Abstract
Neurologic events during left ventricular assist device (LVAD) support are associated with significant morbidity and death. To evaluate this problem, we analyzed neurocognitive function and the frequency and incidence of neurologic events in 21 consecutive patients who were undergoing long-term support with the HeartMate® XVE LVAD (Thoratec Corporation; Pleasanton, Calif). The mean duration of LVAD support was 531 days (range, 55–1,309 d); the cumulative support time was 11,188 days (30.7 yr). No patients received anticoagulant therapy, and most received aspirin. None experienced strokes or transient ischemic attacks. Twenty patients were discharged from the hospital; 2 were later readmitted because of transient changes in neurologic status (metabolic encephalopathy) that ultimately resolved. Neurologic function, as measured by the National Institutes of Health Stroke Scale (NIHSS) and the Modified Rankin Score (MRS), was abnormal before LVAD implantation but normal 6 and 12 months after (mean NIHSS, 23.6 before vs 0 after; mean MRS, 0.68 before vs 0.18 after). Neurocognitive function, as evaluated by the Boston Naming Test, Trail Making Test part B, and Block Design Test, also improved during LVAD support. Together, these findings indicate that few neurologic events occur during long-term HeartMate XVE LVAD support in the absence of anticoagulation therapy. They also suggest that modifications made to the HeartMate LVAD since the REMATCH trial have resulted in fewer complications, and that better patient selection and supportive care have improved outcomes.
Key words: Biocompatible materials, brain injuries, cerebrovascular disorders, equipment design, heart-assist devices, heart failure/surgery/therapy, neurologic examination, thromboembolism/prevention & control, trail making test, word association tests
Neurologic events during support with a left ventricular assist device (LVAD) are of considerable concern because of the potentially negative impact on survival and quality of life. Patients on LVAD support are very susceptible to developing some form of neurologic deficit or complication during their support period. The effects of neurologic events can range from transient mild symptoms to long-lasting symptoms that necessitate prolonged rehabilitation or, in severe cases, can result in death. The most common causes of these events are thromboembolism and hemorrhagic stroke; less frequent causes are ischemia due to low perfusion, septic emboli, metabolic derangement, and air embolism.
The reported prevalence of neurologic events in LVAD-supported patients ranges from 2% to 48%, and the incidence ranges from 0.009 to 5.73 events per patient year.1,2 The main reasons for this large variability are the heterogeneity of patient populations and the significant differences in thrombogenicity among pump designs. Smooth-surfaced blood pumps—the smooth surfaces of which are interrupted at conduit and valve connection points—are extremely susceptible to thrombus development because of the technical impossibility of fabricating a system that has no surface interruptions. Thrombus development within these pumps greatly increases the risk of cerebral embolism, because any thrombus that forms will adhere weakly to the pump's internal surface and eventually embolize. Moreover, the higher levels of anticoagulation required for smooth-surfaced LVADs adds to the risk of hemorrhagic stroke. Smooth-surfaced pumps have the highest reported rates of neurologic events; in contrast, textured pumps have the lowest.
The HeartMate® XVE LVAS (Thoratec Inc.; Pleasanton, Calif) has textured blood-contacting surfaces that were uniquely designed to promote the adherence of circulating cells and the proliferation of a pseudoneointimal lining throughout the pump.3 The lower thrombogenicity of these surfaces has enabled the support of patients without anticoagulation for long periods.4 This clinically relevant feature of the HeartMate became an important factor in the decision to evaluate the device for use as destination therapy in the seminal Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial.5 In that trial, patients were not required to receive anticoagulation therapy, and the neurologic event and stroke rates were high (44% and 16%, respectively).6 However, as noted in a recent report7 on the European LionHeart Clinical Utility Baseline Study (CUBS) trial of the smooth-surfaced LionHeart LVAD, the neurologic event and stroke rates were even higher than those in the REMATCH trial (57% and 35%, respectively), even though all patients in that study received anticoagulation therapy. In the bridge-to-transplantation experience,7 similar differences were observed across a variety of pump designs.
