Abstract
Background
Embryonic stem cell (ESC)–derived cardiomyocytes are anticipated to serve as a useful source for future cell-based cardiovascular disease therapies. Research emphasis is currently focused on determining methods to direct the differentiation of ESCs to a large population of cardiomyocytes with high purity. To this aim, understanding the molecular mechanisms that control ESC-to-cardiomyocyte differentiation should play a critical role in the development of this methodology. The Wnt/β-catenin signaling pathway has been implicated in both embryonic cardiac development and in vitro ESC differentiation into cardiomyocytes. Chibby is a recently identified nuclear protein that directly binds to β-catenin and antagonizes its transcriptional activity.
Methods and Results
Chibby was ubiquitously expressed in early stages of ESC differentiation but upregulated during cardiomyocyte specification. Of interest, the Chibby gene promoter has multiple binding sites for the cardiac-specific homeodomain protein Nkx2.5, and its promoter activity was indeed positively regulated by Nkx2.5. Furthermore, overexpression of Chibby increased cardiac differentiation of ESCs, whereas loss of Chibby by RNAi impaired cardiomyocyte differentiation.
Conclusions
These data illustrate the regulation and function of Chibby in facilitating cardiomyocyte differentiation from ESCs. By revealing molecular mechanisms that control ESC-to-cardiomyocyte differentiation, this study will allow for the future development of technologies to improve cardiomyocyte differentiation from ESCs.
Keywords: myocytes, signal transduction, stem cells
The Wnt/β-catenin signaling pathway has been described as being important for cardiomyocyte development from both in vivo and in vitro studies. Most studies have pointed to the inhibition of this pathway as necessary for cardiomyocyte differentiation, proliferation, or repair. For example, in vivo studies using Xenopus laevis or mice with the Wnt antagonists Dkk1 and sFRP lead to enhanced cardiac development or repair after myocardial infarction.1–3 Furthermore, the conditional deletion of β-catenin in the endoderm of mice leads to ectopic heart development.4 In contrast to the above reports, the addition of Wnt3a has been suggested to induce cardiomyocyte differentiation of embryonic carcinoma cells.5 However, this Wnt3a addition has recently been suggested to improve cardiomyocyte differentiation in a temporally regulated manner in which Wnt3a is critical during the very early stages of differentiation to mesoderm lineages and repressive during later stages of differentiation to cardiomyocytes.6,7
Clinical Perspective p 626
Chibby (Cby) was identified in a protein-protein interaction screen (yeast Ras recruitment system) using the C-terminal region of β-catenin as bait.8 Cby is a nuclear protein that has been conserved throughout evolution. Cby competes with Tcf/Lef for binding to β-catenin and thus represses β-catenin–mediated transcriptional activation. From Northern blotting, Cby was found to be highly expressed in multiple human tissues, including skeletal muscle, kidney, liver, placenta, and heart. The identification of Cby expression in heart tissue warranted the further study of this newly identified protein during cardiac differentiation.
Embryonic stem cells (ESCs) have become a useful tool to study in vitro differentiation as a model for development. On aggregation of these cells by the “hanging drop” method, the cells differentiate into multiple lineages, including beating cardiomyocytes. Furthermore, ESCs have the potential for clinical therapy. Because these cells have a capacity for unlimited self-renewal, they may serve as a valuable source for cell replacement strategies.9 Specifically, these cells may be useful in cases such as heart disease in which the availability of donor hearts is a major limitation. The ultimate goal of ESC biology is to differentiate these cells into a pure, high-percentage population useful for cell transplantation. However, for the potential of ESCs to be realized clinically, more studies must be done to understand the molecular mechanisms controlling cell lineage specification.9
Because the Wnt/β-catenin signaling cascade has been suggested to be important for murine ESC self-renewal and the differentiation to cardiomyocytes, we sought to determine how Cby, a new player in the Wnt/β-catenin pathway, would be implicated in this process.
Methods
ESCs
The murine ESCs used were α-cardiac myosin heavy chain-EGFP (MHC-GFP) CGR8,10 α-fetoprotein (Afp)-GFP,11 Brachyury-GFP,12 and R1. MHC-GFP tetracycline-off inducible cell line was established by the cotransfection of pCAG20−1 (CAG promoter driving the Tet activator-tTA2) and pUHDIO-3 (cytomegalovirus minimal promoter with Tet-operator driving the puromycin resistance gene) into MHC-GFP ESCs and subsequent selection with puromycin. The MHC-GFP Cby-TRE–off inducible ESC line was developed by first cloning flag-tagged human Cby (hCby) into pTRE2-hyg vector (Clontech, Mountain View, Calif), which contains the Tet operator followed by a minimal cytomegalovirus promoter. pTRE2-Cby was then transfected into MHC-GFP Tet-off inducible cell line, and hygromycin B–resistant clones were selected. To develop the Cby RNAi vector, mouse Cby short-hairpin RNA (shRNA) construct was developed by subcloning the targeting sequence (5′-GTGGCAGACTCCGTGATTAGT-3′) into the pSuppressor Retro vector (Imgenex, Sorrento Valley, Calif). The Cby knockdown ESC and control cell lines were developed by transfecting the empty vector or Cby knockdown vector into R1 ESCs and subsequent selection with G418. The hCby rescue of the mouse Cby RNAi ESC line was developed by cloning flag-tagged hCby into a pCAG-hyg vector and transfection into the Cby knockdown clone and subsequent selection with hygromycin B.
