Abstract
Introduction
During periodontitis, an innate immune response to bacterial challenge is primarily mediated by neutrophils. We compared neutrophilic content and the level of neutrophil-derived antimicrobial peptides in gingival crevicular fluid (GCF) in two clinical forms of severe periodontitis.
Methods
GCF was collected from 14 patients with aggressive periodontitis, 17 patients with chronic periodontitis, and nine healthy subjects. Samples were analyzed for periodontopathogen load using real-time polymerase chain reactions. The amounts of myeloperoxidase and α-defensins (HNP1–3) were determined by enzyme-linked immunosorbent assay, and the level of cathelicidin (hCAP18/LL-37) was assayed by Western blot.
Results
Myeloperoxidase concentration was not correlated with levels of LL-37 and HNP1–3 in samples from patients, compared to controls. The amount of HNP1–3 was twofold and fourfold higher in patients with aggressive and chronic periodontitis, respectively. Those with chronic disease had significantly elevated amounts of mature LL-37. The increased concentration of both peptides in chronic periodontitis correlated with the load of Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola.
Conclusion
The lack of a correlation between LL-37, HNP1–3, and myeloperoxidase content suggests that neutrophils are not the sole source of these bactericidal peptides in the GCF of patients with periodontitis; and that other cells contribute to their local production. The bacterial proteases of P. gingivalis, T. forsythia, and T. denticola might degrade hCAP18/LL-37, because the 11-kDa cathelicidin-derived fragment was present in GCF collected from pockets infected with these bacteria. Collectively, it appears that a local deficiency in LL-37 can be considered as a supporting factor in the pathogenesis of severe cases of periodontitis.
Keywords: defensins, bactericidal peptide, cathelicidin LL-37, crevicular fluid, periodontitis, periodontopathogenic bacteria
The initiation and progression of periodontal disease is related to the colonization of the gingival crevice by specific microorganisms and their subsequent subgingival proliferation. These organisms include Aggregatibacter (Actinobacillus) action-mycetemcomitans and members of the so-called ‘red complex’ of periodontopathogenic bacteria (Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola) (6, 18, 22). An effective host response to this bacterial challenge is primarily mediated by neutrophils and is characterized by an influx of neutrophils into the gingival crevice (16).
It is recognized that an extremely diverse group of antimicrobial peptides participate in the innate immune response of all multicellular organisms (5). In humans, the best characterized of these are α-defensins, β-defensins, and the cathelicidin hCAP18/LL-37. Neutrophils are the most abundant source of α-defensins 1–4 (human neutrophil peptides HNP1–4) and hCAP18/LL-37. In neutrophils, HNP1–4 are stored in azurophilic (primary) granules in a fully processed, biologically active form. These peptides are also produced by B and T lymphocytes (3, 14, 41). The sole human cathelicidin (hCAP18/LL-37) is encoded by the CAMP gene, and encompasses two distinct domains. The N-terminal, ‘cathelin-like’ domain is structurally conserved among vertebrates (53), which is in stark contrast to the highly diverse antimicrobial peptide constituting the C-terminal domain. The gene for human cathelicidin is highly expressed in the myeloid-lineage of bone marrow cells and also in many types of epithelial cells. In neutrophils, hCAP18/LL-37 is stored in specific (secondary) granules as a biologically inactive precursor. During phagocytosis, or in otherwise stimulated neutrophils, bactericidal peptide LL-37 is released from hCAP18/LL-37 by limited proteolysis, which is exerted by protease 3 (46).
In humans, cathelicidin seems to play a key role in protecting the human periodontium against dental plaque bacteria. Patients with morbus Kostmann syndrome who are treated with granulocyte colony-stimulating factor have normal neutrophil counts and are cured of recurrent infections, although they still suffer from severe periodontitis (9). In 2002, Pütsep et al. (35) showed that neutrophils from these patients were deficient in hCAP18/LL-37 and seemingly carried reduced amounts of the α-defensins (HNP1–3) while otherwise remaining fully functional. To further elucidate the role of LL-37 and HNP1–3 in periodontal disease, we have quantified the levels of these bactericidal peptides in gingival crevicular fluid (GCF) from severe periodontitis patients who have no known congenital deficiency or dysfunction of their neutrophils. We have then correlated the levels of antimicrobial peptides with neutrophil abundance and the presence of specific periodontopathogenic bacteria.
Material and methods
Subjects
Patients attending the Clinic of Periodontology at the University Hospital of Jena were recruited for this study. The subjects included 14 patients with aggressive periodontitis (mean age 32.8 years) and 17 patients with chronic periodontitis (mean age 59.1 years) (Table 1). The patients were diagnosed according to the classifications recommended by the American Academy of Periodontology in 1999 (3). Chronic periodontitis, a disease that is more prevalent in adults, is characterized by a steady amount of destruction with the presence of local factors and a general slow to moderate progression of periodontal disease (27). In contrast, aggressive periodontitis is characterized by rapid attachment loss and bone destruction, where the amounts of microbial deposits are inconsistent with the severity of periodontal tissue destruction (4). Inclusion criteria for the diagnosis of generalized chronic periodontitis were attachment loss ≥5 mm at more than 30% of sites and a patient age of ≥35 years. For diagnosis of aggressive periodontitis patients had to fulfill the following criteria: radiographic bone loss ≥50% for at least two different teeth, age ≤35 years at the onset of the disease (anamnesis), and clinically healthy (no systemic diseases, e.g. diabetes mellitus). Nine periodontally healthy subjects (mean age 30.9 years) were included as a control group. Subjects were free of systemic diseases, and had at least 20 teeth in occlusion. Less than 35% of the patients were active smokers. Patients who had received systematic periodontal treatment in the preceding year, who had taken antibiotics within the previous 3 months, or who were pregnant or nursing were excluded from this study. Clinical examinations included a plaque record index (33), a bleeding-on-probing index, and measurements of probing depths and of attachment loss at six sites per tooth. The study protocol was approved by the Ethics Committee of the University of Jena, Germany. All participants gave their informed written consent.
