, Fanelli MR, Antonini E. Preparation and properties of apohaemoglobin and reconstituted haemoglobins. Methods Enzymol. 1981;76:72–87. doi: 10.1016/0076-6879(81)76115-9. [DOI] [PubMed] [Google Scholar]
- Beaven GH, Chen SH, d’Albis A, Gratzer WB. A spectroscopic study of the haemin-human-serum-albumin system. Eur. J. Biochem. 1974;41:539–546. doi: 10.1111/j.1432-1033.1974.tb03295.x. •••reference is not cited in the text•••. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Jandl JH. Exchange of heme among hemoglobins and between hemoglobin and albumin. J. Biol. Chem. 1968;243:465–475. [PubMed] [Google Scholar]
- Crumton MJ, Polson A. A comparison of the conformation of sperm whale metmyoglobin with that of apomyoglobin. J. Mol. Biol. 1965;11:722–729. doi: 10.1016/s0022-2836(65)80030-4. [DOI] [PubMed] [Google Scholar]
- Diaz PI, Rogers AH. The effect of oxygen on the growth and physiology of Porphyromonas gingivalis. Oral Microbiol. Immunol. 2004;19:88–94. doi: 10.1046/j.0902-0055.2003.00121.x. [DOI] [PubMed] [Google Scholar]
- Gatoni M, Boffi A, Sarti P, Chiancone E. Stability of the heme-globin linkage in αβ dimers and isolated chains of human haemoglobin. A study of heme transfer reaction from the immobilized proteins to albumin. J. Biol. Chem. 1996;271:10130–10136. doi: 10.1074/jbc.271.17.10130. [DOI] [PubMed] [Google Scholar]
- Hanioka T, Tanaka M, Takaya K, Matsumori Y, Shizukuishi S. Pocket oxygen tension in smokers and nonsmokers with periodontal disease. J. Periodontol. 2000;71:550–554. doi: 10.1902/jop.2000.71.4.550. [DOI] [PubMed] [Google Scholar]
- Hargrove MS, Olson JS. The stability of holomyoglobin is determined by heme affinity. Biochemistry. 1996;35:11310–11318. doi: 10.1021/bi9603736. [DOI] [PubMed] [Google Scholar]
- Hargrove MS, Barrick D, Olson JS. The association rate constant for heme binding to globin is independent of protein structure. Biochemistry. 1996;35:11293–11299. doi: 10.1021/bi960371l. [DOI] [PubMed] [Google Scholar]
- Kawahara K, Kirshner AG, Tanford C. Dissociation of human CO-hemoglobin by urea, guanidine hydrochloride, and other reagents. Biochemistry. 1965;4:1203–1213. doi: 10.1021/bi00883a001. [DOI] [PubMed] [Google Scholar]
- Kimura H, Tsudzuki T, Murachi T. Proteolytic degradation of hemoglobin-haptoglobin complex by lysosomal enzymes from rat liver. J. Biochem. 1975;77:909–912. doi: 10.1093/oxfordjournals.jbchem.a130814. [DOI] [PubMed] [Google Scholar]
- Kimura H, Yamato S, Murachi T. Different susceptibilities to intracellular proteases of haemoglobin and haemoglobin-haptoglobin complex. Physiol. Chem. Phys. 1976;8:101–106. [PubMed] [Google Scholar]
- Kimura H, Yamato S, Murachi T. Increase in susceptibility of haemoglobin to proteases upon treatment with p-mercuribenzoate. J. Biochem. 1978;84:205–211. doi: 10.1093/oxfordjournals.jbchem.a132109. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Murachi T. Enhanced susceptibility of haemoglobin to proteases upon ascorbic acid oxidation of the heme moiety. J. Biochem. 1978;84:83–91. doi: 10.1093/oxfordjournals.jbchem.a132122. [DOI] [PubMed] [Google Scholar]
- Perutz MF. Stereochemistry and cooperative effects in haemoglobin. Nature. 1970;228:726–734. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Pike RN, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J. Biol. Chem. 1994;269:406–411. [PubMed] [Google Scholar]
- Potempa J, Pike R, Travis J. Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biol. Chem. 1997;378:223–230. doi: 10.1515/bchm.1997.378.3-4.223. •••reference is not cited in the text•••. [DOI] [PubMed] [Google Scholar]
- Potempa J, Banbula A, Travis J. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontology. 2000;24:153–192. doi: 10.1034/j.1600-0757.2000.2240108.x. [DOI] [PubMed] [Google Scholar]
- Smalley JW, Thomas MF, Birss AJ, Withnall R, Silver J. A combination of both arginine- and lysine-specific gingipain activity of Porphyromonas gingivalis is necessary for the generation of the µ-oxo bishaem-containing pigment from haemoglobin. Biochem. J. 2004;379:833–840. doi: 10.1042/BJ20031221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley JW, Birss AJ, Szmigielski B, Potempa J. The HA2 haemagglutinin domain of the lysine-specific gingipain (Kgp) of Porphyromonas gingivalis promotes µ-oxo bishaem formation from monomeric iron(III) protoporphyrin IX. Microbiology. 2006;152:1839–1845. doi: 10.1099/mic.0.28835-0. [DOI] [PubMed] [Google Scholar]
- Smalley JW, Birss AJ, Szmigielski B, Potempa J. Sequential action of R- and K-specific gingipains of Porphyromonas gingivalis in the generation of the haem-containing pigment from oxyhaemoglobin. Arch. Biochem. Biophys. 2007;465:44–49. doi: 10.1016/j.abb.2007.05.011. [DOI] [PubMed] [Google Scholar]