ESC Maintenance and Differentiation
All ESC lines were maintained and differentiated as previously described.11,13
Flow Cytometry and Fluorescent-Assisted Cell Sorting
Differentiated cells were prepared into a single-cell suspension by treatment with 0.05% trypsin/EDTA and incubation at 37°C for 5 to 10 minutes. Flow cytometric analysis was performed with a fluorescent-assisted cell sorting (FACS) machine (Sort, Becton Dickinson, Franklin Lakes, NJ) using CellQuest Acquisition data analysis software (Becton Dickinson). Sorted cells were collected by Vantage machine (Becton Dickinson) also using CellQuest Acquisition data analysis software (Becton Dickinson). Quantification of MHC-GFP flow cytometry was determined by the ratio of GFP+ cells for each condition to the GFP+ cells for Cby-TRE+doxycycline (Dox; noninduced control) for each independent experiment.
Reverse-Transcriptase and Real-Time Polymerase Chain Reaction
Reverse-transcriptase polymerase chain reaction (RT-PCR) was performed as previously described.11 Real-time PCR reaction was performed using the TaqMan Gene Expression Assay (Applied Biosystems, Foster City, Calif) according to the manufacturer's instructions. Primers sequences are listed in the supplementary table.
Immunoblotting
Immunoblotting was performed by first lysing cells in RIPA buffer and then normalizing total protein using a Lowry assay (Bio-Rad, Hercules, Calif). Next, 20 μg to 30 μg total protein was separated on 15% SDS-PAGE gels and transferred to nitrocellulose membranes. The following primary antibodies were used: Flag M2 (Stratagene, La Jolla, Calif), actin (Santa Cruz Biotechnology, Inc, Santa Cruz, Calif), and Cby.2 Species-specific peroxidase-conjugated IgG (Santa Cruz) was used as the secondary antibody, followed by enhanced chemiluminescence detection (Amersham, Piscataway, NJ).
Immunofluorescence Staining
Immunofluorescence staining was performed as described previously.11,13
Luciferase Reporter Assays
Luciferase reporter assays were performed as described previously.13 The Cby promoter (2.0 kb) was cloned into pGL3-basic luciferase reporter vector (Promega, Madison, Wis). The Nkx2.5 and mutant R190H Nkx2.5 were cloned into pcDNA3 as described previously.14
Electrophoretic Mobility Shift Assays
Electrophoretic mobility shift assays were performed as previously described.15 Briefly, end-labeled and annealed oligonucleotides (≈50 000 cpm) were incubated with 3-fold serial dilutions of 66 ng MBP-Nkx2.5 or MBP-Nkx2.5 homeodomain fusion protein, 50 μg BSA, 0.5 μg poly(dG-dC) in 10 mmol/L HEPES, pH 8.0, 50 mmol/L KCl, 1 mmol/L EGTA, 10% glycerol, 2.5 mmol/L DTT, and 7 mmol/L MgCl2 in a 15-μL reaction for 20 minutes at room temperature and separated on a 5% polyacrylamide gel in 0.5× Tris-glycine buffer. Protein-DNA binding affinity was estimated by the protein concentration at which 50% of the DNA probe had become bound.16 The probes used here are as follows: distal, TTTGGACAAACCGAGTTAAGTGCAACAATAGTC (−1734 to −1701); mutant distal, TTTGGACAAACCGAGTTGGGTGCAACAATAGTC (−1734 to −1701); proximal, TCCAGCCTGGGTCACCACTTAACATTTTTAACACAAC (−385 to −348); and mutant proximal, TCCAGCCTGGGTCACCACCCAACATTTTTAACACAAC (−385 to −348).
Statistical Analyses
Real-time PCR, luciferase assays, and flow cytometry data are presented as means and SDs of experiments in triplicate. Data were analyzed by Student's t test or 1-way ANOVA with repeated-measures analysis and Tukey's posttest analysis. For all analyses, values of P<0.05 were considered statistically significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
The β-Catenin Antagonist Cby Is Ubiquitously Expressed in ESCs and in Early Lineage Specification but Is Gradually Downregulated During Differentiation
Cby is a recently identified nuclear protein that antagonizes the transcriptional activity of β-catenin. Because the expression pattern of Cby during embryonic development has not been elucidated, we initially studied the levels of Cby expression in undifferentiated mouse ESCs and during their in vitro differentiation. ESCs may be differentiated in vitro by forming aggregates, called embryoid bodies, on the lids of Petri dishes using the hanging drop method.17 To determine the levels of Cby expression in ESCs and during in vitro differentiation, R1 ESCs were differentiated by the hanging drop method, and cells were collected at days 0, 4, 7, 10, and 14. Total RNA was extracted, and Cby expression, along with that of other genes, was analyzed by RT-PCR. Our data suggest that Cby is expressed in ESCs but gradually decreases through 14 days of differentiation (Figure 1A). In comparison, multiple Wnts become upregulated during ESC differentiation, whereas cardiac markers Nkx2.5, β-MHC, and Mef2c begin to be expressed at day 7. Real-time PCR confirmed the gradual decrease in Cby expression during ESC differentiation and Nkx2.5 upregulation at day 7 (Figure 1B). Because Cby may be expressed in a temporal or lineage-restricted manner, we analyzed Cby expression in primitive endoderm or mesendoderm progenitor cells. To determine whether Cby is expressed in primitive endoderm, Afp-GFP ESCs were aggregated in media for 4 days. Similarly, to determine whether Cby is expressed in mesendoderm progenitors, Brachyury-GFP ESCs were differentiated in monolayer adherent culture for 4 days. All GFP-positive and -negative cells were isolated by FACS, and Cby RNA expression was detected by RT-PCR (Figure 1C and 1D). Cby was found to be expressed in all positive and negative GFP cell populations. These data suggest that Cby is expressed throughout early lineage differentiation.
Figure 1.