Table 1.
Clinical data (mean and standard deviation) of the subjects
| Aggressive periodontitis | Chronic periodontitis | Periodontally healthy subjects | |
|---|---|---|---|
| n | 14 | 17 | 9 |
| Age (years) | 32.8 ± 6.8 | 59.1 ± 8.14 | 30.9 ± 7.2 |
| % Male | 43 | 53 | 44 |
| Pocket depth (mm) of tested sites | 6.10 ± 1.55 | 6.10 ± 1.46 | 1.51 ± 0.19 |
| Attachment level (mm) of tested sites | 6.89 ± 0.97 | 6.55 ± 1.42 | 1.55 ± 0.22 |
| Full mouth probing depth (mm) | 4.40 ± 1.22 | 3.92 ± 0.67 | 1.45 ± 0.21 |
| Full mouth attachment level (mm) | 4.60 ± 1.37 | 4.14 ± 0.73 | 1.57 ± 0.30 |
| Bleeding on probing (%) | 84.09 ± 19.18 | 83.96 ± 18.35 | 14.00 ± 13.05 |
| Plaque record index (%) | 45.0 ± 32.0 | 85.0 ± 39.0 | 30.0 ± 25.0 |
Sampling of crevicular fluid and blood collection
Crevicular washes were obtained using a previously described method (20, 45). Briefly, samples were collected in the morning, 2–3 h after breakfast. The sites to be sampled were isolated with cotton rolls and gently air-dried. A gel-loading capillary tip was carefully inserted into the crevice at a level of approximately 1 mm below the gingival margin. In each case, seven sequential washes with 10 µl phosphate-buffered saline were performed using a micropipette. The crevicular fluid was obtained as a pooled sample from the deepest site in each quadrant, transferred into a microcentrifuge tube, immediately frozen, and kept at −20°C until analyzed.
From all subjects included in this study, samples of the peripheral blood were collected by venepuncture onto heparin as the anticoagulant. From selected subjects peripheral mononuclear cells (PMNs) were isolated using dextran sedimentation followed by hypotonic lysis of erythrocytes.
Microflora
DNA was extracted from 5 µl crevicular fluid using the High Pure PCR Template Preparation Kit (Roche, Mannheim, Germany) according to the manufacturer’s recommendations. Real-time polymerase chain reaction (PCR) was carried out using a RotorGene 2000 (Corbett Research, Sydney, Australia). Primers specific for 16S ribosomal DNA from P. gingivalis, Prevotella intermedia, T. forsythia, and T. denticola were designed as described by Ashimoto et al. (4), whereas those for A. actinomycetemcomitans were designed as described by Tran and Rudney (48). PCR amplification was carried out in a reaction volume of 20 µl, consisting of 2 µl template DNA solution and 18 µl reaction mixture, containing 2 µl 10 × PCR buffer, 2.75 mm MgCl2, 0.2 mm nucleotides, 0.5 µm of each primer, 10−4 SybrGreen and 1 U Taq polymerase (Fermentas Life Sciences, St. Leon-Rot, Germany). Positive and negative controls were included in each set of analyzed samples. Each analysis was performed in duplicate. The positive control consisted of 2 µl genomic DNA from reference strains, equivalent to a range of bacteria from 102 to 107. The negative control constituted of 2 µl of sterile water added to 18 µl of reaction mixture. The cycling conditions comprised an initial denaturation step at 95°C for 5 min, followed by 45 cycles at 95°C for 15 s, 65°C (apart from A. actinomycetemcomitans and P. intermedia which used 62°C) for 20 s, followed by a touch-down for five cycles at 72°C for 20 s. The sensitivity and specificity of the method were checked using well-characterized bacterial strains and subgingival plaque samples in which the pathogen presence was quantified using the commercially available certified micro-Ident® kit (HAIN LIFESCIENCE GmbH, Nehren, Germany). The specificity of the amplification was always assayed using melting curves. For quantification, the results from unknown plaque samples were projected using a standard threshold curve from counted and pure cultures of the target bacterium. Bacterial loads were quantified using log-stages.
Enzyme-linked immunosorbent assay
Isolated PMN and GCF samples were subjected to three cycles of thawing and freezing to lyze cells and release intracellular granule content. Myeloperoxidase (MPO) and α-defensin were detected in GCF and saliva samples using Human MPO and Human HNP 1–3 (Neutrophil Defensins) enzyme-linked immunosorbent assay (ELISA) kits, respectively, according to the manufacturer’s protocol. Both kits were obtained from HyCult Biotechnology (Uden, the Netherlands). Samples were diluted 10-fold and 100-fold in phosphate-buffered saline for MPO determination, and 10,000-fold in a plasma dilution buffer provided in the assay kit for defensin determination.