Meanwhile, LVAD systems have been evolving. New designs are now in clinical trials, and older designs have been refined to minimize complications. Along with improvements in technology, clinicians have gained important experience in patient selection and supportive medical care, which has in turn resulted in improved outcomes. Here, we report the results of a recent retrospective study that demonstrates a very low incidence of neurologic dysfunction and consistent improvement in neurocognitive function in advanced heart failure patients who received long-term LVAD support.
Patients and Methods
Twenty-four consecutive patients with advanced heart failure were supported with the HeartMate XVE LVAD from 2003 through 2007. There were 3 deaths (12.5%) within 30 days of LVAD implantation (2 of right-heart failure and 1 of recurrent respiratory failure). The remaining 21 patients were evaluated. Nineteen patients (90%) completed both the 6- and 12-month neurocognitive evaluations.
The HeartMate XVE LVAD was implanted in standard fashion, under cardiopulmonary bypass.8 Although the HeartMate XVE can provide up to 10 L/min of continuous cardiac output, it normally operates in automatic mode. The automatic-mode setting makes the flow rate dependent upon left ventricular filling volume but also enables the level of support to vary in response to physiologic demand. For each patient in this study, the device was set to operate in automatic mode. Standard supportive agents (inotropic solutions, and vasoactive and antibiotic drugs) were administered after implantation. No anticoagulants were administered during support because of the established biocompatibility of the HeartMate's textured surfaces. However, aspirin (81 or 325 mg) or clopidogrel (75 mg) was administered daily as antiplatelet therapy. Patients intolerant to aspirin were given clopidogrel. Antiplatelet therapy was discontinued in 3 patients for up to 30 days, due to diffuse gastritis and gastrointestinal bleeding. Antiplatelet therapy was resumed in these patients once bleeding stopped.
Neurologic status and neurocognitive function were prospectively evaluated before LVAD implantation and at 6 and 12 months after implantation. These routine assessments included the National Institutes of Health Stroke Scale (NIHSS), the Modified Rankin Score (MRS), the Boston Naming Test (BNT), the Trail Making Test part B (TMT), and the Block Design Test (BDT). The NIHSS and MRS objectively measure the severity of neurologic deficits in stroke patients and the amount of disability due to motor dysfunction.9,10 The BNT, TMT, and BDT are used to evaluate general mental ability (for example, word recall, language ability, and speed of cognitive function).11-13 All of these tests were performed by an independent examiner.
Results
The study population was predominantly male (20/21; 95%), with a mean age of 60.7 years (range, 29–79 yr) and a mean body surface area of 2.09 m2 (range, 1.62– 2.74 m2). In most patients (17/21; 81%), LVAD implantation was intended as destination therapy; in all other patients (4/21; 19%), it was intended as a bridge to heart transplantation. Diagnostic indications for LVAD implantation included ischemic cardiomyopathy (12/21; 57%) and dilated cardiomyopathy (9/21; 43%). Most of the study population had either an unknown (n=2) or no prior (n=17) history of neurologic disorders. The rest had a prior history of cerebral vascular accident (n= 2).
Before LVAD implantation, the mean left ventricular ejection fraction was 0.19 (range, 0.10–0.25), the mean oxygen-consumption maximum was 9.78 L/min (range, 6.5–12 L/min), and the left ventricular end diastolic dimension was 69.9 mm (range, 54–110 mm). At the time of implantation, all patients had New York Heart Association functional class IV heart failure and were dependent upon inotropic medications.
All 21 patients recovered from the implantation procedure, and all but one were discharged from the hospital. The mean time to hospital discharge after LVAD implantation was 33.7 days (range, 12–115 d), and the average outpatient time was 522 days (range, 47–1,259 d). The mean duration of LVAD support was 531 days (range, 55–1,309 d). The cumulative support time was 11,188 days (30.7 yr), during which no strokes or transient ischemic attacks occurred. All discharged patients were able to manage their home medication and LVAD equipment without problems. Two patients (10%) subsequently experienced transient changes in mental status that necessitated readmission. In both cases, the NIHSS and MRS values were normal, and computed tomographic scans of the head were negative. There was no significant carotid stenosis.