Cby expression patterns during ESC differentiation. A, RNA expression of Cby; cardiac markers Nkx2.5, β-MHC, and Mef2c; and various Wnts determined by RT-PCR for ESCs and embryoid bodies during differentiation. B, Real-time PCR analysis of Cby (black) and Nkx2.5 (white), normalized to β-actin, in ESCs and embryoid bodies during differentiation. Experiments were performed in triplicate and are representative of multiple experiments. Mean fold change and SDs are shown. C, Purification of primitive endoderm cells (Afp-GFP) by FACS show expression of Cby by RT-PCR in GFP+/− populations. D, Purification of mesendoderm progenitor cells (Brachyury-GFP) by FACS shows expression of Cby by RT-PCR in GFP+/− populations. *P<0.05 vs Cby at day 4, 7, 10, or 14; †P<0.05 vs Cby at day 0, 10, or 14; **P<0.05 vs all other Nkx2.5 time points (ANOVA).
High Cby Expression Is Restricted to Cardiomyocytes During Late Stages of Differentiation and Development
We next sought to determine the expression pattern of Cby during late stages of ESC differentiation. After 10 to 15 days of differentiation by the hanging drop method, ESC-derived cardiomyocytes can be detected by a spontaneous beating phenotype. To enhance visualization of the cardiomyocytes, ESCs containing a stable transgene for EGFP under the control of the cardiac promoter MHC-GFP cells may be used. Using day 15 embryoid bodies, high Cby expression was specifically colocalized only with the MHC-GFP cardiomyocytes as determined by immunocytochemistry compared with the GFP-negative cells in which Cby expression was found to be low or nonexistent (Figure 2A). It should also be noted that Cby may be able to shuttle between the nucleus and the cytoplasm (K.-I. Takemaru et al, unpublished observations, 2006). To further confirm the increased expression in cardiomyocytes compared with nonmyocytes, we performed FACS on MHC-GFP ESCs differentiated for 12 days. Real-time PCR was then used to compare the expression of Cby and Nkx2.5 in the GFP-negative and GFP-positive sorted populations (Figure 2B). Cby was determined to be 2.7-fold higher in cardiomyocytes compared with noncardiomyocytes. Together, these data suggest that Cby is expressed fairly ubiquitously during the early stages of ESC differentiation, but at later stages, high Cby expression is restricted to cardiomyocytes.
Figure 2.
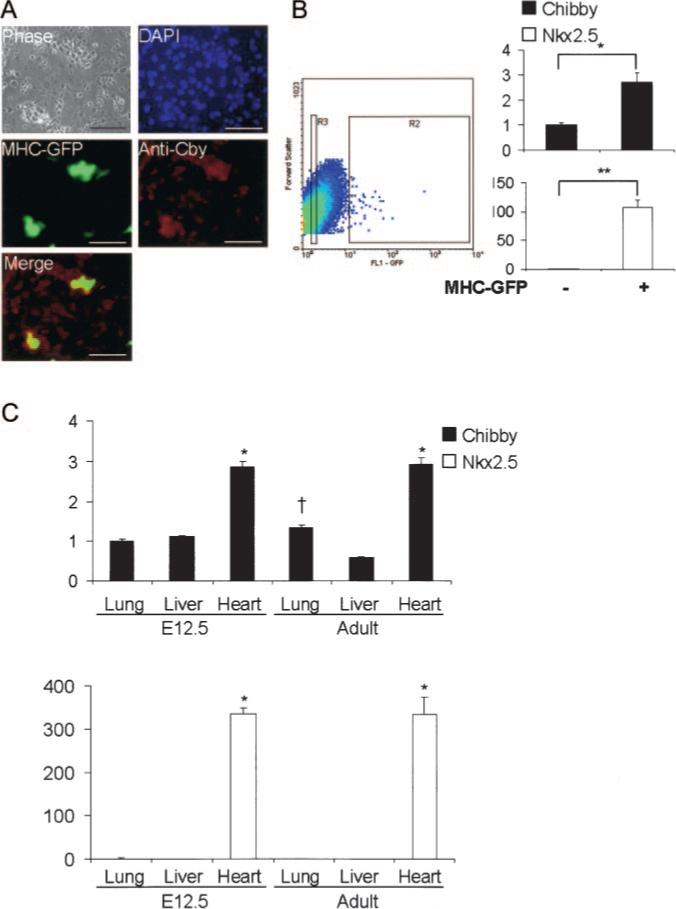
Cby is highly expressed in cardiomyocytes. A, Cby is expressed in ESC-derived cardiomyocytes by immunocytochemistry. Bar=0.1 mm. B, Purification of cardiomyocytes (MHC-GFP) by FACS shows Cby and Nkx2.5 expression increased significantly in the GFP+ cells vs GFP− cells by real-time PCR. Mean fold change of samples in triplicate and SDs are shown. *P<0.005; **P<0.001. C, Real-time PCR analysis of embryonic day 12.5 and adult mice organs shows statistically increased expression of Cby and Nkx2.5 in the heart vs lung and liver. Mean fold change of experiment in triplicate and SDs are shown. *P<0.05 vs lung and liver samples; †P<0.05 vs adult liver (ANOVA).
To determine the expression of Cby in vivo, lung, liver, and heart were isolated from E12.5 mice embryos and 8- to 10-week-old mice. RNA was extracted from the tissues, and real-time PCR was performed to determine the expression of Cby and Nkx2.5 (Figure 2C). Cby was ≈3-fold higher in both embryonic and adult heart compared with the lung and liver. These data suggest that high levels of Cby are expressed in cardiac tissue compared with other tissues.