Western blot
GCF samples were diluted four times with sample buffer (0.125 m Tris–HCl, 20% glycerol, 4% sodium dodecyl sulfate), and resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis [16% peptide gel (49.5% T/6% C)] using the Tris–Tricine discontinuous buffer system (38). LL-37 was synthesized on an Applied Biosystems model 433A synthesizer (Applied Biosystems, Forester City, CA), and purified by preparative reverse-phase high-performance liquid chromatography (42, 43); it was kindly supplied to us as a gift by Dr Jan Pohl (Department of Microbiology and Immunology, Emory University School of Medicine, Atlanta, GA). The concentration of the peptide was determined by quantitative amino acid analysis, and peptide was used at a concentration of 4 µg/ml (6 ng/well) as a standard. Electrophoresed gels were electroblotted (Mini Trans-Blot System; Bio-Rad) on to polyvinylidene difluoride membranes (Amersham-Pharmacia Co., Piscataway, NJ). Non-specific binding sites on the membranes were blocked overnight in 5% skimmed milk (Difco, Detroit, MI) and immunoblotted. The blots were probed with a monoclonal mouse antibody against LL-37 (clone 1.1C12; 46) and goat anti-mouse immunoglobulin G horseradish-peroxidase-conjugated antibodies (Sigma, St Louis, MO). Immunoreactive peptides were detected with ECL Plus (Amersham-Pharmacia Co.) according to manufacturer’s protocol before membranes were exposed to X-ray films (Kodak). The intensity of LL-37-immunoreactive bands in each sample was assessed semi-quantitatively by the densitometric analysis of immunoblots using serial dilutions of synthetic LL-37 as a reference.
Data analysis
Data were expressed as means and standard deviation (SD). Full mouth probing depths, an attachment level, bleeding on probing, and the plaque record index were recorded to characterize participants included in this study. Furthermore, the means (± SD) of pocket depth and the attachment level were calculated for those sites from which GCF and microbiological samples were collected. All data were entered into the SPSS 13.0 program and were analyzed using the Mann–Whitney U-test and the Spearman test. The level of significance was set at P < 0.05 with 95% confidence intervals.
Results
Clinical data and microflora
Demographic and clinical data are presented in Table 1. Not unexpectedly, patients with periodontal disease demonstrated significantly higher mouth mean probing depths compared to healthy control subjects. However, there were no statistically significant differences in mouth mean probing depths, nor in probing depths at the tested sites, between chronic and aggressive periodontitis.
None of the five investigated periodontopathogens, P. gingivalis, T. forsythia, T. denticola, A. actinomycetemcomitans, and P. intermedia, was detected in samples from the control group. In stark contrast, patients suffering from periodontitis carried at least two pathogens with P. gingivalis and T. forsythia at the highest prevalence in chronic and aggressive forms of the disease, respectively. Interestingly, the only significant difference between the two patient groups was the level of P. gingivalis, which was much higher in chronic than in aggressive periodontitis (Table 2).
Table 2.
Prevalence and high load (≥106/sample) of the periodontopathogens Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, and Prevotella intermedia in gingival crevicular fluid obtained from periodontitis patients as well as healthy controls
| Positive ≥106 |
||||||
|---|---|---|---|---|---|---|
| Species | Aggressive periodontitis | Chronic periodontitis | Periodontally healthy subjects | |||
| A. actinomycetemcomitans | 6 (43%) | 2 (14%) | 6 (35%) | 0 (0%) | 0 (0%) | 0 (0%) |
| P. gingivalis | 5 (36%) | 2 (14%) | 13 (76%) | 10 (59%) | 0 (0%) | 0 (0%) |
| T. denticola | 6 (43%) | 2 (14%) | 13 (76%) | 4 (24%) | 0 (0%) | 0 (0%) |
| T. forsythia | 10 (71%) | 3 (21%) | 16 (94%) | 6 (35%) | 0 (0%) | 0 (0%) |
| P. intermedia | 5 (36%) | 1 (7%) | 9 (53%) | 7 (41%) | 0 (0%) | 0 (0%) |
MPO as an equivalent for neutrophil numbers
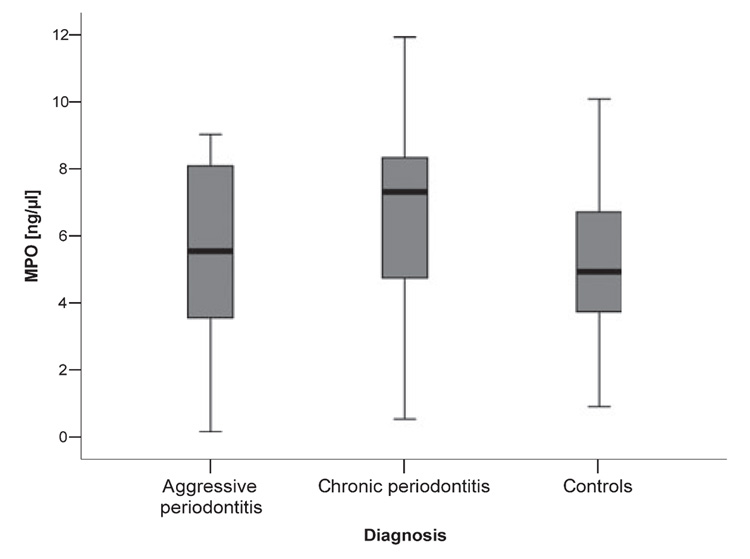
The method used for GCF collection did not allow for direct counting of neutrophils in GCF samples. Instead, we have determined the concentration of MPO, a protein that is abundant in neutrophils and of which they are the only source; this fact allows a direct correlation of MPO with neutrophil numbers (7). To lyze neutrophils and release the intracellular MPO, the GCF samples were subjected to three cycles of freezing and thawing. The median concentration of MPO was found to be 5.56 ng/µl (range 1.80–9.00 ng/µl), 6.90 ng/µl (range 0.54–11.93 ng/µl) and 4.57 ng/µl (range 0.90–10.08 ng/µl) in samples collected from aggressive periodontitis, chronic periodontitis, and periodontally healthy controls, respectively (Fig. 1). Differences in MPO concentration between the groups were not significant and did not correlate with clinical parameters, with the exception of patients with aggressive periodontitis, in whom the amount of MPO was negatively associated with the bleeding on probing index (r = − 0.575; P = 0.03, data not shown).
Fig. 1.
Level of myeloperoxidase in gingival crevicular fluid obtained from periodontitis patients and healthy controls. The results present median, and 25th and 75th percentiles.