A subgroup of 7 patients in this study had chronic atrial fibrillation, putting them at higher risk for thromboembolic events. These patients received antiplatelet therapy (aspirin, 325 mg daily) but no anticoagulants, and they experienced no neurologic events during the study.
Neurologic and neurocognitive function improved with LVAD support, as shown by routine periodic testing. The general neurologic dysfunction observed before implantation improved markedly after 6 months of support and remained so at 12 months (mean NIHSS, 23.6 before vs 0 after; mean MRS, 0.68 before vs 0.18 after) (Fig. 1). The same was also true of neurocognitive function (Fig. 2). As evaluated by BNT and BDT, neurocognitive function improved significantly after 6 months of support but then declined at 12 months, although not to preimplantation levels. Meanwhile, as evaluated by TMT, neurocognitive function continued to improve between the 6-month and 12-month assessments (mean, 167.3 sec [before] vs 145.1 sec [6 mo] vs 119.7 sec [12 mo]).
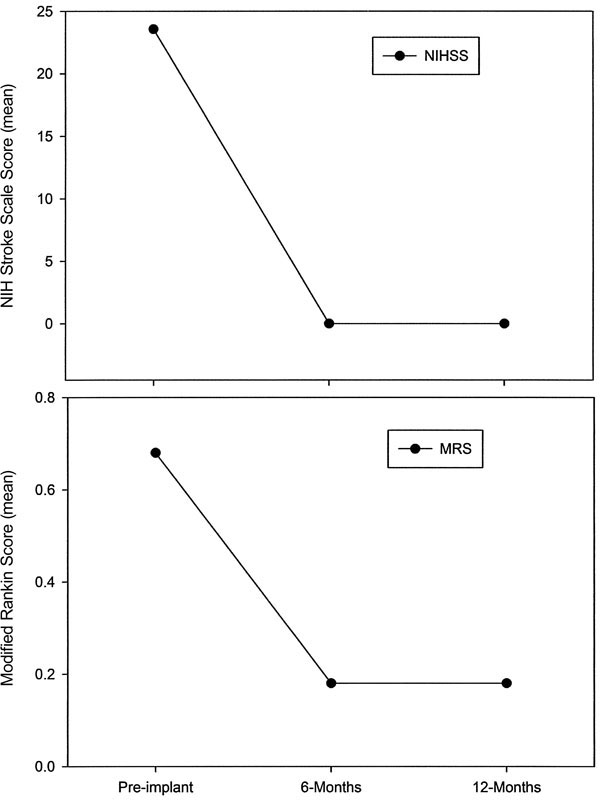
Fig. 1 Improvement in neurologic function during long-term left ventricular assist device support. The National Institutes of Health Stroke Scale (NIHSS) and Modified Rankin Score (MRS) were used to measure the severity of neurologic deficits and disability in 19 patients. The abnormal neurologic function observed before implantation had normalized by 6 and 12 months after implantation.
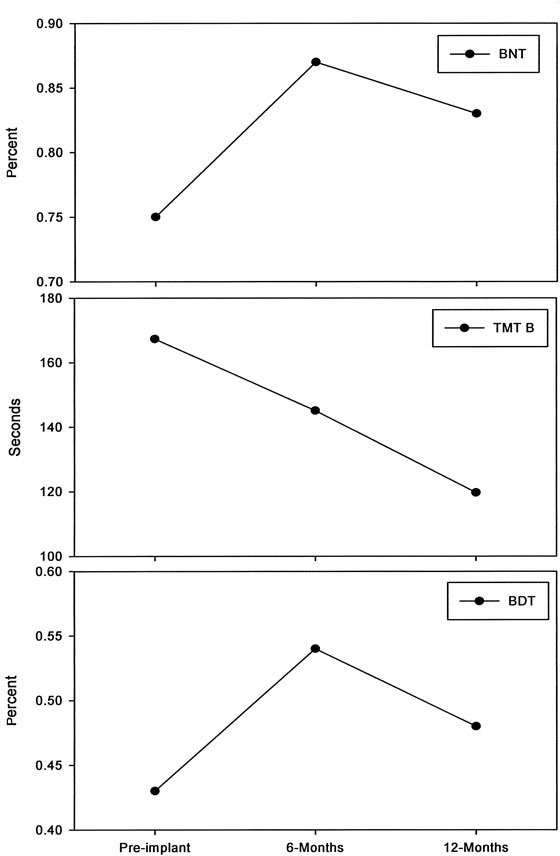
Fig. 2 Improvement in neurocognitive function during long-term left ventricular assist device support. The Boston Naming Test (BNT), Trail Making Test part B (TMT), and Block Design Test (BDT) were used to evaluate function in 19 patients. The greatest improvement was seen at 6 months after implantation.