Cby Expression Is Upregulated by the Cardiac-Specific Transcription Factor Nkx2.5
Because Cby expression appeared to be highly expressed in cardiomyocytes during late-stage embryoid body differentiation, we analyzed the Cby promoter region for any specific cardiac regulatory elements by computer in silico analysis. Within 2 kb upstream of the transcriptional start site for murine Cby, we identified 5 potential bindings site for Nkx2.5 (Data Supplement Figure I). Of these 5 sites, 2 were highly significant, with a score of 97 of 100 on the TRANSFAC database (http://motif.genome.jp/).
To determine whether Nkx2.5 can directly bind to the Cby promoter, electrophoretic mobility shift assays were performed using probes that included the 2 highly significant putative binding sites (Figure 3A). As predicted, full-length Nkx2.5 and the Nkx2.5 homeodomain were able to induce shifts for both probes with high efficiency (Kd ranged from 0.7 to 6.4×10−9 mol/L). However, neither a mutant Nkx2.5 nor the MBP alone was able to induce a shift. Interestingly, we found multiple shifts, suggesting that Nkx2.5 may bind to multiple positions within these probes. Finally, probes that contained mutated sites (AA to GG) in the Nkx2.5 consensus sequences showed significantly decreased affinity for Nkx2.5 or Nkx2.5 homeodomain binding (Kd >5.8×10−8 mol/L; data not shown).
Figure 3.
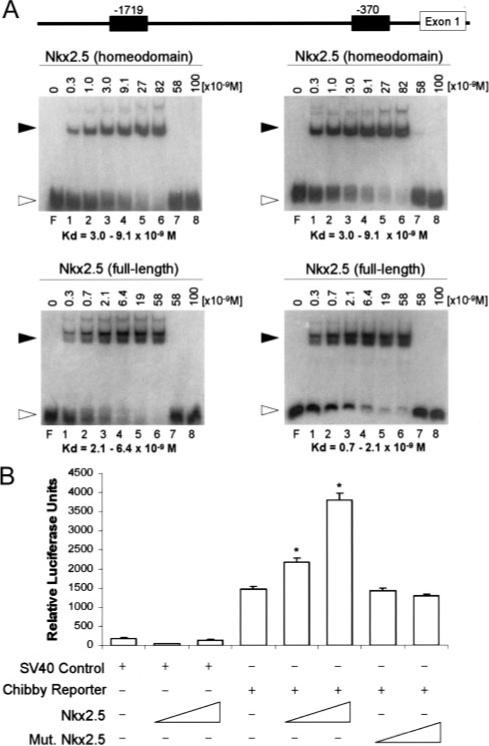
Nkx2.5 activates the Cby promoter. A, Schematic of the Cby promoter showing 2 putative binding sites for Nkx2.5 (top). Nkx2.5 can bind to the distal site (−1719) shown with 3-fold serial increasing concentrations (lanes 1 through 6) of Nkx2.5 homeodomain (middle, left) or full-length Nkx2.5 (bottom, left). Nkx2.5 can bind to the proximal site (−370) shown with 3-fold serial increasing concentrations (lanes 1 through 6) of Nkx2.5 homeodomain (middle, right) of full-length Nkx2.5 (bottom, right). Closed arrowhead indicates shifted probe; open arrowhead, free probe; F, free probe only lane; lane 7, mutated Nkx2.5; lane 8, MBP. B, Increasing concentrations of Nkx2.5 (0.6 and 1.2 μg DNA) failed to activate the SV40 control luciferase but activated Cby luciferase reporter dose dependently in NIH3T3 cells. An Nkx2.5 R190H mutant, incapable of DNA binding, does not activate the Cby reporter. *P<0.05 vs all samples (ANOVA).
To further determine whether Nkx2.5 can directly activate Cby expression, the 2-kb promoter region of mouse Cby was cloned into a luciferase reporter vector. Transfection of an Nkx2.5 expression vector activated the mouse Cby luciferase reporter in a dose-dependent manner in NIH3T3 cells (Figure 3B). Furthermore, an Nkx2.5 R190H mutant, incapable of DNA binding, failed to enhance the activity of the Cby luciferase reporter. Together, these data suggest that Nkx2.5 binds to the Cby promoter to activate its expression during cardiomyocyte differentiation.
Cby Antagonizes β-Catenin Activity in ESCs
To confirm the function of Cby as an antagonist of β-catenin signaling in pluripotent, nontransformed cells, we performed luciferase activity assays in mouse ESCs. Initially, we found that the activity of β-catenin in ESCs by the Topflash/Fopflash system (a luciferase reporter with wild-type or mutated Tcf-binding sites) was undetectable (data not shown).18 To enhance this activity, a constitutive active β-catenin vector with or without a Cby expression vector was transiently transfected into ESCs, and Topflash/Fopflash activity was measured. As expected, Cby overexpression significantly reduced β-catenin–dependent reporter activity by >3-fold (Data Supplement Figure II).
Loss of Cby Inhibits Cardiomyocyte Differentiation of ESCs
Previous studies have suggested that activation of Wnt/β-catenin signaling can inhibit cardiac differentiation.6,7,19
Consistent with these findings, activation of the Wnt/β-catenin signaling cascade during late ESC differentiation by either 5 mmol/L LiCl or 10 ng/mL rWnt 3a significantly reduced cardiomyocyte formation (supplementary Figure III). We therefore decided to test whether the loss of the β-catenin antagonist Cby also may reduce cardiomyocyte differentiation.