α-Defensins
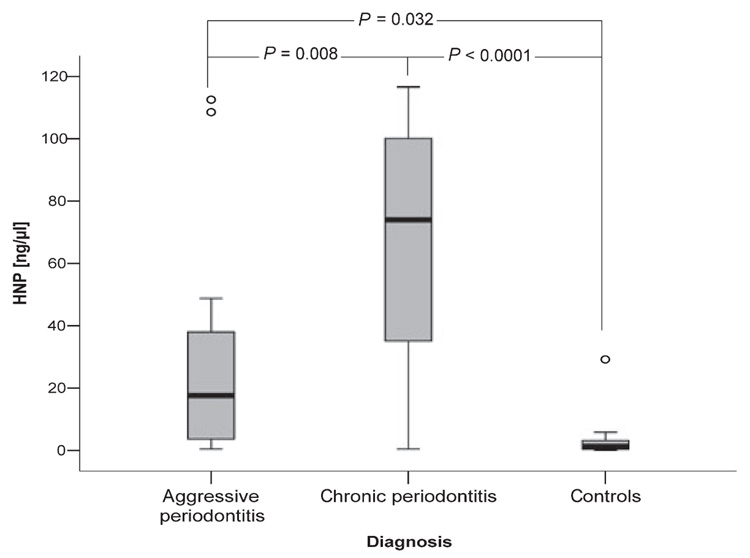
HNP1–3 were detected in all GCF samples, however at different levels. The highest concentration was found in GCF from patients with chronic periodontitis (73.92 µg/µl, range 0.40–116.17 µg/µl), with only moderate concentrations in samples from patients with aggressive periodontitis (median 17.64 µg/µl, range 0.36–112.52 µg/µl), and low concentrations (1.20 µg/µl, range 0.01–29.18 µg/µl) in healthy controls.
Further and more detailed statistical analysis of these data revealed that in periodontally healthy subjects, the levels of MPO and HNP1–3 were correlated with each other (r = 0.731; P = 0.025), indicating that only in this group were GCF neutrophils the main source of bactericidal peptides. Conversely, the concentration of α-defensins in GCF was found to be closely associated with the load of the periodontopathogens P. gingivalis, T. denticola, and T. forsythia, but not with the A. actinomycetemcomitans or P. intermedia load (Table 3).
Table 3.
Correlation coefficients (Spearman test) between periodontopathogenic species and myeloperoxidase (MPO) as well as antimicrobial peptides
| MPO | HNP1–3 | Unprocessed cathelicidin | 11-kDa fragment | Mature LL-37 | |
|---|---|---|---|---|---|
| A. actinomycetemcomitans | 0.156 | 0.129 | 0.011 | 0.075 | 0.126 |
| P. gingivalis | 0.061 | 0.575** | 0.518** | 0.360* | 0.405* |
| T. forsythia | 0.212 | 0.676** | 0.344* | 0.514** | 0.484** |
| T. denticola | 0.013 | 0.538** | 0.338* | 0.492** | 0.525** |
| P. intermedia | 0.263 | 0.260 | − 0.178 | 0.289 | 0.324* |
P < 0.05
P < 0.01.
Cathelicidin LL-37
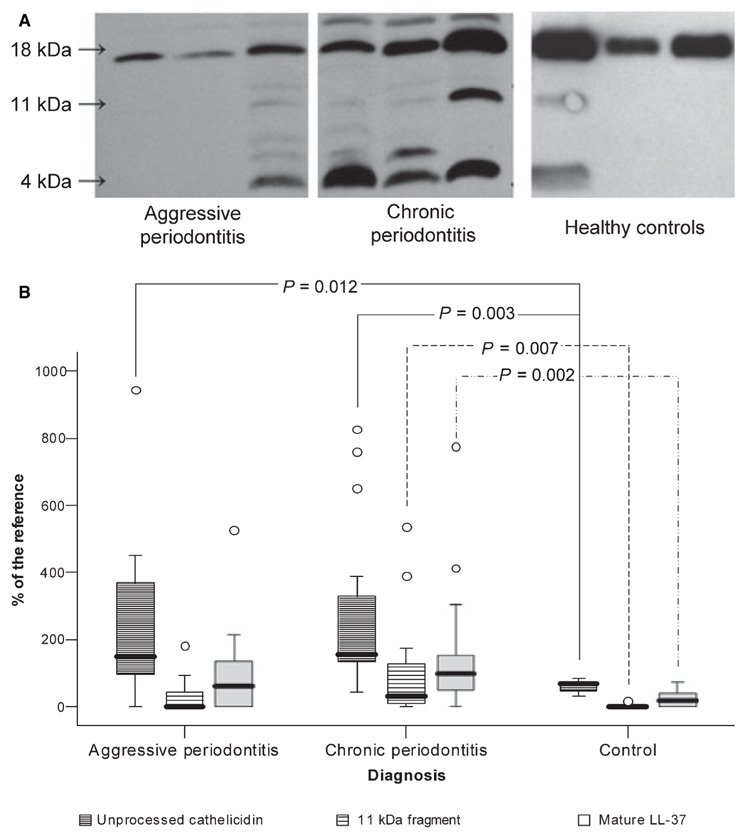
The presence of intact cathelicidin, the cathelicidin-derived bactericidal peptide LL-37, and fragments containing LL-37 in GCF samples was determined by Western blot using the monoclonal antibody anti-LL37. As shown in Fig. 2, GCF samples differed considerably with respect to both the band pattern and the intensity of the individual immunoreactive bands. Despite significantly higher levels of unprocessed cathelicidin among GCF samples collected from patients with aggressive periodontitis compared to healthy controls, two out of 14 patients (14%) lacked any trace of a protein or peptides immunoreactive with antibody against LL-37. As indicated by high MPO concentrations (approximately 6 ng/µl), these samples contained abundant neutrophils as well as being rich in HNP1–3 (data not shown). Furthermore, in five out of 14 patients (36%) suffering from aggressive periodontitis, the mature LL-37 peptide was absent. In chronic periodontitis, the levels of both hCAP18/LL-37 and mature LL-37 were significantly higher compared to levels in healthy controls, despite the fact that three of the 17 patients (18%) lacked the mature bactericidal peptide.
