Abstract
Since the molecular cloning of the vzg-1/Edg-2/LPA1 gene, studies have attempted to characterize LPA1 receptor functionality into a single categorical role, different from the other Edg-family LPA receptors. The desire to categorize LPA1 function has highlighted its complexity and demonstrated that the LPA1 receptor does not have one absolute function throughout every system. The central nervous system is highly enriched in the LPA1 receptor, suggesting an integral role in neuronal processes. Metastatic and invasive breast cancer also appears to have LPA-mediated LPA1 receptor functions that enhance phenotypes associated with tumorigenesis. LPA1 possesses a number of motifs conserved among G protein-coupled receptors (GPCRs): a DRY-like motif, a PDZ domain, Ser/Thr predicted sites of phosphorylation, a dileucine motif, double cysteines in the tail and conserved residues that stabilize structure and determine ligand binding. The third intracellular loop of the LPA1 receptor may be the crux of receptor signaling and attenuation with phosphorylation of Thr-236 potentially a key determinant of basal LPA1 signaling. Mutagenesis data supports the notion that Thr-236 regulates this process since mutating Thr-236 to Ala-236 increased basal and LPA-mediated serum response factor (SRF) signaling activity and Lys-236 further increased this basal signaling. Here we describe progress on defining the major functions of the LPA1 receptor, discuss a context dependent dualistic role as both a negative regulator in cancer and a proto-oncogene, outline its structural components at the molecular amino-acid level and present mutagenesis data on the third intracellular loop of the receptor.
Keywords: LPA1 receptor, LPA, AKT, mutagenesis, ovarian cancer, breast cancer, ICL3
Introduction
The vzg-1/Edg-2/LPA1 receptor was the first identified [1] of a subsequent seven lysophosphatidic acid (LPA) receptors, LPA2-7, numbered in the order of their deorphaning, that bind LPA [1-7] and PPARγ, another reported LPA receptor [8]. The LPA1 receptor, having 364 amino acids, is most closely related to LPA2 and LPA3 endothelial differentiation gene (Edg) family receptors, sharing 50-57% sequence homologies. In contrast, it has no significant homology (less than 20%) with the LPA4-6 receptors [6]. Many systems are affected by LPA signaling and the LPA1 receptor can contribute to these, either directly by binding ligand, or indirectly through receptor transactivation or heterodimerization. The LPA1 receptor forms functional homo- and differential hetero-dimers with LPA1-3 receptors and the homologous sphingosine-1-phosphate receptors 1-3, GR4 and OGR1 [9], which makes it difficult to determine independent functions for LPA1 versus receptor homodimers. The LPA1 receptor belongs to a larger class of GPCRs that span the membrane seven times and connect extracellular ligands to intracellular signaling cascades, evoking functional outcomes. There are over 1000 GPCRs and these receptors are proven pharmaceutical targets because of their wide-ranging ability to affect systems and processes within the human body.
1. LPA1 receptor is abundantly expressed in CNS and functions in olfactant detection and schizophrenia
The discovery of the LPA1 receptor occurred in a cell line that resembled cortical neuroblasts with the LPA1 receptor subsequently being found highly expressed in regions of the central nervous system during embryonic neurogenesis [1]. This seems appropriate given the fact that comparing LPA receptor expression in multiple different tumor cell lines showed a consistent and predominant, although not exclusive, high level of LPA1 receptor mRNA in the central nervous system [10]. Among cells of the central nervous system, LPA1 receptor mRNA expression was highest in the spinal cord [11]. After identification of the LPA1 receptor, subsequent studies emphasized its role in postnatal development of neurons and oligodendrocytes [12]. Much work has been accomplished to better understand the relationship of the LPA1 receptor and the central nervous system. To summarize data reviewed elsewhere, LPA signaling effects numerous cells and nervous system processes, such as mobilization of intracellular calcium, neuroblast actin polymerization, changes in neuroblast and neuron conductance, growth cone collapse in neurons, proliferation in astrocytes and oligodendrocytes, chemotaxis in microglia, process retraction in oligodendrocytes, survival and migration in Schwann cells [13].
Functional aberrations demonstrated by LPA1 receptor null-mutant mice include a lack of olfactant detection or olfaction processing among post-natal surviving pups which caused an inability to locate nipples for milk suckling and therefore half of these postnatal survivors died. Other differences between null-mutant and wild-type mice included a reduced postnatal growth rate with a 30% reduction in size, blunted snouts, widely spaced eyes and altered development of the cerebral cortex [14]. Additional studies of null-mutant LPA1 receptor mice suggested that incisor overgrowth might contribute to postnatal death, especially when untreated [11]. Interestingly, the changes in basal amino acid and monoamine levels in the brains of null-mutant mice were similar to neurochemical changes that occur in schizophrenia, suggesting that mice lacking the LPA1 receptor provide a model for this disease [15]. Their reduced ability to evoke gamma-aminobutyric acid (GABA) and glutamate release from the hippocampus, dysmorphic craniofacial features and decrease in 5-HT utilization is consistent with features implicated in the pathology of schizophrenia [11, 15]. Thus, a major role of the LPA1 receptor occurs during central nervous system development and maintenance of a normal operation system.
2. The LPA1 receptor as a negative regulator of ovarian tumor progression
The LPA1 receptor is expressed in many human tumors including the lung, breast, stomach, kidney and prostate [10]. It is present in normal ovarian epithelial cells but mRNA from ovarian cancer cell lines varies dramatically [16, 17]. Data generated from gene expression microarray experiments using serous ovarian tumors at varying stages and grades of cancer show a significant reduction in the expression of the LPA1 receptor (Fig. 1). The LPA1 receptor is represented three times on this microarray and overall shows an 84% decrease in its expression when compared to benign controls in this experiment. This is in stark contrast with the LPA2 and LPA3 receptors which are dramatically increased in their expression, here 70% and 83%, respectively (Fig. 1 and [17]). Previous data from our lab indicated that overexpression of the LPA1 receptor induced apoptosis and anoikis in A2780 ovarian carcinoma and Jurkat T cells [18]. Together, these data suggest that the LPA1 receptor might be a negative regulator for ovarian epithelial cell growth.
Figure 1. The LPA1 receptor is decreased in ovarian cancer while the LPA2 and LPA3 receptors are increased.

Thirty samples from serous ovarian cancer patients and five benign controls were analyzed using Affymetrix gene expression profiling microarrays. Results were analyzed using the median from controls and an in-house clustering program developed by the Bioinformatics Department at the University of Texas M.D. Anderson Cancer Center. Hierarchical clustering demonstrates a dramatic decrease (red) in the genes in the bottom cluster: LPA1 (location indicated by asterisk*), GPR23/LPA4 and LPP3. The upper middle cluster includes genes that are increased (green): LPA2, LPA3, LPP2 and SPHK1. Bar on the bottom left indicates the outcome color scheme.
In contrast, we examined gene expression array data from five breast cancer patient sample databases for LPA1 receptor expression to determine whether similar patterns existed in breast compared to ovarian and found no trend among breast tumors (Fig. 2). Each breast cancer database consisted of at least 100 patient samples with some having over 250 samples, representing over 800 total breast cancer samples. Similarly, an analysis of data collected from compiled studies in Oncomine (www.Oncomine.org) revealed no distinct trend among existing tumors in the database with LPA1 expression (Table 1) [19-28]. This suggests that the patterns of LPA1 receptor expression between ovarian, breast and other cancer types is markedly different but it does not rule out the possibility that there is a connection between LPA1 receptor functionality and breast or any other cancer progression.
Figure 2. No major expression pattern is observed for the LPA1 receptor in breast cancer.

Over 100 samples from breast cancer patients and seven normal controls were analyzed using Affymetrix expression profiling microarrays. Results were analyzed using the median from controls and an in-house clustering program developed by the Bioinformatics Department at the University of Texas M.D. Anderson Cancer Center. Hierarchical clustering demonstrates no significant trend in the expression of the LPA1 receptor (location indicated by asterisk*), among patient samples. Results were repeated in five datasets and a representative experiment is shown here. Bar on the bottom left shows the outcome color scheme.
TABLE I.