R1 ESCs were transfected with an siRNA vector against Cby to knock down its expression, and stable cell lines were developed. Immunoblot analysis demonstrates that Cby RNAi efficiently reduced endogenous protein levels by >80% (Figure 4A). Although Cby is expressed in ESCs, its knockdown did not seem to have any adverse effects on ESC maintenance. However, on differentiation of the Cby knockdown cell line, we found that the normal in vitro differentiation patterning between days 5 and 14 was dramatically altered. Specifically, embryoid bodies showed smaller outgrowths and fewer beating cells (Figure 4B and 4C). To confirm that the differentiation defect was due directly to the loss of Cby, hCby was re-expressed in the Cby knockdown clone (Figure 4E). hCby is not susceptible to the effect of the siRNA against the mouse Cby gene. The expression of hCby was able to rescue both the differentiation of outgrowths from the embryoid bodies and the beating cardiomyocytes (Figure 4B and 4C).
Figure 4.

Loss of Cby leads to decreased cardiac differentiation of murine ESCs. A, Western blot showing Cby was successfully knocked down by RNAi. β-Actin was used as a loading control. Densitometry analysis shows >80% reduction in Cby protein. B, In vitro differentiation of Cby RNAi clone showed severely decreased differentiation during days 5 through 14. Re-expression of hCby was able to compensate for the loss of endogenous mouse Cby and restore normal outgrowth size for embryoid bodies. Bar=1 mm. C, Percentage of embryoid bodies containing beating areas. Cby RNAi failed to develop beating areas compared with the empty RNAi vector control. Introduction of hCby compensated for loss of mouse Cby to restore beating areas. More than 25 embryoid bodies were counted per condition per day (>75 embryoid bodies per condition in total). Open bars indicate control vector; closed bars, Cby RNAi; and shaded bars, +hCby. Data are representative from multiple experiments. D, Marker expression at days 0 and 4 analyzed by RT-PCR. In Cby knockdown or rescue cells, expression of ES cell markers Oct4 and Nanog, early mesoderm marker Brachyury, and primitive endoderm markers Afp and transthyretin appeared normal. E, Cby RNAi blocks induction of cardiac markers Nkx2.5, β-MHC, and Mef2c and endodermal markers Afp, albumin, and α-antitrypsin. Expression of these markers was rescued by introduction of hCby. Neuroectodermal markers Nestin and Sox2 were upregulated in the Cby knockdown. Markers collagen II, Flk1, and cytokeratin-19 were unaffected by the knockdown of Cby. All data are representative from multiple experiments. The effect of the loss of Cby (outgrowth formation, cardiomyocyte differentiation defect, and reduced marker expression) was confirmed in 3 independent knockdown clones (data not shown).
To confirm that the loss of Cby did not affect ESC maintenance or early differentiation but did affect late stages of differentiation, RNA expression of various markers was determined by RT-PCR. Pluripotency markers Oct4 and Nanog were not affected by Cby RNAi in ESCs. Similarly, neither early mesendoderm marker Brachyury nor endoderm markers Afp and transthyretin appeared affected by the knockdown of Cby within 4 days of differentiation (Figure 4D). However, by day 12 of differentiation, the cardiac markers Nkx2.5, β-MHC, and Mef2c were downregulated in the Cby knockdown but not in the negative control cells (Figure 4E). Furthermore, expression of hCby rescued the expression of the cardiac markers. Interestingly, endoderm differentiation at day 12, as determined by Afp, albumin, and α-antitrypsin expression, was also downregulated by the Cby RNAi and rescued by hCby expression. Additionally, there appeared to be an increase in neuroectoderm differentiation in the Cby knockdown, as determined by Nestin and Sox2 expression. Finally, the chondrocyte marker collagen II, vascular endothelial marker Flk1, and epithelial marker cytokeratin 19 were unaffected by the RNAi. Together, these data indicate that Cby facilitates the formation of late-stage mesoderm/endoderm lineages such as cardiomyocytes but is not required for Brachyury-positive mesendoderm progenitors.
Cby Overexpression Promotes Cardiomyocyte Differentiation of ESCs
To investigate how ectopic expression of Cby would affect cardiac differentiation of ESCs, we developed a stable cell line overexpressing Cby using R1 ESCs as the parental line. Sustained Cby overexpression in ESCs, like the loss of Cby by RNAi, did not have any adverse effects on ESC maintenance. On differentiation of this cell line, we found an increase in the number of beating cardiomyocytes (data not shown). To further evaluate the effect of Cby on cardiomyocyte differentiation of ESCs, we developed a tetracycline-off, inducible cell line using the MHC-GFP ESCs. As shown in Figure 5A, low levels of Cby expression could be detected in the presence of Dox. However, on the removal of Dox, Cby protein expression successively increased after 24, 48, and 72 hours. The addition of Dox for 24 hours led to a rapid reduction in protein to nearly background levels. On the differentiation of these cells in the presence or absence of Dox by the hanging drop method, we found that when Cby was induced consecutively for 15 days, there was an increase in the number of GFP-positive cells (Figure 5B). To confirm this increase in cardiomyocytes, we performed flow cytometric analysis on the Cby-inducible cell line and the parental cell line, with or without Dox, after 15 days of differentiation. From multiple trials, we found that there was an ≈2-fold increase in the number of GFP+ cardiomyocytes among differentiated cells overexpressing Cby (Figure 5C and 5D). Finally, we confirmed the increased differentiation of ESCs to cardiomyocytes by checking the RNA expression of cardiac markers by RT-PCR (Figure 5E). Cardiac markers Nkx2.5, β-MHC, and Mef2c were significantly increased in the Cby-overexpressing cells compared with the nonoverexpressing cells. Other lineage markers, collagen II and Nestin, appeared unchanged. These data suggest that sustained Cby overexpression will increase cardiomyocyte differentiation without significantly affecting other cell lineages.
Figure 5.