Fig. 2.
Levels of the hCAP18, 11-kDa fragment, as well as LL-37 in gingival crevicular fluid (GCF) obtained from periodontitis patients and healthy controls. (A) Representative Western blot patterns of LL-37 immunoreactive proteins/peptides in GCF samples collected from patients with chronic and aggressive periodontitis as well as from healthy controls. Arrows point to immunoreactive bands against anti-LL-37 antibody, representing intact hCAP18/LL-37, 11-kDa fragment of hCAP18/LL-37, and the LL-37 peptide. Each blot was developed independently. Therefore, the intensity of immunoreactive bands on different panels cannot be used directly to compare the amount of hCAP18/LL-37-derived fragments in analysed samples. (B) The relative concentrations of hCAP18/LL-37, 11-kDa fragment of hCAP18/LL-37, and the LL-37 peptide in GCFs inferred from the densitometric analysis of Western blots using serial dilutions of synthetic LL-37 as a reference included on each blot. The results present median, including 25th and 75th percentiles and extreme values (circles), as well as P-values of statistical significance determined by Mann–Whitney test.
In healthy subjects, the unprocessed 18-kDa form of cathelicidin was present at moderate concentrations and substantial amounts of free LL-37 peptide were detected in only 22% of samples. In this group, there was a significant correlation between the concentrations of immunoreactive LL-37 with both MPO (r = 0.731; P = 0.025) and HNP1–3 (r = 0.696; P = 0.037) concentrations. So in the healthy periodontium, neutrophils were the main source of cathelicidin.
Unlike in healthy controls, GCF samples from patients with periodontitis often contained a clear immunoreactive band around 11 kDa. This band apparently represented an alternatively processed form of hCAP18. Interestingly, in patients with aggressive periodontitis, the intensity of this 11-kDa fragment was always associated with a high level of the LL-37 peptide (r = 0.789; P = 0.001). In chronic periodontitis, an association between the 11-kDa fragment and the LL-37 peptide was not as clear and among the six samples that were rich in the 11-kDa fragment, three showed low levels of LL-37. Analysis of the relationship between LL-37 immunoreactive band patterns and specific bacteria in periodontal plaque revealed that the 11-kDa band was correlated with the presence of P. gingivalis, T. forsythia, and T. denticola (Table 3). Additionally, the load of P. intermedia was found to correlate with levels of mature LL-37 in GCF samples (Table 3).
Discussion
Neutrophils with their content of antimicrobial peptides are indispensable for the elimination of localized bacterial infections. Here we have shown that GCF from periodontally healthy subjects contained very low levels of HNP1–3 (Fig. 3), which correlated well (P = 0.033) with MPO concentrations. This correlation indicates that in the healthy periodontium, infiltrating neutrophils are the main source of α-defensins. In contrast, HNP1–3 concentrations in GCF collected from periodontitis patients were much higher; patients with the chronic form of the disease had significantly more α-defensins in their GCF than those with aggressive periodontitis. In both cases, there was no association between neutrophil content and α-defensin levels in the patient’s GCF. The differences in HNP1–3 concentration between the groups were statistically significant (Fig. 3), which is interesting considering the lack of variation in neutrophil numbers found in the GCF of the three studied groups. This suggests that sources other than neutrophils are responsible for α-defensin production in inflamed periodontal/gingival tissues. Indeed, it has been shown that HNP1–3 are also expressed in lymphocytes and monocytes (1), which are abundant in periodontitis sites (15). The difference in the HNP1–3 content in GCF may be related to the composition of bacterial flora in dental plaque from both disease forms. In the samples analyzed, we found abundant P. gingivalis in chronic periodontitis but its presence was low in aggressive periodontitis. The opposite was found for the prevalence of A. actinomycetemcomitans, which is in accordance with previous reports on bacterial etiology of periodontitis (8, 37). These two bacterial species may exert divergent effects on the local production of α-defensins, and in line with this, we found that the concentration of HNP1–3 in GCF correlated with the P. gingivalis load, but not with that of A. actinomycetemcomitans (Table 3).
Fig. 3.
Level of α-defensins (HNP1–3) in gingival crevicular fluid obtained from periodontitis patients and healthy controls. The results present median, including 25th and 75th percentiles and extreme values (circles), as well as P-values of statistical significance determined by Mann–Whitney test.
The high concentration of α-defensins in severe periodontitis and the low abundance in healthy subjects challenges the results of an HNP1–3 analysis in GCF using a mass spectroscopy approach (29). That study demonstrated that α-defensins were more abundant, and in higher proportion, in GCF samples collected from the healthy sites, rather than diseased sites. This discrepancy is most likely because two different analytical techniques were used in the studies. While mass spectroscopy only allows for the relative quantification of intact defensins, the ELISA method employed here might detect various forms of HNP1–3, including proteolytically generated fragments of α-defensins. Therefore, and most interestingly, the discrepancies may indicate that in GCF from periodontitis patients, most α-defensins are partially degraded. This assumption correlates with the finding that GCF contains many tissue- and bacteria-derived proteinases (17), including the gingipains of P. gingivalis (30, 50), for which defensins are excellent substrates (Puklo et al., 2007 manuscript in preparation). Therefore, it is likely that α-defensins are not fully active within the periodontal pocket. In addition to proteolytic degradation, the bactericidal activity of the α-defensins can be abrogated by the dermatan sulfate-containing fragments of proteoglycans that are released locally by bacterial proteases (40). On the other hand, the high local levels of α-defensins in periodontal tissues might have a destructive effect. HNP1–3, at concentrations of 5–8 µg/ml, promotes the proliferation of periodontal epithelial cells, and at very high concentrations (of 50 µg/ml) induces cell-death of human oral fibroblasts (32). In addition, HNP1–3 may hinder neutrophil chemotaxis (19) and, together with lower expression of CD38, may contribute to the attenuation of the physiological functions of neutrophils observed in patients suffering from aggressive periodontitis (13, 49).