Summary of correlation HeatMap data representing differential expression analysis in cancerversus normal controls from independent studies analyzing LPA1 mRNA
| Normal or Cancer type (no. samples) | Differential Expression |
|||
|---|---|---|---|---|
| High | Low | Neutral | Study | |
| Brain | ||||
| Normal brain (6) | 6 | 0 | 0 | [19] |
| Anaplastic Oligoastrocytoma (4) | 1 | 1 | 2 | [19] |
| Anaplastic Oligodendroglioma (23) | 4 | 10 | 9 | [19] |
| Normal brain, epilepsy (23) | 19 | 4 | 0 | [20] |
| Astrocytoma (26) | 7 | 8 | 11 | [20] |
| Glioblastoma multiforme (77) | 18 | 18 | 41 | [20] |
| Oligodendroglioma (50) | 9 | 19 | 22 | [20] |
| Normal brain (4) | 4 | 0 | 0 | [21] |
| Anaplastic Oligoastrocytoma (6) | 2 | 1 | 3 | [21] |
| Glioblastoma (31) | 11 | 8 | 13 | [21] |
| Oligodendroglioma (8) | 1 | 4 | 3 | [21] |
| Astrocytic Tumor (5) | 0 | 2 | 3 | [21] |
| Breast | ||||
| Normal (7) | 5 | 0 | 2 | [22] |
| Breast carcinoma (40) | 6 | 10 | 24 | [22] |
| Liver | ||||
| Non-tumor liver (76) | 8 | 7 | 61 | [23] |
| Benign liver disease (7) | 2 | 2 | 3 | [23] |
| Metastatic liver cancer (10) | 6 | 2 | 2 | [23] |
| Hepatocellular carcinoma (104) | 17 | 40 | 47 | [23] |
| Melanoma | ||||
| Normalskin (7) | 7 | 0 | 0 | [24] |
| Benign Nevus (18) | 16 | 0 | 2 | [24] |
| Melanoma (45) | 3 | 23 | 19 | [24] |
| Normal mole (10) | 9 | 1 | 0 | [25] |
| Primary melanoma (5) | 0 | 0 | 5 | [25] |
| Metastatic melanoma (17) | 1 | 9 | 7 | [25] |
| Ovarian | ||||
| Normal ovary (4) | 4 | 0 | 0 | [26] |
| Ovarian clear cell adenocarcinoma (8) | 0 | 6 | 2 | [26] |
| Ovarian endometrioid adenocarcinoma (37) | 8 | 7 | 22 | [26] |
| Ovarian mucinous adenocarcinoma (13) | 1 | 4 | 8 | [26] |
| Ovarian serous adenocarcinoma (41) | 9 | 13 | 19 | [26] |
| Prostate | ||||
| Normal prostate (23) | 15 | 0 | 8 | [27] |
| Prostate carcinoma (64) | 13 | 22 | 29 | [27] |
| Metastatic prostate cancer (25) | 0 | 18 | 7 | [27] |
| Seminoma | ||||
| Normal testis (6) | 0 | 6 | 0 | [28] |
| Adult male germ cell tumor (91) | 23 | 27 | 41 | [28] |
| Adult male germ cell tumor metastases (7) | 2 | 4 | 1 | [28] |
Prior to the discovery of additional LPA receptors, such as LPA4 and LPA5, the rat brain-derived neuroblastoma cells, B103, and the rat hepatoma cells, RH7777, were accepted as LPA receptor null expressing model systems. Studies now show that B103 cells express LPA4 and RH7777 cells express both LPA5 and PPARγ [29]. While these cells express only selective LPA receptors, they still maintain the genes for others. A recent publication indicated that the absence of the LPA1 receptor expression in both B103 and RH7777 rat cell lines was the result of hypermethylation in the 5′ upstream region of the gene since treatment with a DNA methyltransferase inhibitor lead to re-expression of the LPA1 receptor gene and protein [30]. Whether this occurs in other cell types and human cancers is currently unknown. It is tempting to speculate on the possibility that this mechanism is responsible for the reduced LPA1 receptor expression that happens concurrently with malignant transformation in ovarian cancer and other cell types. If the answer to this hypothesis is yes, the more intriguing question is, why does this occur?
3. GPCRs involved in pathogenesis and oncogenic signaling
Numerous examples exist of GPCRs that are agents associated with diseases such as atherosclerosis, the human immunodeficiency virus/acquired immune deficiency syndrome and cancer (Table 2) [31-44]. Some GPCRs are known oncogenes related to cancer, having the ability to transform cells, while others are suspected oncogenes awaiting definitive proof of their transforming potential. Quintessential GPCRs that possess a compelling role in the development of cancer can arise through herpesvirus infection.
TABLE II.
Examples of G protein-coupled receptors associated wit pathophysiological conditions
| GPCR | Associated pathology | Study |
|---|---|---|
| α-1 adrenergic | Prostate cancer | Canonical |
| BILF1 | Epstein-Barr encoded | [31] |
| CCR6, CCR7, CCR10 | Upregulated by Epstein-Barr virus | [32, 33] |
| CXCR2 | Transforming activity | [34] |
| FPR | Malignant glioma | [35] |
| Grm1 | Melanoma | [36] |
| GPR56 | Glioblastoma multiforme | [37] |
| GPR87 | Non-small cell lung carcinoma | [38] |
| KSHV-GPCR | Kaposi’s sarcoma and primary effusion lymphoma | [39, 40] |
| MC1R | Melanoma | [41, 42] |
| ORF74 | Lymphomas | [43] |
| PSGR | Human prostate neoplasia and prostate tumors | [44] |
Certain herpesviruses, like Kaposi’s sarcoma and Epstein Barr virus, infect the host and encode their own GPCRs which signal constitutively to activate a variety of cellular cascades, essentially “hijacking” the network system [31, 39, 45]. For example, the Kaposi’s sarcoma herpesvirus that produces aberrantly-proliferating lesions frequently observed on HIV-infected individuals encodes the KSHV-GPCR which activates an inflammatory-cytokine-like signaling pathway that contributes to angiogenesis and cellular transformation [46]. Similarly, the Epstein-Barr virus is well known to cause infectious mononucleosis, Burkitt’s lymphoma, Non-Hodkin’s lymphomas and Hodkin’s disease, among other diseases. Resembling Kaposi’s sarcoma, the Epstein-Barr virus encodes it own GPCR, BILF1, which is constitutively active and likely precipitates disease progression [31]. Interestingly, infecting Hodgkin lymphoma cells with the Epstein-Barr virus results in autotaxin induction [47], the enzyme that produces LPA.
Infection with the Epstein-Barr virus also leads to the upregulation of several chemokine receptors [32, 33] that are presumed to facilitate cellular transformation by enhancing proliferation, migration and cell survival [45]. Affymetrix microarray expression profiling has improved the discovery of aberrant GPCR expression within patient tissues, uncovering unexpected changes in both orphan and characterized GPCRs, potentially leading to the identification of easily-accesible (GPCR) drug targets overexpressed on the surface of tumorigenic cells. Examples of upregulated GPCRs include human gliomas that overexpress GPR56 [37], prostate tumors that overexpress prostate-specific G-protein coupled receptor [44] and ovarian cancers that overexpress the LPA2 and LPA3 receptors [17]. Another connection between specific GPCRs and other oncogenic pathways is the cross-talk between epidermal growth factor (EGF) receptors whereby GPCRs can increase activation of the receptors either through release of membrane anchored growth factors or through intracellular cross talk.
4. The LPA1 receptor is a proto-oncogene in breast cancer
Studies have hypothesized roles for GPCRs in disease progression where mounting evidence supports aberrant receptor expression or signaling. Accumulating evidence supports the hypothesis that the LPA1 receptor contributes to the metastatic capability of breast cancers, but thus far not to the development of ovarian cancers. To this extent, whether the LPA1 receptor is a negative or positive mediator contributing to tumor progression likely depends on the specific cell or tumor type. Results from a comprehensive screen to determine the functional consequences of overexpressing 1000 breast cancer candidate cDNAs in immortalized MCF-10A parental and derivative cells with an inducibly active variant of the ErbB2/HER2 receptor, revealed that the LPA1 receptor exhibited potent proto-oncogene-like properties [48]. The inducible system was used during migration assays and employed derivative MCF-10A.B2 cells expressing a chimeric ErbB2/HER2 receptor activated through dimerization mediated by a small molecule (AP1510). The ErbB2/HER2 receptor is an oncogenic tyrosine kinase receptor which is amplified in 15% of all breast cancers and prior to the advent of the targeted therapeutic antibody Herceptin was correlated with poor prognosis [49]. In this study examining breast carcinogenesis, the LPA1 receptor scored positively in three assays: proliferation, morphogenesis and migration. The authors used a 3-dimensional acinar morphogenesis assay and noted that the LPA1 receptor induced large, protrusive, disorganized acinar structures, features that resembled invasive tumor cells. In addition, because the receptor induced migration in the absence of ErbB2/HER2 activation but not in the absence of the ErbB2/HER2 inducible system, the data suggests the LPA1 receptor may require weak signals from ligand-independent dimerization of ErbB2/HER2 to induce migration. [48] In this study, no exogenous LPA was added in any assay indicating either that LPA is induced endogenously, present in the media or that the LPA1 receptor, at least when overexpressed, can signal in the absence of exogenous LPA. These results may unravel the disparity seen among LPA1 receptor-mediated outcomes in breast cancer. In terms of the lipid profiles in plasma of breast cancer patients, a comprehensive analysis comparing LPA, LPC, LPI, LPS, SPC and S1P levels between normal healthy controls, women with benign breast lesions and women with breast cancer failed to detect significant differences [50].
Multiple studies performed both in vitro and in vivo support the mechanistic relationship of the LPA1 receptor to motility and metastasis in breast cancer. Transcriptional down-regulation of the LPA1 receptor by the metastasis suppressor gene, Nm23-HI, demonstrated that the LPA1 receptor was critical for cell motility in several breast cancer lines and also the metastatic MDA-MB-435 cell line [51]. It is important to note that the origin of the MDA-MB-435 line is controversial as it is identical to the M14 cell line which is designated as a melanoma. A follow-up study correlated this in vitro mechanism to a suppression of in vivo metastasis by Nm23-HI inversely affecting the expression of the LPA1 receptor [52]. Other studies show that knocking-down LPA1 receptor expression or inhibiting the LPA1 receptor through a non-specific antagonist reduces human breast cancer cell metastasis to bone in murine models [53]. Taken together, these studies suggest a connection between the LPA1 receptor and LPA signaling to metastatic breast cancer.