Overexpression of Cby increases cardiac differentiation of ESCs. A, Induction and clearance of tetracycline-off inducible Cby in MHC-GFP ESCs by immunoblotting. Densitometry analysis shows a >3-fold increase after Dox removal for 72 hours and rapid reduction back to nearly background levels after 24 hours of Dox addition. B, Parental cell line (MHC-GFP TopES) with and without Dox and MHC-GFP Cby-TRE with and without Dox. Bar=0.25 mm. C, Flow cytometry analysis of parental and Cby-inducible cells. D, Average of 3 independent flow cytometry experiments normalized to Cby with Dox. E, RNA expression by RT-PCR for cardiac markers Nkx2.5, β-MHC, and Mef2c; mesoderm marker collagen II; and neuroectoderm marker Nestin. β-Actin is the loading control. *P<0.05.
To further define the time window in which Cby overexpression best increases the cardiomyocyte population, we induced Cby expression by removing Dox during the early and late stages of differentiation. Overexpression of Cby in only the first 4 days of differentiation led to a cardiomyocyte population of 3.8% of the total cells, which was not significantly different from the control cell population of 3.6% (data not shown). On the other hand, overexpression of Cby by Dox removal during the later stages of differentiation, days 4 through 15, led to a cardiomyocyte population of 7.0%, which represented an increase over the controls but still was less than sustained Cby overexpression through days 0 through 15, which was found to be 9.9%. These data suggest that sustained Cby overexpression throughout ESC differentiation leads to the largest increase in the cardiomyocyte population.
Discussion
Our data suggest that the β-catenin antagonist Cby facilitates cardiomyocyte differentiation from mouse ESCs. We found that ectopic expression of Cby will lead to a 2-fold increase in the number of cardiomyocytes formed. Cby overexpression did not have any adverse effects on ESC maintenance or differentiation. The expression of multiple lineage markers in the Cby-overexpressing embryoid bodies suggests that Cby was not increasing cardiomyocyte percentages by simply inducing cell death in noncardiac lineages. Indeed, the general morphology of embryoid bodies with or without Cby overexpression displayed no significant differences in the size of outgrowths, variety of cell lineages that developed, or total number of cells. These findings are in contrast to other reports of an increase in the purity of cardiomyocytes formed but not necessarily the total percentage of cardiomyocytes by purifying progenitor cell populations or by inducing the cell death of noncardiomyocyte lineages.20,21 These data suggest that by modifying signal transduction cascades, we may influence the resulting lineage specification. Because the ultimate goal of ESC research is to develop a high percentage and pure population of specific lineage cells useful for transplantation, genetic manipulation by overexpression of exogenous proteins may not be feasible. However, by understanding the signaling pathways critical to cardiomyocyte differentiation, we may then use specific factors or chemicals to influence these pathways and improve the differentiation. To this aim, we have attempted to use several chemicals previously reported to directly inhibit the interaction between β-catenin and Tcf.22–24 Unfortunately, the chemicals tested so far, Sulindac, Quercetin, and EGCG, have proved too toxic for use in ESC culture. Recently, Wnt11 was identified as a factor to increase ESC-derived cardiomyocyte formation by 2-fold.25 Wnt11 promotes noncanonical signaling through JNK and PKC while inhibiting canonical signaling through β-catenin.26 These data therefore support our findings with Cby. Several groups have suggested a role for Wnts and β-catenin in myocyte maturation or regeneration, which may seem at odds with findings presented here.27,28 However, the role of Cby may not negate a role for Wnt or β-catenin. Wnts may serve to stabilize β-catenin to promote its role in adherens junctions to support contractility in myocytes.29 Cby may thus serve to repress the unwar-ranted β-catenin–dependent transcriptional regulation. Other groups have identified factors such as Noggin that significantly promote cardiomyocyte differentiation from ESCs.30 Noggin was found to promote differentiation preferentially in the early phases of differentiation to Brachyury+ cells and lead to a 100-fold increase in cardiomyocyte differentiation. However, it should be noted that the true percentage of cardiomyocytes within the embryoid body population was not determined in that study. The 100-fold increase was determined by immunostaining and confocal microscopy. Because embryoid bodies grow in a 3-dimensional structure, the total cardiomyocytes within the population cannot be determined unless the embryoid body is dispersed into single cells and analyzed by flow cytometry. The 100-fold increase in ESC-derived cardiomyocyte formation most likely does not represent 100% efficiency. However, by using a combination of methods such as those described by Yuasa and colleagues30 and in our study, we may further promote cardiac differentiation to reach this ultimate goal.
In contrast to our gain-of-function studies, which increased cardiac differentiation of ESCs, the loss of Cby by RNAi led to an inhibition of normal embryoid body formation, along with a strong decrease in the number of cardiomyocytes. These data, coupled with our findings that Cby expression is ubiquitous during early stages of embryoid body differentiation but that high Cby expression is found primarily in ESC-derived cardiomyocytes at late stages of differentiation, implicate Cby in the cardiac differentiation cascade. Thus, Cby likely inhibits β-catenin activity of downstream genes in precardiac mesoderm to permit the differentiation toward the cardiomyocyte lineage. Currently, it is unclear what genes that are transcriptionally regulated by the Tcf/β-catenin complex may be repressive to cardiomyocyte development.
Interestingly, in addition to the loss of cardiac differentiation by the knockdown of Cby, there was also a loss of endoderm during the late stages of differentiation (day 12) but not during the early stages of differentiation (day 4). This suggests that although Brachyury+ mesendoderm progenitors form normally, their subsequent differentiation to specified mesoderm or endoderm lineages is defective. Additionally, we found an increase in neuroectoderm marker expression in the Cby RNAi. Because β-catenin activity has been reported to be required for neural differentiation of ESCs, the loss of the β-catenin antagonist Cby may promote neural differentiation at the expense of mesoderm and endoderm differentiation.31 Alternatively, neuroectoderm lineages may simply be overrepresented because of the loss of other cell types.