As for HNP1–3, the presence of hCAP18/LL-37 correlated with neutrophil content only in periodontally healthy controls, suggesting that neutrophils are the main source of LL-37 in the non-inflamed periodontium. Significantly, substantial amounts of the full-length, precursor protein were present in all healthy subjects, and detectable amounts of mature LL-37 were found only in five out of nine subjects (56%). This suggests that the majority of hCAP18/LL-37 in healthy tissues originated from lyzed PMNs and that the majority of neutrophils traversing the healthy periodontium/gingiva into the gingival crevice are not activated. In aggressive periodontitis, the inflammatory response does not account for the severity of tissue destruction (26). The lack of a significant increase in the level of mature LL-37 in GCF from this group of patients, despite the presence of a relatively higher amount of full-length hCAP18/LL-37, supports the clinical observations. Surprisingly, two out of 14 aggressive periodontitis patients lacked any trace of immunoreactive LL-37, even though normal levels of unprocessed hCAP18/LL-37 were simultaneously detected in the peripheral blood of these subjects (data not shown). These findings suggest that both the LL-37 portion of full-length cathelicidin and the released bactericidal peptide can be degraded in the GCF of this subset of patients. This hypothesis is supported by the susceptibility of LL-37 to digestion by proteases of several common bacteria, including Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pyogenes, all of which cleave LL-37 (39, 44). On the other hand, A. actinomycetemcomitans, the major pathogen in the aggressive form of periodontitis, has very low proteolytic activity and leukotoxin is the key virulence factor. Leukotoxin triggers a rapid release of PMN granule components (25). It can be speculated that a unique composition of host-derived proteases in aggressive periodontitis is responsible for the cathelicidin degradation, which is in keeping with the observation that in normal human epidermis, hCAP18/LL-37 or mature LL-37 are subject to proteolytic processing by tissue kallikreins (51).
Upon neutrophil stimulation, hCAP18/LL-37 is proteolytically processed by protease 3 into LL-37 and the cathelin protein (16 kDa) (46). The hCAP18/LL-37 gene, however, is expressed in various types of epithelial cells (12), including gingival epithelial cells, in which its expression is stimulated by different periodontal pathogens (23, 34). This stimulated local production of hCAP18/LL-37, together with the enhanced influx of neutrophils, may explain the positive correlation between the concentration of immunoreactive LL-37 protein in the inflamed gingiva and the depth of periodontal pockets as a measure of the severity of periodontal disease (23). It is, however, unclear which protease causes the release of bactericidal LL-37 from the precursor in addition to protease 3. In this context, it is interesting to note that in GCF from patients with periodontitis, we have found aberrantly processed hCAP18/LL-37 as an 11-kDa fragment that still bears the LL-37 peptide at its C terminus. This fragment is different from that of the 14-kDa fragment generated by neutrophil elastase or cathepsin G (46), and is most likely to be generated by bacterial protease(s), especially as its occurrence is correlated with the presence of the highly proteolytic organisms P. gingivalis, T. forsythia, and T. denticola.
Despite in vitro findings that HNPs and LL-37 are totally ineffective (2, 21, 36), or possess only marginal bactericidal activity against A. actinomycetemcomitans and P. gingivalis (24, 34, 47), clinical observations strongly argue for the importance of at least LL-37 in the protection of gingival/periodontal tissues against bacterial challenge. Specifically, the congenital deficiency of hCAP18/LL-37 in morbus Kostmann syndrome (35) and the absence of mature LL-37 in Papillon–Lefèvre syndrome (10) are the primary reasons for early development of severe aggressive periodontitis in these patients. In keeping with this, our data here suggest that cathelicidin LL-37 is an important, but still insufficient, part of the innate host defense in severe periodontitis. Combining these findings, it is plausible to conclude that cathelicidin is a key effector molecule of the innate immunity in the human periodontium/gingival tissues although its protective role may be unrelated to bactericidal activity. Conversely, in the hypoxic environment of the inflamed gingiva (11), which suppresses oxygen-dependant bactericidal activity of phagocytes (28), cathelicidin may synergize with HNPs to efficiently kill periodontopathogens, as they do in the case against Escherichia coli and S. aureus (31). This intriguing possibility is the subject of research in our laboratories.
Acknowledgments
The authors would like to thank Claudia Ranke, University Hospital of Jena, for technical assistance in the determination of microflora as well as Dr Jürgen W. Einax, University of Jena, for advice on statistics. Furthermore, we are indebted to Dr Lindsey N. Shaw from the University of South Florida, Tampa, FL and Dr Ky-Anh Nguyen from the Institute of Dental Research, Sydney, Australia, for language corrections, and critical reading of this paper, respectively. The authors acknowledge the support of the German Academic Exchange Service (DAAD) through a grant to M.P. (no. A/05/15130), and of the State Committee for Scientific Research (KBN, Warszawa, Poland) and the National Institutes of Health through grants 2 P04C 024 29 (to M.P. and J.P.) and DE 09761 (to J.P.), respectively.