5. Regions of the LPA1 receptor recognize LPA and stabilize its structure in the plasma membrane
Mutational structure-function studies have uncovered informative components of many different GPCRs. Each component of the GPCR functions in a combined effort to regulate ligand binding, receptor internalization (endocytosis), receptor localization to the membrane, proper folding and stabilization of the serpentine structure and G protein coupling which promotes receptor signaling. The light receptor rhodopsin is the classic example of a stable GPCR and serves as a model for other studies because it was the first GPCR to have a characterized three-dimensional crystal structure depicting its conformation in an activated state [54].
The LPA1 receptor has a total of 364 amino acids that weave back-and-forth through the plasma membrane, seven times in all, forming a slinking “serpentine”-like structure commonly used to describe GPCRs. The protein structure of the LPA1 receptor is divided into three areas in relation to the plasma membrane where it is embedded: extracellular, transmembrane-spanning (TM) and intracellular (cytoplasmic) domains (Fig. 3). Weaving through the plasma membrane seven times leaves three loops on the outside (extracellular loops - ECL1, ECL2, ECL3) and three loops on the inside of the cell-surface (intracellular loops - ICL1, ICL2, ICL3). The N and C terminals of LPA1 are extracellular and intracellular respectively. The functionality of a GPCR can be further described by three regions: a mostly-extracellular trigger region comprising amino acid residues required for ligand binding, a linking core middle region that stabilizes the TM domains for proper protein folding and an intracellular cytoplasmic coupling region that regulates G protein coupling and signaling of the receptor [55].
Figure 3. Model of the LPA1 receptor depicted in the plasma membrane.
The LPA1 receptor is shown here with all 364 amino acids represented by a letter. The acidic amino acids are red, basic are purple, neutral are green, unique and hydrophobic are blue. Asterisks next to the amino acid indicates a mention of the residue or motif in the text (i.e. ERH/DRY-like motif, NPXXY, PDZ/HSVV, etc.). Labels include the three regions: extracellular, transmembrane (TM) and intracellular and within those are loop labels for extracellular loops 1-3 (ECL1, ECL2, ECL3) and intracellular loops 1-3 (ICL1, ICL2, ICL3). The transmembrane regions TMI-TMVII are ordered from left to right.
Beginning with the trigger region, computer models and structure-function studies have determined the residues required for LPA binding to the LPA1 receptor. While 14 residues are known to affect the ligand binding of rhodopsin [55], there are at least three critical residues necessary for LPA binding to LPA1: Arg-124, Gln-125 and Lys-294 [56, 57]. Arg-124 and Gln-125 lie at the junction of the first extracellular loop (ECL1) and the TMIII while Lys-294 is in the TMVII. Of these, Arg-124 is conserved throughout many GPCRs and stabilizes the TMIII region by interacting with lipid headgroups, such as that of LPA [58]. Other ligand-binding residues conserved within the LPA1 receptor include Asn-109 (also reported Asn-112 in other receptors), Arg-116 and Asp-204, but these residues have not been verified experimentally, yet are reported to function in binding, ligand affinity and proper folding in other receptors [58]. In LPA1 the Gln-125 hydrogen bonds with the LPA hydroxyl group and interchanging this to Glu-125 allows the LPA1 receptor to bind either LPA or S1P [57]. In addition, the ECL2 is required by some GPCRs for conformational flexibility that is needed for efficient receptor activation [59]. The function of these residues within the ECL2 is unknown, but the LPA1 receptor shares this commonality at Asp-191 and Glu-193, with the vasotocin, vasopressin, isotocin, mesotocin and oxytocin receptors [58]. Thus, the critical regions responsible for LPA binding to the LPA1 receptor are found at the junctions of the ECL1 and ECL2 with TMIII and TMV, respectively, along with TMVII.
The core region functions mainly to stabilize proper GPCR structure but also segregates within the middle region and can affect GPCR expression and activity. The most conserved residues in the TM regions of GPCRs are found in TMII, TMIII and TMVII [55]. Within the TMII, the LPA1 receptor has Asp-96, which is conserved among many GPCRs [60]. TMVII in the LPA1 receptor contains Lys-294, which was mentioned previously and serves in ligand recognition. The core region of TMVII contains a conserved NPXXY (where X can substitute for any amino acid residue) sequence. In the beta-adrenergic receptor this sequence plays a role in receptor desensitization and resensitization [61 1995 Biochem J]. In the formyl peptide receptor (FPR), mutating Asn to Ala within the NPXXY motif creates an endocytosis-defective mutant that does not desensitize with this mutant activating ERK1/2 at 10-100 fold higher levels than the wild-type receptor [60]. While the role of this residues has not been tested experimentally in the LPA1 receptor, it could affect similar processes.
The purpose of the ICL1 and ICL2 in the LPA1 receptor are inferred from studies using other GPCRs. On the border of TMIII and in the ICL2, the LPA1 receptor contains a DRY-like motif, ERH. This motif is important because the basic Arg forms a stable interaction with Glu and constrains the GPCR in an inactive form. Mutations at these residues cause some GPCRs to signal constitutively and others to increase their agoinst affinity 2-10 fold adopting an “active-like” conformation [62]. A few residues past the DRY-like motif the LPA1 receptor possesses other residues, Val and Leu, which serve as G protein coupling sites in muscarinic receptors since substitutions at these residues cause defects in G protein coupling [63]. Overall by analogy with other receptors, the ICL2 region likely functions in the activation and desensitization of intracellular signaling and a general site for coupling. The ICL1 of LPA1 receptor is the smallest ICL comparatively, having only nine amino acids. This loop reportedly is required for proper receptor processing through cytosolic organelles and expression on the cell surface (in collaboration with the C-terminal tail which will be discussed later) [64].
6. The ICL3 of the LPA1 receptor is at the crux of receptor signal transduction
In the LPA1 receptor, the ICL3 is the largest of the intracellular or extracellular loops, comprising 33 amino acids (ECL2 = 20, ICL2 = 19) and likely constitutes a major component of the “business end” of this receptor. In other GPCR, the ICL2 and ICL3 loops interact to regulate functions, like glucagon-induced G protein signaling in the glucagon receptor since simultaneous replacement of both loops was required to completely abolish signal transduction [64]. In contrast, other studies suggest that the ICL3 dictates most of the coupling and signaling of GPCRs [65] and contains most of the Ser/Thr residues involved in regulation of GPCR trafficking [66] and GRK-dependent receptor desensitization [67]. In at least the M2 muscarinic acetylcholine receptor, the ICL3 is the primary site of arrestin binding to desensitize the receptor, prevent further signaling and facilitating endocytosis [68]. For the melanocortin 4 receptor, the ICL3 is critical for its constitutive and functional activity [69]. Mutating residues in the central portion of the ICL3 within the angiotensin II receptor disrupted ERK signaling mediated by the receptor [70]. Taken together, these results suggest that the ICL3 is at the crux of receptor functioning in a number of GPCRs.
Mutagenesis studies on the ICL3 in a closely-related receptor to LPA1, the sphingosine-1-phosphate 1 receptor (S1P(1R)), indicates that activated AKT binds to S1P(1R) and phosphorylates Thr-236 within the ICL3 [71]. The LPA1 receptor also has a Thr residue at this exact position in the ICL3. Sequence alignments of the LPA2 and LPA3 receptors determined that the LPA3 receptor also shares the conserved Thr at position 217, but the LPA2 receptor has a Val residue in that position. Mutating the S1P(1R) Thr-236 to Ala-236 resulted in a dominant-inhibitory S1P(1R) mutant, causing a reduction in S1P-mediated Rac activation, chemotaxis and angiogenesis without altering the association with AKT [71]. Furthermore, an activated and constitutively-active AKT bound to S1P(1R), even in the absence of S1P ligand, with a dominant-inhibitory AKT not being able to associate with the receptor [71].
In light of these results and our own predictive models suggesting Ser and Thr residues within the ICL3 had a high probability of phosphorylation and receptor signaling, we created mutant LPA1 receptor constructs. We mutated Thr-236, Ser-240, Ser-243 and Ser-244 to Ala-236 (T236A), Ala-240 (S240A) and Ala-243/Ala-244 (S243/244A) in order to prevent phosphorylation at these sites and determine the effect on LPA-mediated serum response factor (SRF) promoter activity using a SRE luc plasmid as a read-out for receptor signaling with these constructs. We manipulated the HepG2 liver cell line, which in our hands did not express the LPA1 receptor and did not respond to LPA. All mutant receptors were checked for equivalent expression levels, proper cell-surface targeting and endocytosis (data not shown). Of these constructs, only the Thr-236 to Ala-236 (T236A) showed a significant change in SRF activation, increasing normal receptor activity by 1.5 fold even in the absence of added LPA ligand (Fig. 4). Thus, phosphorylation at Thr-236 may be critical for dampening receptor signaling; since this residue affects signaling outcomes and Ala-236 substitutions (which prevent phosphorylation) increase SRF signaling activity.