The finding that the homeodomain containing protein Nkx2.5 can bind to and activate the Cby promoter is of significant interest. Nkx2.5 plays a critical role in early cardiac development in that the knockout mice are embryonic lethal at day 9 to 10 postcoitum with a strong defect in looping morphogenesis.32 Nkx2.5 also has been shown to be essential for in vitro cardiac differentiation through the use of Nkx2.5 dominant negatives in P19 embryonal carcinoma cells.33 Because Cby knockdown led to reduced Nkx2.5 expression during ESC differentiation and Cby overexpression increased Nkx2.5 expression, it is possible that Nkx2.5 and Cby may regulate each other through a positive feedback loop to enhance cardiac differentiation. The regulation of Cby by Nkx2.5 also may represent a novel mechanism by which Nkx2.5 can affect the Wnt/β-catenin signaling cascade.
Canonical Wnt signaling has been shown to be essential for primitive streak induction and mesoderm formation in vivo while also directly activating mesodermal genes such as Brachyury.34–36 Recent studies also have shown a requirement for Wnt signaling during mesoderm differentiation of ESCs.37 Our data suggest that although Cby is expressed in Brachyury+ cells, neither the overexpression of Cby nor its knockdown has a significant effect on the development of early mesodermal lineages. This apparent conflict could be delineated by the fact that Cby likely functions in a cell-type–specific manner. For example, the ectopic expression of Cby represses activation by β-catenin in ESCs, 293T, NIH3T3, and COS7 cells but not in C2C12, HeLa, HepG2, Neuro2a, and U2OS cells (H.-I. Takemaru, unpublished observations, 2006). Unidentified tissue-specific cofactors may be required for the function of Cby.
Conclusions
Our data strongly indicate that the inhibition of β-catenin signaling by Cby, most likely after the onset of Brachyury-positive mesendoderm progenitors, is a key step in forming cardiomyocytes from ESCs. Inhibition of the Wnt/β-catenin pathway, perhaps by the use of small inhibitory molecules, may provide a useful tool to increase cardiomyocyte differentiation from ESCs. By understanding the intracellular signaling pathways that control cardiomyocyte differentiation from ESCs, we may further facilitate the potential use of ESCs in cell replacement therapies for cardiovascular injury and disease.
Acknowledgments
We would like to thank Dr Gordon Keller, Mount Sinai School of Medicine, New York, NY, for the Brachyury-GFP ESCs; Dr Richard Lee, Harvard University, Boston, Mass, for the MHC-GFP ESCs; and Dr Randall Moon, University of Washington, Seattle, for the Topflash/Fopflash reporters.
Sources of Funding
This work was supported by the American Heart Association with a grant-in-aid to Dr Terada, a predoctoral fellowship to A. Singh, a Carol M. Baldwin Breast Cancer Research Award and grant NIH R01 DK073191 to Dr Takemaru, and grants AHA 03352528N and NIH R01 HL081577 to Dr Kasahara.
Footnotes
CLINICAL PERSPECTIVE Cell-based therapeutics for the treatment and repair of damaged myocardium, after injury resulting from cardiovascular disease, is one of most promising avenues in stem cell research. Embryonic stem cell–derived cardiomyocytes are considered one of the cell types with the highest potential to be useful for these therapies. However, many obstacles need to be overcome before we can take these cells to the clinical-trials stage. One such obstacle is the heterogeneity of cell types formed during the differentiation of embryonic stem cells. We believe that by understanding the molecular mechanisms and signaling pathways that control the differentiation of embryonic stem cells to cardiomyocytes, we can apply this knowledge to directly differentiate the cells to cardiomyocytes and reduce the subsequent heterogeneity during the differentiation process. We have found that Chibby, a recently identified protein antagonist of the Wnt/β-catenin signaling pathway, is an important regulator of cardiomyocyte differentiation in vitro. The loss of Chibby will reduce cardiomyocyte differentiation of embryonic stem cells, whereas increasing Chibby levels will enhance cardiomyocyte differentiation. These data suggest that antagonizing the Wnt/β-catenin pathway, probably at a stage after initial mesoderm formation, will promote cardiomyocyte differentiation in vitro, which may be useful for future cell-based therapies. By further delineating those signaling pathways that control the differentiation to cardiomyocytes, embryonic stem cell–derived cardiac replacement therapies may be achievable in the future.
Disclosures None.