References
- 1.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 2.Altman H, Steinberg D, Porat Y, et al. In vitro assessment of antimicrobial peptides as potential agents against several oral bacteria. J Antimicrob Chemother. 2006;58:198–201. doi: 10.1093/jac/dkl181. [DOI] [PubMed] [Google Scholar]
- 3.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Ashimoto A, Chen C, Bakker I, Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol. 1996;11:266–273. doi: 10.1111/j.1399-302x.1996.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 5.Boman HG. Antibacterial peptides: basic facts and emerging concepts. J Intern Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 6.Borrell LN, Papapanou PN. Analytical epidemiology of periodontitis. J Clin Periodontol. 2005;32 Suppl 6:132–158. doi: 10.1111/j.1600-051X.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- 7.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 8.Bragd L, Dahlen G, Wikström M, Slots J. The capability of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius to indicate progressive periodontitis; retrospective study. J Clin Periodontol. 1985;14:95–99. doi: 10.1111/j.1600-051x.1987.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson G, Wahlin YB, Johansson A, et al. Periodontal disease in patients from the original Kostmann family with severe congenital neutropenia. J Periodontol. 2006;77:744–751. doi: 10.1902/jop.2006.050191. [DOI] [PubMed] [Google Scholar]
- 10.de Haar SF, Hiemstra PS, van Steenbergen MT, Everts V, Beertsen W. Role of polymorphonuclear leukocyte-derived serine proteinases in defense against Actinobacillus actinomycetemcomitans. Infect Immun. 2006;74:5284–5291. doi: 10.1128/IAI.02016-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskow RN, Loesche WJ. Oxygen tensions in the human oral cavity. Arch Oral Biol. 1971;16:1127–1128. doi: 10.1016/0003-9969(71)90218-4. [DOI] [PubMed] [Google Scholar]
- 12.Frohm Nilsson M, Sandstedt B, Sorensen O, Weber G, Borregaard N, Stahle-Backdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita T, Kantarci A, Warbington ML, et al. CD38 expression in neutrophils from patients with localized aggressive periodontitis. J Periodontol. 2005;76:1960–1965. doi: 10.1902/jop.2005.76.11.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallo RL, Murakami M, Ohtake T, Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J Allergy Clin Immunol. 2002;110:823–831. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- 15.Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Characterization of cellular infiltrate, detection of chemokine receptor CCR5 and interleukin-8 and RANTES chemokines in adult periodontitis. J Periodontal Res. 2001;36:194–203. doi: 10.1034/j.1600-0765.2001.360309.x. [DOI] [PubMed] [Google Scholar]
- 16.Garant PR. Oral cells and tissues. Carol Stream, IL: Quintessence Publishing Co, Inc.; 2003. [Google Scholar]
- 17.Gazi MI, Cox SW, Clark DT, Eley BM. A comparison of cysteine and serine proteinases in human gingival crevicular fluid with tissue, saliva and bacterial enzymes by analytical isoelectric focusing. Arch Oral Biol. 1996;41:393–400. doi: 10.1016/0003-9969(96)00007-6. [DOI] [PubMed] [Google Scholar]
- 18.Genco RJ, Kornman K, Williams R, et al. Consensus report periodontal diseases: pathogenesis and microbial factors. Ann Periodontol. 1996;1:926–932. doi: 10.1902/annals.1996.1.1.926. [DOI] [PubMed] [Google Scholar]
- 19.Grutkoski PS, Graeber CT, Lim YP, Ayala A, Simms HH. Alpha-defensin 1 (human neutrophil protein 1) as an antichemotactic agent for human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 2003;47:2666–2668. doi: 10.1128/AAC.47.8.2666-2668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guentsch A, Erler M, Preshaw PM, Sigusch BW, Klinger G, Glockmann E. Effect of smoking on crevicular polymorphonuclear neutrophil function in periodontally healthy subjects. J Periodontal Res. 2006;41:184–188. doi: 10.1111/j.1600-0765.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- 21.Guthmiller JM, Vargas KG, Srikantha R, et al. Susceptibilities of oral bacteria and yeast to mammalian cathelicidins. Antimicrob Agents Chemother. 2001;45:3216–3219. doi: 10.1128/AAC.45.11.3216-3219.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 23.Hosokawa I, Hosokawa Y, Komatsuzawa H, et al. Innate immune peptide LL-37 displays distinct expression pattern from beta-defensins in inflamed gingival tissue. Clin Exp Immunol. 2006;146:218–225. doi: 10.1111/j.1365-2249.2006.03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isogai E, Isogai H, Matuo K, et al. Sensitivity of genera Porphyromonas and Prevotella to the bactericidal action of C-terminal domain of human CAP18 and its analogues. Oral Microbiol Immunol. 2003;18:329–332. doi: 10.1034/j.1399-302x.2003.00083.x. [DOI] [PubMed] [Google Scholar]
- 25.Johansson A, Claesson R, Hänström L, Sandström G, Kalfas S. Polymorphonuclear leukocyte degranulation induced by leukotoxin from Actinobacillus actinomycetemcomitans. J Periodontal Res. 2000;35:85–92. doi: 10.1034/j.1600-0765.2000.035002085.x. [DOI] [PubMed] [Google Scholar]
- 26.Lang N, Bartold PM, Cullinan M, et al. Consensus report: aggressive periodontitis. Ann Periodontol. 1999;4:53. [Google Scholar]
- 27.Lindhe J, Ranney R, Lamster I, et al. Consensus report: chronic periodontitis. Ann Periodontol. 1999;4:38. [Google Scholar]