Figure 4. LPA1-T236A exhibits elevated basal signaling activity in comparison to wild-type LPA1.
HepG2 cells were transiently transfected in serum-free medium with the SRF reporter plasmid, pSRE-luc, and the transfection control plasmid, pRL-TK, plus the indicated FLAG-tagged LPA1 expression plasmids. Twenty-four hours after transfection, cells were stimulated with or without 1 μM LPA for 16 h prior to detection of luciferase activity using a dual luciferase assay. The data above was expressed as the mean ± S.E. (n=3) of the ratio of induced firefly luciferase activity (in relative light units, RLU) to constitutive renilla luciferase activity (this is a measure of transfection efficiency). Similar results were obtained in six independent experiments. **P<0.01, comparing the SRF activity between LPA1- and LPA1 T236A-transfected cells in the absence of agonist and LPA1- and LPA1 T236A-transfected cells in the presence of agonist.
Further data in support of this hypothesis was attained during subsequent mutagenesis experiments. In addition to the T236A mutant, we created three mutants that would introduce a positive charge (T236K), mimic phosphorylation (T236D) or introduce a bulky base (T236F) at this position. Changing the Thr-236 to Asp-236, T236D, in order to mimic phosphorylation very slightly elevated untreated SRE activity above the wild-type LPA1 receptor, but treatment with LPA was unable to induce further receptor signaling activation (Fig. 5A). This reinforces the importance of Thr-236 in the regulation of LPA1 signaling and raises the possibility that phosphorylation of this residue negatively modulates receptor signaling. The bulky base introduction, T236F, did not change SRE activity from the wild-type LPA1 receptor. Interestingly, introducing a positive charge with Lys at 236, T236K, increased receptor activation 2 fold above wild-type and further increased activity with LPA stimulation. A dose response experiment comparing the LPA1 receptor wild type to T236A and T236K mutants demonstrated these mutants’ ability to increase SRF activation beyond that of the wild-type LPA1 receptor with increasing doses of LPA (Fig. 5B). Taken together, our data suggest that Thr-236 is a critical residue in the ICL3 that mediates signal transduction and attenuation for the LPA1 receptor.
Figure 5. Both LPA1 T236A and LPA1 T236K exhibit increased basal SRF activity relative to wild-type LPA1.
(A) HepG2 cells were transiently transfected in serum-free medium with the SRF reporter plasmid, pSRE-luc, and the transfection control plasmid, pRL-TK, plus the indicated FLAG-tagged LPA1 expression plasmids. Twenty-four hours after transfection, cells were stimulated with or without 1 μM LPA for 16 h prior to detection of luciferase activity using a dual luciferase assay. The data above was expressed as the mean ± S.E. (n=3) of the ratio of induced firefly luciferase activity (in relative light units, RLU) to constitutive renilla luciferase activity (this is a measure of transfection efficiency). Similar results were obtained in four independent experiments. **P<0.01, comparing the SRF activity between LPA-treated and untreated cells for each expression condition showing a statistical difference: LPA1 T236K, LPA1 T236A, and LPA1 T236F. (B) HepG2 were transfected as described above and then incubated with 0.01 μM, 0.1 μM, 1 μM, 10 μM LPA for 16 h prior to detection of luciferase activity for the transfection conditions: control (Bluescript), diamond; FLAG-LPA1, square; FLAG-LPA1 T236A, triangle; FLAG-LPA1 T236K, ex. Shown is a representative example of three experiments, which gave similar results; the data is expressed as the mean ± S.E. (n=3).
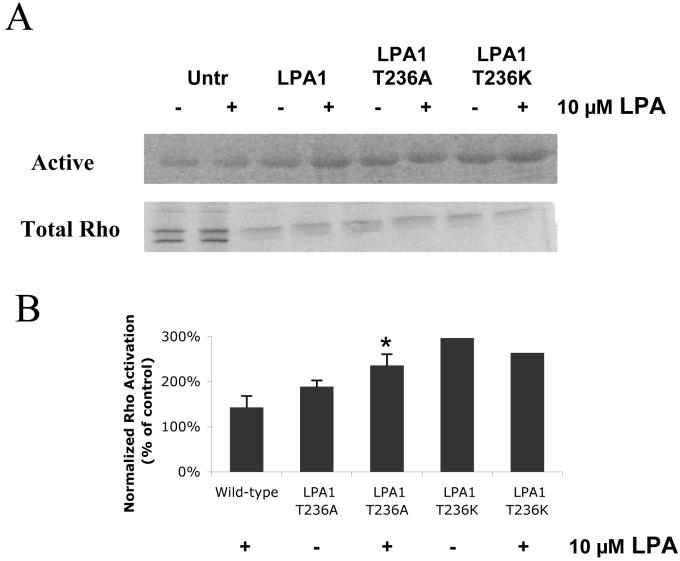
LPA stimulation of SRF is mediated primarily through the G protein Gα13, which directly activates the small GTPase, RhoA [72]. We next tested the effects of these LPA1 mutants on RhoA activation. Previous data indicated that the dominant inhibitory Rho construct, T19N, completely inhibited LPA induction of SRF in OVCAR-3 ovarian cancer cells [73]. This indicates that Gα13/Rho signaling is likely the predominant pathway for SRF activation. Indeed, both the LPA1 receptor mutants T236A and T236K demonstrated an increase in the basal activation of RhoA when compared to controls; addition of LPA did not further increase RhoA activation (Fig. 6). These results support the idea that Thr-236 is a critical determinant in regulating the basal activity of LPA1 potentially through alter coupling to RhoA.
Figure 6. Both LPA1 T236A and LPA1 T236K exhibit elevated basal activation of RhoA.
(A) Rh7777 cells were transfected with no plasmid (Untreated), wild-type LPA1, LPA1 T236A or LPA1 T236K plasmid in serum-free medium. Forty-eight hours after transfection, these cells were treated with or without 10 μM LPA for 30 min. Cell extracts were prepared and incubated with glutathione beads that were pre-bound with GST-Rhotekin-RBD fusion protein to isolate Rho-GTP. The levels of Rho-GTP present in the cell extracts (active) as well as the levels of total Rho present in these samples were analyzed by western blotting. The blot presented is from a representative experiment that was repeated three times with the same results. (B) The band intensities of Rho-GTP in the samples in (A) were quantified and normalized to intensities of total Rho in three separate experiments. Bars show the average result of pooled data from the three experiments for wild-type and LPA1 T236A and two for LPA1 T236K. *p<0.05 for experimental groups vs. wild-type LPA1 (no LPA treatment).
7. The C-terminal tail and PDZ domain aid receptor trafficking
Precise biosynthesis is necessary for a GPCR to function in cell signaling. The receptor must be synthesized, processed and folded by intracellular machinery, then escape from the endoplasmic reticulum and be transported to the cell surface where it can bind extracellular ligand. Several sites within the GPCR are reported to regulate targeting to the cell surface. In ECL3 between the borders of TMVI and TMVII, the LPA1 receptor has two conserved Asp residues. In the vasopressin receptor, mutating the Asp to Ala reduces cell-surface expression by nearly 50% [58]. The C-terminal tail of the LPA1 receptor also contains two putative dileucine tail motifs; one that is proximal to the canonical di-cysteine palmitoylation motif (a.a. 328-329) with an upstream Glu residue and a second distal dileucine motif (a.a. 352-353) that is downstream of a serine-rich domain (a.a. 341-347). The proximal dileucine motif is similar to the sequence, ELRSLLCC, found in the vasopressin receptor, which aids this receptor in escaping from the endoplasmic reticulum and targets it to the cell surface [74].
The function of the double cysteine residues in the C-terminal tail of the LPA1 receptor is unclear. The double cysteines are presumed to be the palmitoylation site based on other class A GPCRs; however, there is no formal proof that these sites are palmitoylated. If they are in fact palmitoylated, the acyl tails of the palmitate will insert into the cytoplasmic leaflet of the plasma membrane to form a pseudo fourth intracellular loop. Otherwise, the double cysteines aid post-biosynthesis receptor transport since mutating these residues in other GPCRs reduced receptor transport to the cell surface significantly [75]. Disulfide bonds can form between extracellular cysteine residues, creating an internal scaffold within GPCR structure to aid conformation and stabilize the structure [76].
Finally, at the C-terminal tail end of the LPA1 receptor lies a Class I PDZ-binding motif, HSVV, that could interact with PDZ-domain containing proteins in the cytosol. PDZ domain-mediated protein interactions are important to promote rapid recycling of endocytosed GPCRs back to the cell surface, sorting through this recycling pathway and GPCR signal regulation [77]. Recent work determined that removing the PDZ motif from the LPA1 receptor caused an increase in signaling of AKT, GSK3β and an increase in the rate of cell proliferation, resulting in a constitutively-active mutant [78]. Thus far, the reported interaction between the LPA1 receptor’s PDZ domain and intracellular proteins include PDZ-RhoGEF and RhoGEF (LARG) [79]. The only other reported data is the inability of the LPA1 receptor to interact with the PDZ domains of MAGI-3 [80]. This is in contrast to LPA2 receptor PDZ binding motif, DSTL, that interacts with NHERF2 [81], PDZ-RhoGEF [79], RhoGEF (LARG) [79], MAGI-3/PDZ5, MAGI-2/PDZ5, NHERF1/PDZ1 and neurabin [80].