Supplementary Material
References
- 1.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley AC, Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 2005;19:387–396. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barandon L, Couffinhal T, Ezan J, Dufourcq P, Costet P, Alzieu P, Leroux L, Moreau C, Dare D, Duplaa C. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation. 2003;108:2282–2289. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- 4.Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R. Formation of multiple hearts in mice following deletion of beta-catenin in the embryonic endoderm. Dev Cell. 2002;3:171–181. doi: 10.1016/s1534-5807(02)00206-x. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura T, Sano M, Songyang Z, Schneider MD. A Wnt- and beta-catenin-dependent pathway for mammalian cardiac myogenesis. Proc Natl Acad Sci U S A. 2003;100:5834–5839. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koyanagi M, Haendeler J, Badorff C, Brandes RP, Hoffmann J, Pandur P, Zeiher AM, Kuhl M, Dimmeler S. Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J Biol Chem. 2005;280:16838–16842. doi: 10.1074/jbc.M500323200. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita JK, Takano M, Hiraoka-Kanie M, Shimazu C, Peishi Y, Yanagi K, Nakano A, Inoue E, Kita F, Nishikawa S. Prospective identification of cardiac progenitors by a novel single cell-based cardiomyocyte induction. FASEB J. 2005;19:1534–1536. doi: 10.1096/fj.04-3540fje. [DOI] [PubMed] [Google Scholar]
- 8.Takemaru K, Yamaguchi S, Lee YS, Zhang Y, Carthew RW, Moon RT. Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature. 2003;422:905–909. doi: 10.1038/nature01570. [DOI] [PubMed] [Google Scholar]
- 9.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, Lee RT. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107:1912–1916. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
- 11.Hamazaki T, Oka M, Yamanaka S, Terada N. Aggregation of embryonic stem cells induces Nanog repression and primitive endoderm differentiation. J Cell Sci. 2004;117:5681–5686. doi: 10.1242/jcs.01489. [DOI] [PubMed] [Google Scholar]
- 12.Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, Kouskoff V. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 13.Minamino T, Yujiri T, Papst PJ, Chan ED, Johnson GL, Terada N. MEKK1 suppresses oxidative stress-induced apoptosis of embryonic stem cell-derived cardiac myocytes. Proc Nat Acad Sci U S A. 1999;96:15127–15132. doi: 10.1073/pnas.96.26.15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasahara H, Benson DW. Biochemical analyses of eight NKX2.5 homedomain missense mutations causing atrioventricular block and cardiac anomalies. Cardiovasc Res. 2004;64:40–51. doi: 10.1016/j.cardiores.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Kasahara H, Usheva A, Ueyama T, Aoki H, Horikoshi N, Izumo S. Characterization of homo- and heterodimerization of cardiac Csx/ Nkx2.5 homeoprotein. J Biol Chem. 2001;276:4570–4580. doi: 10.1074/jbc.M004995200. [DOI] [PubMed] [Google Scholar]
- 16.Carey J. Gel retardation. Methods Enzymol. 1991;208:103–117. doi: 10.1016/0076-6879(91)08010-f. [DOI] [PubMed] [Google Scholar]
- 17.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 18.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B. Hans Clevers Constitutive transcriptional activation by a b-catenin-Tcf complex in APC–/– colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt MM, Guan K, Wobus AM. Lithium influences differentiation and tissue-specific gene expression of mouse embryonic stem (ES) cells in vitro. Int J Dev Biol. 2001;45:421–429. [PubMed] [Google Scholar]
- 20.Kouskoff V, Lacaud G, Schwantz S, Fehling HJ, Keller G. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc Natl Acad Sci U S A. 2005;102:13170–13175. doi: 10.1073/pnas.0501672102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanno S, Kim PK, Sallam K, Lei J, Billiar TR, Shears LL., 2nd Nitric oxide facilitates cardiomyogenesis in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12277–12281. doi: 10.1073/pnas.0401557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice PL, Kelloff J, Sullivan H, Driggers LJ, Beard KS, Kuwada S, Piazza G, Ahnen DJ. Sulindac metabolites induce caspase- and proteasome-dependent degradation of beta-catenin protein in human colon cancer cells. Mol Cancer Ther. 2003;2:885–892. [PubMed] [Google Scholar]
- 23.Park CH, Chang JY, Hahm ER, Park S, Kim HK, Yang CH. Quercetin, a potent inhibitor against beta-catenin/Tcf signaling in SW480 colon cancer cells. Biochem Biophys Res Commun. 2005;328:227–234. doi: 10.1016/j.bbrc.2004.12.151. [DOI] [PubMed] [Google Scholar]
- 24.Dashwood WM, Orner GA, Dashwood RH. Inhibition of beta-catenin/Tcf activity by white tea, green tea, and epigallocatechin-3-gallate (EGCG): minor contribution of H(2)O(2) at physiologically relevant EGCG concentrations. Biochem Biophys Res Commun. 2002;296:584–588. doi: 10.1016/s0006-291x(02)00914-2. [DOI] [PubMed] [Google Scholar]
- 25.Terami H, Hidaka K, Katsumata T, Iio A, Morisaki T. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Commun. 2004;325:968–975. doi: 10.1016/j.bbrc.2004.10.103. [DOI] [PubMed] [Google Scholar]
- 26.Maye P, Zheng L, Li L, Wu D. Multiple mechanisms for Wnt11-mediated repression of canonical Wnt signaling pathway. J Biol Chem. 2004;279:24659–24665. doi: 10.1074/jbc.M311724200. [DOI] [PubMed] [Google Scholar]
- 27.Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 28.Cossu G, Borello U. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 1999;18:6867–6872. doi: 10.1093/emboj/18.24.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toyofuku T, Hong Z, Kuzuya T, Tada M, Hori M. Wnt/frizzled-2 signaling induces aggregation and adhesion among cardiac myocytes by increased cadherin–β-catenin complex. J Cell Biol. 2000;150:225–242. doi: 10.1083/jcb.150.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, Hattori F, Fukami S, Shimazaki T, Ogawa S, Okano H, Fukada K. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotech. 2005;23:607–611. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- 31.Otero JJ, Fu W, Kan L, Cuadra AE, Kessler JA. Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development. 2004;131:3545–3557. doi: 10.1242/dev.01218. [DOI] [PubMed] [Google Scholar]
- 32.Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeobox gene Nkx2−5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 33.Jamali M, Rogerson PJ, Wilton S, Skerjanc IS. Nkx2−5 activity is essential for cardiomyogenesis. J Biol Chem. 2001;276:42252–42258. doi: 10.1074/jbc.M107814200. [DOI] [PubMed] [Google Scholar]
- 34.Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 35.Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnold SJ, Stappert J, Bauer A, Kispert A, Herrmann BG, Kemler R. Brachyury is a target gene of the Wnt/beta-catenin signaling pathway. Mech Dev. 2000;91:249–258. doi: 10.1016/s0925-4773(99)00309-3. [DOI] [PubMed] [Google Scholar]
- 37.Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