- 28.Loesche WJ, Robinson JP, Flynn M, Hudson JL, Duque RE. Reduced oxidative function in gingival crevicular neutrophils in periodontal disease. Infect Immun. 1988;56:156–160. doi: 10.1128/iai.56.1.156-160.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundy FT, Orr DF, Shaw C, Lamey PJ, Linden GJ. Detection of individual human neutrophil alpha-defensins (human neutrophil peptides 1, 2 and 3) in unfractionated gingival crevicular fluid–a MALDI-MS approach. Mol Immunol. 2005;42:575–579. doi: 10.1016/j.molimm.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Mailhot JM, Potempa J, Stein SH, et al. A relationship between proteinase activity and clinical parameters in the treatment of periodontal disease. J Clin Periodontol. 1998;25:578–584. doi: 10.1111/j.1600-051x.1998.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 31.Nagaoka I, Hirota S, Yomogida S, Ohwada A, Hirata M. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm Res. 2000;49:73–79. doi: 10.1007/s000110050561. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura M, Abiko Y, Kurashige Y, et al. Effect of defensin peptides on eukaryotic cells: primary epithelial cells, fibroblasts and squamous cell carcinoma cell lines. J Dermatol Sci. 2004;36:87–95. doi: 10.1016/j.jdermsci.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 33.O’Leary T, Drake R, Naylor J. The plaque control records. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 34.Ouhara K, Komatsuzawa H, Yamada S, et al. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, β-defensins and LL37, produced by human epithelial cells. J Antimicrob Chemother. 2005;55:888–896. doi: 10.1093/jac/dki103. [DOI] [PubMed] [Google Scholar]
- 35.Pütsep K, Carlsson G, Boman HG, Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–1149. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 36.Raj PA, Antonyraj KJ, Karunakaran T. Large-scale synthesis and functional elements for the antimicrobial activity of defensins. Biochem J. 2000;347:633–641. [PMC free article] [PubMed] [Google Scholar]
- 37.Schacher B, Baron F, Rossberg M, Wohlfeil M, Arndt R, Eickholz P. Aggregatibacter actinomycetemcomitans as indicator for aggressive periodontitis by two analyzing strategies. J Clin Periodontol. 2007;34:566–573. doi: 10.1111/j.1600-051X.2007.01080.x. [DOI] [PubMed] [Google Scholar]
- 38.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 39.Schmidtchen A, Frick IM, Andersson E, Tapper H, Björck L. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol Microbiol. 2002;46:157–168. doi: 10.1046/j.1365-2958.2002.03146.x. [DOI] [PubMed] [Google Scholar]
- 40.Schmidtchen A, Frick IM, Björck L. Dermatan sulphate is released by proteinases of common pathogenic bacteria and inactivates antibacterial α-defensin. Mol Microbiol. 2001;39:708–713. doi: 10.1046/j.1365-2958.2001.02251.x. [DOI] [PubMed] [Google Scholar]
- 41.Schneider JJ, Unholzer A, Schaller M, Schafer-Korting M, Korting HC. Human defensins. J Mol Med. 2005;83:587–595. doi: 10.1007/s00109-005-0657-1. [DOI] [PubMed] [Google Scholar]
- 42.Shafer WM, Hubalek F, Huang M, Pohl J. The bactericidal activity of a synthetic peptide (CG 117–136) of human lysosomal cathepsin G is dependent on arginine content. Infect Immun. 1996;64:4842–4845. doi: 10.1128/iai.64.11.4842-4845.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shafer WM, Pohl J, Onunka VC, Bangalore N, Travis J. Human lysosomal cathepsin G and granzyme B share a functionally conserved broad spectrum antibacterial peptide. J Biol Chem. 1991;266:112–116. [PubMed] [Google Scholar]
- 44.Sieprawska-Lupa M, Mydel P, Krawczyk K, et al. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother. 2004;48:4673–4679. doi: 10.1128/AAC.48.12.4673-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sigusch B, Klinger G, Holtz H, Süss J. In vitro phagocytosis by crevicular phagocytes in various forms of periodontitis. J Periodontol. 1992;63:496–501. doi: 10.1902/jop.1992.63.6.496. [DOI] [PubMed] [Google Scholar]
- 46.Sorensen OE, Follin P, Johnsen AH, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka D, Miyasaki KT, Lehrer RI. Sensitivity of Actinobacillus actinomycetemcomitans and Capnocytophaga spp. to the bactericidal action of LL-37: a cathelicidin found in human leukocytes and epithelium. Oral Microbiol Immunol. 2000;15:226–231. doi: 10.1034/j.1399-302x.2000.150403.x. [DOI] [PubMed] [Google Scholar]
- 48.Tran SD, Rudney JD. Improved multiplex PCR using conserved and species-specific 16S rRNA gene primers for simultaneous detection of Actinobacillus actinomycetemcomitans, Bacteroides forsythus, and Porphyromonas gingivalis. J Clin Microbiol. 1999;37:3504–3508. doi: 10.1128/jcm.37.11.3504-3508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Dyke TE, Hoop GA. Neutrophil function and oral disease. Crit Rev Oral Biol Med. 1990;1:117–133. doi: 10.1177/10454411900010020201. [DOI] [PubMed] [Google Scholar]
- 50.Wikstrom M, Potempa J, Polanowski A, Travis J, Renvert S. Detection of Porphyromonas gingivalis in gingival exudate by dipeptide enhanced trypsin-like activity. J Periodontol. 1994;65:47–55. doi: 10.1902/jop.1994.65.1.47. [DOI] [PubMed] [Google Scholar]
- 51.Yamasaki K, Schauber J, Coda A, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 52.Yang D, Chertov O, Oppenheim JJ. The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell Mol Life Sci. 2001;58:978–989. doi: 10.1007/PL00000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaiou M, Nizet V, Gallo RL. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J Invest Dermatol. 2003;120:810–816. doi: 10.1046/j.1523-1747.2003.12132.x. [DOI] [PubMed] [Google Scholar]