8. Phosphorylation and desensitization of LPA1
Multiple post-translational mechanisms appear to regulate the function of LPA1 including those that impact its phosphorylation and desensitization state as well as those that regulate its intracellular trafficking and cellular localization. Like other Class-A GPCRs, LPA1 signaling is desensitized through both homologous and heterologous mechanisms [82]. Avendano-Vasquez et al. [82] first demonstrated that GFP-tagged LPA1, when expressed in rat hepatic C9 cells, is phosphorylated in response to LPA and phorbol ester stimulation. Indeed, phorbol 12-myristate 13-acetate (PMA) pre-treatment inhibited subsequent LPA1-induced calcium mobilization suggesting that LPA1 can undergo heterologous desensitization. This was further supported by the finding that both bradykinin and angiotensin-II stimulation promoted LPA1 phosphorylation. [82] In a recent follow-up to this work, studies showed that 17β-estradiol, acting through the estrogen receptor-α, leads to heterologous desensitization, phosphorylation, and internalization of LPA1 in both C9 cells and in ERα+ T47D human breast cancer cells [83].
β-arrestins are involved in agonist-induced desensitization and internalization of many GPCRs [84]. We previously demonstrated that β-arrestins were critical for both receptor desensitization and endocytosis of LPA1 in response to agonist stimulation [85]. LPA stimulated phosphoinositide hydrolysis was greatly elevated in transiently transfected β-arrestin 1/2 double knockout mouse embryo fibroblasts (MEFs) that express LPA1 when compared to wild-type MEFs. Re-expression of either β-arrestin-1 or 2 into the knockout MEFs restores LPA1 signaling to levels observed in wild type MEFs. Thus, LPA1 activity is subject to both homologous and heterologous desensitization.
9. Endocytic trafficking of LPA1
Many GPCRs are rapidly internalized into cells through endocytosis following agonist stimulation. Wang et al. [57] first showed that LPA1 is internalized in HEK cells following LPA stimulation, but not sphingosine-1-phosphate stimulation. We later showed that LPA1 is rapidly internalized in HeLa cells after agonist stimulation and delivered to transferrin receptor-positive endosomes through β-arrestin and clathrin-dependent endocytosis [85, 86]. Removal of LPA promotes LPA1 recycling back to the cell surface after acute agonist stimulation (e.g., 0.5-1 hr) [86]. As previously mentioned, studies have shown that PMA stimulation promotes LPA1 internalization and that receptor phosphorylation is required for internalization [82].
Recent studies indicate that the serine-rich domain (a.a., 341-347) within the C-terminal tail is critical for LPA1 association with β-arrestin [87]. Thus, both signal attenuation and LPA-dependent internalization is severely inhibited in an LPA1 mutant that is truncated to remove this Ser-rich domain, along with the distal di-leucine motif (a.a. 352-353) and PDZ-binding domain. Interestingly, neither the Ser-rich domain nor β-arrestin is required for PMA-induced endocytosis of LPA1. Instead, the distal di-leucine motif and clathrin AP-2 adaptor proteins are essential for PMA-induced internalization of LPA1. A truncation mutant lacking the distal di-leucine motif and PDZ-binding domain, but which retains the Ser-rich domain, is internalized in response to LPA but not PMA and can associate with β-arrestin. Taken together, one might speculate that homologous and heterologous desensitization of LPA1 is mediated by distinct mechanisms that utilize different kinases and adaptor proteins.
10. Nuclear localization and function of LPA1
One of the most intriguing observations about LPA1 is the finding that this receptor is also present within the nucleus and/or nuclear envelope of mammalian cells [88-90]. Gobeil et al. [90] first showed that a pool of LPA1 is constitutively localized in the nucleus of porcine cerebral microvascular endothelial cells, rat hepatoma cells, and in rat liver tissue. Stimulation of isolated nuclei with LPA led to calcium mobilization and increased transcription of cyclooxygenase-2 and inducible nitric oxide synthase genes in a pertussis toxin and PI3K-dependent manner. Interestingly, co-immunoprecipitation experiments and plasma membrane fractionation experiments indicated that LPA1 was present within both caveolin-1 enriched and clathrin-enriched fractions. This is consistent with our previous observations that cholesterol is required for LPA1 stimulation of phosphoinositide hydrolysis and for its association with β-arrestin, which directs the receptor to clathrin-coated pits for endocytosis [85].
Further evidence for a role for LPA1 signaling in the nucleus comes from the observations that in rat PC12 neuronal cells, endogenous LPA1 is associated with the nerve growth factor receptor, TrkA, and upon stimulation with either nerve growth factor (NGF) or LPA1 these receptors translocate to the nucleus [88, 89]. LPA1 potentiates NGF-induced MAPK activation in a temporal fashion [88]. Whereas LPA stimulation of MAPK is rapid but transient (e.g., maximal stimulation between 5 and 10 min and little activation after 30 min), NGF stimulation of MAPK is robust only after 10 to 30 min. Stimulation with sub-maximal doses of NGF and LPA leads to a synergistic and robust stimulation of MAPK that is apparent after 5 min. Recent work indicates that integrin signaling is critical for the maintenance of LPA1 within the nucleus [89]. Disruption of integrin-matrix association using Arg-Gly-Asp-Ser (RGDS) peptides greatly reduces nuclear LPA1 localization. Most interestingly, LPA stimulation of isolated nuclei from PC12 cells leads to changes in the Ser/Thr and Tyr phosphorylation pattern of several proteins [89]. Isolation and characterization of these proteins should shed more light on the physiological functions of nuclear LPA1 receptors. Evidence was also provided, which suggests that cell-surface LPA1 can translocate to the nucleus upon agonist stimulation [89]. It will be very interesting to investigate the intracellular trafficking pathways involved in this cell surface-to-nucleus transport of LPA1. One can envision a variety of mechanisms ranging from direct translocation of LPA1-containing vesicles into the nucleus, which is not commonly observed, to retrograde transport from endosomes through the Golgi and back to the endoplasmic reticulum and nuclear envelope localization. Indeed, certain pathogenic toxins such as Shiga toxin and ricin have been shown to traverse the endocytic and secretory pathways to the endoplasmic reticulum followed by release into the cytoplasm from the endoplasmic reticulum [91].
11. Future directions
One of the difficulties in defining the functions of individual LPA receptors is that most cells express multiple receptor isoforms. The development of LPA receptor-selective agonists and antagonists as well as siRNA approaches offer the possibility to “tease” out the functions of individual receptors. This however is challenged by potential ligand-independent functions of LPA1 and potential indirect activation of LPA1 through ligand-dependent activation of other GPCRs in heterodimeric complexes. Many antagonists have been developed against LPA receptors and the enzymes involved in LPA biosynthesis and we summarized those compounds elsewhere [92]. Most of these have not been rigorously tested in animal models to determine potential monotherapeutic or combinatorial functionality. Whether specific compounds that target the LPA1 receptor have utility against a wide range of cancer subtypes is unknown. Data suggest that in at least LPA/LPA1 receptor-driven breast metastasis, antagonists are sufficient to inhibit this process [93] but it is unknown what the long-term consequences of LPA1 inhibition might be.
Detailed mutagenesis studies of individual LPA receptors may also provide insights into their specific functions. It was intriguing to find that mutation of a single residue within the third intracellular loop of LPA1, Thr-236, strongly impacted the basal activity of this receptor. Whereas introduction of a neutral or positive charge strongly increased basal signaling, the introduction of a negative charge impaired receptor signaling (Fig. 4 and 5). This raises the possibility that phosphorylation of this residue by, as yet, unknown kinases modulates the extent of basal signaling through this receptor. Since, LPA3 also contains a threonine at the analogous position in the ICL3, it will be interesting to determine whether this receptor would be similarly affected by such mutations. As mentioned, GPCRs that exhibit increased basal activity, such as the KSHV GPCR, are able to transform cells [39]. Thus, it would be interesting to determine the transforming activity of the Ala-236 or Lys-236 mutants of LPA1 in cell culture and mouse models and whether activating mutations at Thr-236 exist in naturally occurring tumors.
Further understanding the post-translational mechanisms that regulate the activity of LPA1 will shed light into the specific roles that this receptor plays in a given cell type. Which types of receptors does LPA1 associate with to coordinate and integrate specific signaling networks? Given the importance of sub-cellular compartmentalization in LPA1 function (e.g., plasma membrane vs. nucleus), are there other signaling events mediated by LPA1 from endosomes? Finally, as the molecular details about the function and regulation of nuclear LPA1 are uncovered, the physiological role of this pool of receptors should become clearer. Are other LPA receptors similarly localized to the nucleus of cells? It is clear from the complexity of the functions and regulation of LPA1 that much remains to be learned about this fascinating receptor.