Discussion
The results of this study indicate that patients with advanced heart failure have some neurologic dysfunction before LVAD implantation that improves with the better perfusion afforded by LVAD support. At 6 and 12 months after LVAD implantation, none of our patients showed any signs of stroke, and all exhibited normal cognitive function. The preexisting neurologic dysfunction seen in most cases was likely due to the cachexia and accompanying metabolic derangement usually associated with chronic heart failure. It is assumed that, by restoring systemic perfusion, LVAD support brought about an overall improvement in organ-system function while simultaneously correcting metabolic abnormalities.14,15
Patients in this study and those in the REMATCH trial5 were all supported with the HeartMate XVE LVAD. Overall, however, our study population experienced much lower rates of neurologic events and stroke than did the REMATCH population (9.5% vs 44% for neurologic events and 0 vs 16% for stroke).6 This important difference is likely due to modifications in the device and to improvements in patient selection and medical management that have been made since the REMATCH trial was conducted. Other post-REMATCH reports of patients undergoing long-term support with the HeartMate XVE have also established a lower incidence of complications and better survival rates.16,17
Unlike the smooth surfaces of other pumps, the textured surfaces of the HeartMate attract circulating cells that adhere to form a less thrombogenic tissue lining.18,19 With the exception of its porcine valves (which are also minimally thrombogenic), all of the HeartMate's blood-contacting surfaces are textured. This unique property makes the HeartMate the only LVAD system safe for use without systemic anticoagulation. The nonthrombogenic surface of the HeartMate XVE is associated with fewer neurologic events than are the smooth surfaces of other LVADs.
Although the probability of HeartMate mechanical failure at 2 years is reportedly as high as 64%, most such failures are manageable and rarely lead to death.20 The management of component failures can range from minor changes in external equipment to complete replacement of the device. Complete replacement involves at least as much surgery and postoperative care as does the original implantation procedure, including cardiopulmonary bypass and many days of intensive care. Recent modifications to the HeartMate include improvements in the durability and flexibility of the percutaneous lead, enhanced locking conduit connectors, the addition of an outflow graft bend relief, improvement in the diaphragm support, strengthening of the sutures that stabilize the inflow valve apparatus, and software changes to reduce transvalvular pressure gradients.16 It is assumed that all of these enhancements have helped to reduce the incidence of complications by minimizing the number of mechanical failures. Indeed, lower rates of mechanical failure will reduce the need for reoperation to repair or replace components, thereby reducing complications such as bleeding, infection, and (ultimately) neurologic events. Furthermore, it is easier to achieve and maintain adequate levels of perfusion throughout the period of support without interruptions in normal LVAD operation. None of the patients in our study required reoperation for device replacement.
The good neurologic outcomes in our study are due mainly to important lessons learned about patient care and selection during the REMATCH trial. REMATCH taught that a patient's age and stage of heart failure at the time of LVAD implantation are key determinants of outcome. The patients in our study were an average of 6 years younger than those in the REMATCH trial. The REMATCH experience also fostered the evolution of infection-prevention measures in most centers—measures that, along with driveline enhancements, have reduced infection rates. In our study, no cases of device endocarditis occurred, a factor that we believe contributed to our population's low neurologic event rate.