Acknowledgments
This work was supported by a training fellowship from the Keck Center Pharmacoinformatics Training Program of the Gulf Coast Consortia, National Institutes of Health grant No.1 T90 070109-01 (to M.M.), Georgia Cancer Coalition grant G-32-6CM (to H.R.), and National Institutes of Health grant HL-16734 (to H.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. The Journal of cell biology. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. The Journal of biological chemistry. 1998;273:7906–7910. doi: 10.1074/jbc.273.14.7906. [DOI] [PubMed] [Google Scholar]
- [3].Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. The Journal of biological chemistry. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- [4].Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. The Journal of pharmacology and experimental therapeutics. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- [5].Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. The Journal of biological chemistry. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- [6].Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochemical and biophysical research communications. 2007;363:861–866. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- [7].Pasternack SM, von Kugelgen I, Aboud KA, Lee YA, Ruschendorf F, Voss K, Hillmer AM, Molderings GJ, Franz T, Ramirez A, Nurnberg P, Nothen MM, Betz RC. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nature genetics. 2008;40:329–334. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- [8].McIntyre TM, Pontsler AV, Silva AR, Hilaire A, St, Xu Y, Hinshaw JC, Zimmerman GA, Hama K, Aoki J, Arai H, Prestwich GD. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zaslavsky A, Singh LS, Tan H, Ding H, Liang Z, Xu Y. Homo- and hetero-dimerization of LPA/S1P receptors, OGR1 and GPR4. Biochimica et biophysica acta. 2006;1761:1200–1212. doi: 10.1016/j.bbalip.2006.08.011. [DOI] [PubMed] [Google Scholar]
- [10].Kishi Y, Okudaira S, Tanaka M, Hama K, Shida D, Kitayama J, Yamori T, Aoki J, Fujimaki T, Arai H. Autotaxin is overexpressed in glioblastoma multiforme and contributes to cell motility of glioblastoma by converting lysophosphatidylcholine to lysophosphatidic acid. The Journal of biological chemistry. 2006;281:17492–17500. doi: 10.1074/jbc.M601803200. [DOI] [PubMed] [Google Scholar]
- [11].Harrison SM, Reavill C, Brown G, Brown JT, Cluderay JE, Crook B, Davies CH, Dawson LA, Grau E, Heidbreder C, Hemmati P, Hervieu G, Howarth A, Hughes ZA, Hunter AJ, Latcham J, Pickering S, Pugh P, Rogers DC, Shilliam CS, Maycox PR. LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Molecular and cellular neurosciences. 2003;24:1170–1179. doi: 10.1016/j.mcn.2003.09.001. [DOI] [PubMed] [Google Scholar]
- [12].Weiner JA, Hecht JH, Chun J. Lysophosphatidic acid receptor gene vzg-1/lpA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. The Journal of comparative neurology. 1998;398:587–598. [PubMed] [Google Scholar]
- [13].Herr DR, Chun J. Effects of LPA and S1P on the nervous system and implications for their involvement in disease. Current drug targets. 2007;8:155–167. doi: 10.2174/138945007779315669. [DOI] [PubMed] [Google Scholar]
- [14].Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13384–13389. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roberts C, Winter P, Shilliam CS, Hughes ZA, Langmead C, Maycox PR, Dawson LA. Neurochemical changes in LPA1 receptor deficient mice--a putative model of schizophrenia. Neurochemical research. 2005;30:371–377. doi: 10.1007/s11064-005-2611-6. [DOI] [PubMed] [Google Scholar]
- [16].Fang X, Gaudette D, Furui T, Mao M, Estrella V, Eder A, Pustilnik T, Sasagawa T, Lapushin R, Yu S, Jaffe RB, Wiener JR, Erickson JR, Mills GB. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Annals of the New York Academy of Sciences. 2000;905:188–208. doi: 10.1111/j.1749-6632.2000.tb06550.x. [DOI] [PubMed] [Google Scholar]
- [17].Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, Hasegawa Y, Tanyi JL, LaPushin R, Eder A, Jaffe R, Erickson J, Mills GB. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochimica et biophysica acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- [18].Furui T, LaPushin R, Mao M, Khan H, Watt SR, Watt MA, Lu Y, Fang X, Tsutsui S, Siddik ZH, Bast RC, Mills GB. Overexpression of edg-2/vzg-1 induces apoptosis and anoikis in ovarian cancer cells in a lysophosphatidic acid-independent manner. Clin Cancer Res. 1999;5:4308–4318. [PubMed] [Google Scholar]
- [19].French PJ, Swagemakers SM, Nagel JH, Kouwenhoven MC, Brouwer E, van der Spek P, Luider TM, Kros JM, van den Bent MJ, Sillevis Smitt PA. Gene expression profiles associated with treatment response in oligodendrogliomas. Cancer research. 2005;65:11335–11344. doi: 10.1158/0008-5472.CAN-05-1886. [DOI] [PubMed] [Google Scholar]
- [20].Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey R, Rosenblum M, Mikkelsen T, Fine HA. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- [21].Bredel M, Bredel C, Juric D, Harsh GR, Vogel H, Recht LD, Sikic BI. Functional network analysis reveals extended gliomagenesis pathway maps and three novel MYC-interacting genes in human gliomas. Cancer research. 2005;65:8679–8689. doi: 10.1158/0008-5472.CAN-05-1204. [DOI] [PubMed] [Google Scholar]
- [22].Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, Liao X, Iglehart JD, Livingston DM, Ganesan S. X chromosomal abnormalities in basal-like human breast cancer. Cancer cell. 2006;9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- [23].Chen X, Cheung ST, So S, Fan ST, Barry C, Higgins J, Lai KM, Ji J, Dudoit S, Ng IO, Van De Rijn M, Botstein D, Brown PO. Gene expression patterns in human liver cancers. Molecular biology of the cell. 2002;13:1929–1939. doi: 10.1091/mbc.02-02-0023.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Talantov D, Mazumder A, Yu JX, Briggs T, Jiang Y, Backus J, Atkins D, Wang Y. Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin Cancer Res. 2005;11:7234–7242. doi: 10.1158/1078-0432.CCR-05-0683. [DOI] [PubMed] [Google Scholar]
- [25].Haqq C, Nosrati M, Sudilovsky D, Crothers J, Khodabakhsh D, Pulliam BL, Federman S, Miller JR, 3rd, Allen RE, Singer MI, Leong SP, Ljung BM, Sagebiel RW, Kashani-Sabet M. The gene expression signatures of melanoma progression. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:6092–6097. doi: 10.1073/pnas.0501564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hendrix ND, Wu R, Kuick R, Schwartz DR, Fearon ER, Cho KR. Fibroblast growth factor 9 has oncogenic activity and is a downstream target of Wnt signaling in ovarian endometrioid adenocarcinomas. Cancer research. 2006;66:1354–1362. doi: 10.1158/0008-5472.CAN-05-3694. [DOI] [PubMed] [Google Scholar]
- [27].Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, McDonald C, Thomas R, Dhir R, Finkelstein S, Michalopoulos G, Becich M, Luo JH. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22:2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- [28].Korkola JE, Houldsworth J, Chadalavada RS, Olshen AB, Dobrzynski D, Reuter VE, Bosl GJ, Chaganti RS. Down-regulation of stem cell genes, including those in a 200-kb gene cluster at 12p13.31, is associated with in vivo differentiation of human male germ cell tumors. Cancer research. 2006;66:820–827. doi: 10.1158/0008-5472.CAN-05-2445. [DOI] [PubMed] [Google Scholar]
- [29].Valentine WJ, Fujiwara Y, Tsukahara R, Tigyi G. Lysophospholipid signaling: Beyond the EDGs. Biochimica et biophysica acta. 2008;1780:597–605. doi: 10.1016/j.bbagen.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tsujiuchi T, Shimizu K, Onishi M, Sugata E, Fujii H, Mori T, Honoki K, Fukushima N. Involvement of aberrant DNA methylation on reduced expression of lysophosphatidic acid receptor-1 gene in rat tumor cell lines. Biochemical and biophysical research communications. 2006;349:1151–1155. doi: 10.1016/j.bbrc.2006.08.159. [DOI] [PubMed] [Google Scholar]
- [31].Paulsen SJ, Rosenkilde MM, Eugen-Olsen J, Kledal TN. Epstein-Barr virus-encoded BILF1 is a constitutively active G protein-coupled receptor. Journal of virology. 2005;79:536–546. doi: 10.1128/JVI.79.1.536-546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Birkenbach M, Josefsen K, Yalamanchili R, Lenoir G, Kieff E. Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. Journal of virology. 1993;67:2209–2220. doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Burgstahler R, Kempkes B, Steube K, Lipp M. Expression of the chemokine receptor BLR2/EBI1 is specifically transactivated by Epstein-Barr virus nuclear antigen 2. Biochemical and biophysical research communications. 1995;215:737–743. doi: 10.1006/bbrc.1995.2525. [DOI] [PubMed] [Google Scholar]
- [34].Burger M, Burger JA, Hoch RC, Oades Z, Takamori H, Schraufstatter IU. Point mutation causing constitutive signaling of CXCR2 leads to transforming activity similar to Kaposi’s sarcoma herpesvirus-G protein-coupled receptor. J Immunol. 1999;163:2017–2022. [PubMed] [Google Scholar]
- [35].Zhou Y, Bian X, Le Y, Gong W, Hu J, Zhang X, Wang L, Iribarren P, Salcedo R, Howard OM, Farrar W, Wang JM. Formylpeptide receptor FPR and the rapid growth of malignant human gliomas. Journal of the National Cancer Institute. 2005;97:823–835. doi: 10.1093/jnci/dji142. [DOI] [PubMed] [Google Scholar]
- [36].Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, Koganti A, Zhu H, Robbins C, Makalowska I, Shin SS, Marin Y, Roberts KG, Yudt LM, Chen A, Cheng J, Incao A, Pinkett HW, Graham CL, Dunn K, Crespo-Carbone SM, Mackason KR, Ryan KB, Sinsimer D, Goydos J, Reuhl KR, Eckhaus M, Meltzer PS, Pavan WJ, Trent JM, Chen S. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nature genetics. 2003;34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- [37].Shashidhar S, Lorente G, Nagavarapu U, Nelson A, Kuo J, Cummins J, Nikolich K, Urfer R, Foehr ED. GPR56 is a GPCR that is overexpressed in gliomas and functions in tumor cell adhesion. Oncogene. 2005;24:1673–1682. doi: 10.1038/sj.onc.1208395. [DOI] [PubMed] [Google Scholar]
- [38].Gugger M, White R, Song S, Waser B, Cescato R, Riviere P, Reubi JC. GPR87 is an overexpressed G-protein coupled receptor in squamous cell carcinoma of the lung. Disease markers. 2008;24:41–50. doi: 10.1155/2008/857474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- [40].Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J, Knowles DM. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- [41].Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science (New York, N.Y. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- [42].Rees JL, Healy E. Melanocortin receptors, red hair, and skin cancer. The journal of investigative dermatology. Symposium proceedings / the Society for Investigative Dermatology, Inc. 1997;2:94–98. doi: 10.1038/jidsymp.1997.18. [DOI] [PubMed] [Google Scholar]
- [43].Guo HG, Browning P, Nicholas J, Hayward GS, Tschachler E, Jiang YW, Sadowska M, Raffeld M, Colombini S, Gallo RC, Reitz MS., Jr. Characterization of a chemokine receptor-related gene in human herpesvirus 8 and its expression in Kaposi’s sarcoma. Virology. 1997;228:371–378. doi: 10.1006/viro.1996.8386. [DOI] [PubMed] [Google Scholar]
- [44].Weng J, Wang J, Cai Y, Stafford LJ, Mitchell D, Ittmann M, Liu M. Increased expression of prostate-specific G-protein-coupled receptor in human prostate intraepithelial neoplasia and prostate cancers. International journal of cancer. 2005;113:811–818. doi: 10.1002/ijc.20635. [DOI] [PubMed] [Google Scholar]
- [45].Sodhi A, Montaner S, Gutkind JS. Viral hijacking of G-protein-coupled-receptor signalling networks. Nature reviews. 2004;5:998–1012. doi: 10.1038/nrm1529. [DOI] [PubMed] [Google Scholar]
- [46].Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- [47].Baumforth KR, Flavell JR, Reynolds GM, Davies G, Pettit TR, Wei W, Morgan S, Stankovic T, Kishi Y, Arai H, Nowakova M, Pratt G, Aoki J, Wakelam MJ, Young LS, Murray PG. Induction of autotaxin by the Epstein-Barr virus promotes the growth and survival of Hodgkin lymphoma cells. Blood. 2005;106:2138–2146. doi: 10.1182/blood-2005-02-0471. [DOI] [PubMed] [Google Scholar]
- [48].Witt AE, Hines LM, Collins NL, Hu Y, Gunawardane RN, Moreira D, Raphael J, Jepson D, Koundinya M, Rolfs A, Taron B, Isakoff SJ, Brugge JS, LaBaer J. Functional proteomics approach to investigate the biological activities of cDNAs implicated in breast cancer. Journal of proteome research. 2006;5:599–610. doi: 10.1021/pr050395r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bacus SS, Stancovski I, Huberman E, Chin D, Hurwitz E, Mills GB, Ullrich A, Sela M, Yarden Y. Tumor-inhibitory monoclonal antibodies to the HER-2/Neu receptor induce differentiation of human breast cancer cells. Cancer research. 1992;52:2580–2589. [PubMed] [Google Scholar]
- [50].Murph M, Tanaka T, Pang J, Felix E, Liu S, Trost R, Godwin AK, Newman R, Mills G. Liquid chromatography mass spectrometry for quantifying plasma lysophospholipids: potential biomarkers for cancer diagnosis. Methods in enzymology. 2007;433:1–25. doi: 10.1016/S0076-6879(07)33001-2. [DOI] [PubMed] [Google Scholar]
- [51].Horak CE, Lee JH, Elkahloun AG, Boissan M, Dumont S, Maga TK, Arnaud-Dabernat S, Palmieri D, Stetler-Stevenson WG, Lacombe ML, Meltzer PS, Steeg PS. Nm23-H1 suppresses tumor cell motility by down-regulating the lysophosphatidic acid receptor EDG2. Cancer research. 2007;67:7238–7246. doi: 10.1158/0008-5472.CAN-07-0962. [DOI] [PubMed] [Google Scholar]
- [52].Horak CE, Mendoza A, Vega-Valle E, Albaugh M, Graff-Cherry C, McDermott WG, Hua E, Merino MJ, Steinberg SM, Khanna C, Steeg PS. Nm23-H1 suppresses metastasis by inhibiting expression of the lysophosphatidic acid receptor EDG2. Cancer research. 2007;67:11751–11759. doi: 10.1158/0008-5472.CAN-07-3175. [DOI] [PubMed] [Google Scholar]
- [53].Boucharaba A, Serre CM, Guglielmi J, Bordet JC, Clezardin P, Peyruchaud O. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9643–9648. doi: 10.1073/pnas.0600979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science (New York, N.Y. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- [55].Madabushi S, Gross AK, Philippi A, Meng EC, Wensel TG, Lichtarge O. Evolutionary trace of G protein-coupled receptors reveals clusters of residues that determine global and class-specific functions. The Journal of biological chemistry. 2004;279:8126–8132. doi: 10.1074/jbc.M312671200. [DOI] [PubMed] [Google Scholar]
- [56].Sardar VM, Bautista DL, Fischer DJ, Yokoyama K, Nusser N, Virag T, Wang DA, Baker DL, Tigyi G, Parrill AL. Molecular basis for lysophosphatidic acid receptor antagonist selectivity. Biochimica et biophysica acta. 2002;1582:309–317. doi: 10.1016/s1388-1981(02)00185-3. [DOI] [PubMed] [Google Scholar]
- [57].Wang DA, Lorincz Z, Bautista DL, Liliom K, Tigyi G, Parrill AL. A single amino acid determines lysophospholipid specificity of the S1P1 (EDG1) and LPA1 (EDG2) phospholipid growth factor receptors. The Journal of biological chemistry. 2001;276:49213–49220. doi: 10.1074/jbc.M107301200. [DOI] [PubMed] [Google Scholar]
- [58].Hawtin SR, Simms J, Conner M, Lawson Z, Parslow RA, Trim J, Sheppard A, Wheatley M. Charged extracellular residues, conserved throughout a G-protein-coupled receptor family, are required for ligand binding, receptor activation, and cell-surface expression. The Journal of biological chemistry. 2006;281:38478–38488. doi: 10.1074/jbc.M607639200. [DOI] [PubMed] [Google Scholar]
- [59].Scarselli M, Li B, Kim SK, Wess J. Multiple residues in the second extracellular loop are critical for M3 muscarinic acetylcholine receptor activation. The Journal of biological chemistry. 2007;282:7385–7396. doi: 10.1074/jbc.M610394200. [DOI] [PubMed] [Google Scholar]
- [60].Gripentrog JM, Jesaitis AJ, Miettinen HM. A single amino acid substitution (N297A) in the conserved NPXXY sequence of the human N-formyl peptide receptor results in inhibition of desensitization and endocytosis, and a dose-dependent shift in p42/44 mitogen-activated protein kinase activation and chemotaxis. The Biochemical journal. 2000;352(Pt 2):399–407. [PMC free article] [PubMed] [Google Scholar]
- [61].Barak LS, Menard L, Ferguson SS, Colapietro AM, Caron MG. The conserved seven-transmembrane sequence NP(X)2,3Y of the G-protein-coupled receptor superfamily regulates multiple properties of the beta 2-adrenergic receptor. Biochemistry. 1995;34:15407–15414. doi: 10.1021/bi00047a003. [DOI] [PubMed] [Google Scholar]
- [62].Rovati GE, Capra V, Neubig RR. The highly conserved DRY motif of class A G protein-coupled receptors: beyond the ground state. Molecular pharmacology. 2007;71:959–964. doi: 10.1124/mol.106.029470. [DOI] [PubMed] [Google Scholar]
- [63].Moro O, Lameh J, Hogger P, Sadee W. Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. The Journal of biological chemistry. 1993;268:22273–22276. [PubMed] [Google Scholar]
- [64].Cypess AM, Unson CG, Wu CR, Sakmar TP. Two cytoplasmic loops of the glucagon receptor are required to elevate cAMP or intracellular calcium. The Journal of biological chemistry. 1999;274:19455–19464. doi: 10.1074/jbc.274.27.19455. [DOI] [PubMed] [Google Scholar]
- [65].Senogles SE, Heimert TL, Odife ER, Quasney MW. A region of the third intracellular loop of the short form of the D2 dopamine receptor dictates Gi coupling specificity. The Journal of biological chemistry. 2004;279:1601–1606. doi: 10.1074/jbc.M309792200. [DOI] [PubMed] [Google Scholar]
- [66].Capeyrou R, Riond J, Corbani M, Lepage JF, Bertin B, Emorine LJ. Agonist-induced signaling and trafficking of the mu-opioid receptor: role of serine and threonine residues in the third cytoplasmic loop and C-terminal domain. FEBS letters. 1997;415:200–205. doi: 10.1016/s0014-5793(97)01124-1. [DOI] [PubMed] [Google Scholar]
- [67].Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacological reviews. 2001;53:1–24. [PubMed] [Google Scholar]
- [68].Lee KB, Ptasienski JA, Pals-Rylaarsdam R, Gurevich VV, Hosey MM. Arrestin binding to the M(2) muscarinic acetylcholine receptor is precluded by an inhibitory element in the third intracellular loop of the receptor. The Journal of biological chemistry. 2000;275:9284–9289. doi: 10.1074/jbc.275.13.9284. [DOI] [PubMed] [Google Scholar]
- [69].Kim DH, Shin SW, Baik JH. Role of third intracellular loop of the melanocortin 4 receptor in the regulation of constitutive activity. Biochemical and biophysical research communications. 2008;365:439–445. doi: 10.1016/j.bbrc.2007.10.170. [DOI] [PubMed] [Google Scholar]
- [70].Lehtonen JY, Daviet L, Nahmias C, Horiuchi M, Dzau VJ. Analysis of functional domains of angiotensin II type 2 receptor involved in apoptosis. Molecular endocrinology (Baltimore, Md. 1999;13:1051–1060. doi: 10.1210/mend.13.7.0303. [DOI] [PubMed] [Google Scholar]
- [71].Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, Wu M, Morales-Ruiz M, Sessa WC, Alessi DR, Hla T. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Molecular cell. 2001;8:693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- [72].Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- [73].Nguyen GH, French R, Radhakrishna H. Protein kinase A inhibits lysophosphatidic acid induction of serum response factor via alterations in the actin cytoskeleton. Cellular signalling. 2004;16:1141–1151. doi: 10.1016/j.cellsig.2004.03.006. [DOI] [PubMed] [Google Scholar]
- [74].Schulein R, Hermosilla R, Oksche A, Dehe M, Wiesner B, Krause G, Rosenthal W. A dileucine sequence and an upstream glutamate residue in the intracellular carboxyl terminus of the vasopressin V2 receptor are essential for cell surface transport in COS.M6 cells. Molecular pharmacology. 1998;54:525–535. doi: 10.1124/mol.54.3.525. [DOI] [PubMed] [Google Scholar]
- [75].Schulein R, Liebenhoff U, Muller H, Birnbaumer M, Rosenthal W. Properties of the human arginine vasopressin V2 receptor after site-directed mutagenesis of its putative palmitoylation site. The Biochemical journal. 1996;313(Pt 2):611–616. doi: 10.1042/bj3130611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cook JV, Eidne KA. An intramolecular disulfide bond between conserved extracellular cysteines in the gonadotropin-releasing hormone receptor is essential for binding and activation. Endocrinology. 1997;138:2800–2806. doi: 10.1210/endo.138.7.5233. [DOI] [PubMed] [Google Scholar]
- [77].Gage RM, Matveeva EA, Whiteheart SW, von Zastrow M. Type I PDZ ligands are sufficient to promote rapid recycling of G Protein-coupled receptors independent of binding to N-ethylmaleimide-sensitive factor. The Journal of biological chemistry. 2005;280:3305–3313. doi: 10.1074/jbc.M406934200. [DOI] [PubMed] [Google Scholar]
- [78].Shano S, Hatanaka K, Ninose S, Moriyama R, Tsujiuchi T, Fukushima N. A lysophosphatidic acid receptor lacking the PDZ-binding domain is constitutively active and stimulates cell proliferation. Biochimica et biophysica acta. 2007 doi: 10.1016/j.bbamcr.2007.11.013. [DOI] [PubMed] [Google Scholar]
- [79].Yamada T, Ohoka Y, Kogo M, Inagaki S. Physical and functional interactions of the lysophosphatidic acid receptors with PDZ domain-containing Rho guanine nucleotide exchange factors (RhoGEFs) The Journal of biological chemistry. 2005;280:19358–19363. doi: 10.1074/jbc.M414561200. [DOI] [PubMed] [Google Scholar]
- [80].Zhang H, Wang D, Sun H, Hall RA, Yun CC. MAGI-3 regulates LPA-induced activation of Erk and RhoA. Cellular signalling. 2007;19:261–268. doi: 10.1016/j.cellsig.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Oh YS, Jo NW, Choi JW, Kim HS, Seo SW, Kang KO, Hwang JI, Heo K, Kim SH, Kim YH, Kim IH, Kim JH, Banno Y, Ryu SH, Suh PG. NHERF2 specifically interacts with LPA2 receptor and defines the specificity and efficiency of receptor-mediated phospholipase C-beta3 activation. Molecular and cellular biology. 2004;24:5069–5079. doi: 10.1128/MCB.24.11.5069-5079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Avendano-Vazquez SE, Garcia-Caballero A, Garcia-Sainz JA. Phosphorylation and desensitization of the lysophosphatidic acid receptor LPA1. The Biochemical journal. 2005;385:677–684. doi: 10.1042/BJ20040891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gonzalez-Arenas A, Avendano-Vazquez SE, Cabrera-Wrooman A, Tapia-Carrillo D, Larrea F, Garcia-Becerra R, Garcia-Sainz JA. Regulation of LPA receptor function by estrogens. Biochimica et biophysica acta. 2008;1783:253–262. doi: 10.1016/j.bbamcr.2007.11.014. [DOI] [PubMed] [Google Scholar]
- [84].Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annual review of physiology. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- [85].Urs NM, Jones KT, Salo PD, Severin JE, Trejo J, Radhakrishna H. A requirement for membrane cholesterol in the beta-arrestin- and clathrin-dependent endocytosis of LPA1 lysophosphatidic acid receptors. Journal of cell science. 2005;118:5291–5304. doi: 10.1242/jcs.02634. [DOI] [PubMed] [Google Scholar]
- [86].Murph MM, Scaccia LA, Volpicelli LA, Radhakrishna H. Agonist-induced endocytosis of lysophosphatidic acid-coupled LPA1/EDG-2 receptors via a dynamin2- and Rab5-dependent pathway. Journal of cell science. 2003;116:1969–1980. doi: 10.1242/jcs.00397. [DOI] [PubMed] [Google Scholar]
- [87].Urs NM, Kowalczyk AP, Radhakrishna H. Different mechanisms regulate lysophosphatidic acid-dependent versus phorbol ester-dependent internalization of the LPA1 receptor. The Journal of biological chemistry. 2008;283:5249–57. doi: 10.1074/jbc.M710003200. [DOI] [PubMed] [Google Scholar]
- [88].Moughal NA, Waters C, Sambi B, Pyne S, Pyne NJ. Nerve growth factor signaling involves interaction between the Trk A receptor and lysophosphatidate receptor 1 systems: nuclear translocation of the lysophosphatidate receptor 1 and Trk A receptors in pheochromocytoma 12 cells. Cellular signalling. 2004;16:127–136. doi: 10.1016/j.cellsig.2003.08.004. [DOI] [PubMed] [Google Scholar]
- [89].Waters CM, Saatian B, Moughal NA, Zhao Y, Tigyi G, Natarajan V, Pyne S, Pyne NJ. Integrin signalling regulates the nuclear localization and function of the lysophosphatidic acid receptor-1 (LPA1) in mammalian cells. The Biochemical journal. 2006;398:55–62. doi: 10.1042/BJ20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Gobeil F, Jr., Bernier SG, Vazquez-Tello A, Brault S, Beauchamp MH, Quiniou C, Marrache AM, Checchin D, Sennlaub F, Hou X, Nader M, Bkaily G, Ribeiro-da-Silva A, Goetzl EJ, Chemtob S. Modulation of pro-inflammatory gene expression by nuclear lysophosphatidic acid receptor type-1. The Journal of biological chemistry. 2003;278:38875–38883. doi: 10.1074/jbc.M212481200. [DOI] [PubMed] [Google Scholar]
- [91].Sandvig K, van Deurs B. Delivery into cells: lessons learned from plant and bacterial toxins. Gene therapy. 2005;12:865–872. doi: 10.1038/sj.gt.3302525. [DOI] [PubMed] [Google Scholar]
- [92].Murph M, Mills GB. Targeting the lipids LPA and S1P and their signalling pathways to inhibit tumour progression. Expert reviews in molecular medicine. 2007;9:1–18. doi: 10.1017/S1462399407000476. [DOI] [PubMed] [Google Scholar]
- [93].Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. The Journal of clinical investigation. 2004;114:1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]