Meanwhile, the recent redesign of the HeartMate inflow cannula and modifications to the automatic-mode software have enabled a more consistent rate of flow over the duration of support. In this study, all patients' LVADs were maintained in automatic mode, which maximized the flow rate in response to changes in filling pressure. We believe that the modified inflow conduit has resulted in more consistent filling and more consistent flow, thereby reducing the number of episodes of diminished flow and stasis.
In conclusion, our findings indicate that the HeartMate XVE LVAD can support patients for prolonged periods with antiplatelet therapy alone, and with a very low incidence of neurologic complications. This is due to improvements in patient selection and to multiple modifications to the HeartMate XVE that have arisen from the REMATCH trial, which has resulted in better outcomes and fewer serious complications. Therefore, our study provides further evidence of the safety and effectiveness of long-term LVAD support of advanced heart-failure patients.
Footnotes
Address for reprints: Mark S. Slaughter, MD, 201 Abraham Flexner Way, Suite 1200, Louisville, KY 40202
E-mail: mscabg@aol.com
References
- 1.Copeland JG 3rd, Smith RG, Arabia FA, Nolan PE, Mehta VK, McCarthy MS, Chisholm KA. Comparison of the CardioWest total artificial heart, the Novacor left ventricular assist system and the Thoratec ventricular assist system in bridge to transplantation. Ann Thorac Surg 2001;71(3 Suppl):S92–7. [DOI] [PubMed]
- 2.McCarthy PM, Smedira NO, Vargo RL, Goormastic M, Hobbs RE, Starling RC, Young JB. One hundred patients with the HeartMate left ventricular assist device: evolving concepts and technology. J Thorac Cardiovasc Surg 1998;115(4): 904–12. [DOI] [PubMed]
- 3.Rose EA, Levin HR, Oz MC, Frazier OH, MacManus Q, Burton NA, Lefrak EA. Artificial circulatory support with textured interior surfaces. A counterintuitive approach to minimizing thromboembolism. Circulation 1994;90(5 Pt 2): II87–91. [PubMed]
- 4.Slater JP, Rose EA, Levin HR, Frazier OH, Roberts JK, Weinberg AD, Oz MC. Low thromboembolic risk without anticoagulation using advanced-design left ventricular assist devices. Ann Thorac Surg 1996;62(5):1321–8. [DOI] [PubMed]
- 5.Rose EA, Moskowitz AJ, Packer M, Sollano JA, Williams DL, Tierney AR, et al. The REMATCH trial: rationale, design, and end points. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure. Ann Thorac Surg 1999;67(3):723–30. [DOI] [PubMed]
- 6.Lazar RM, Shapiro PA, Jaski BE, Parides MK, Bourge RC, Watson JT, et al. Neurological events during long-term mechanical circulatory support for heart failure: the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) experience. Circulation 2004;109(20):2423–7. [DOI] [PubMed]
- 7.Pae WE, Connell JM, Boehmer JP, Korfer R, El-Banayosy A, Hetzer R, et al. Neurologic events with a totally implantable left ventricular assist device: European LionHeart Clinical Utility Baseline Study (CUBS). J Heart Lung Transplant 2007;26(1):1–8. [DOI] [PubMed]
- 8.Radovancevic B, Frazier OH, Duncan JM. Implantation technique for the HeartMate left ventricular assist device. J Card Surg 1992;7(3):203–7. [DOI] [PubMed]
- 9.Spilker J, Kongable G, Barch C, Braimah J, Brattina P, Daley S, et al. Using the NIH Stroke Scale to assess stroke patients. The NINDS rt-PA Stroke Study Group. J Neurosci Nurs 1997;29(6):384–92. [DOI] [PubMed]
- 10.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19(5):604–7. [DOI] [PubMed]
- 11.Suzuki M, Omori M, Hatakeyama M, Yamada S, Matsushita K, Iijima S. Predicting recovery of upper-body dressing ability after stroke. Arch Phys Med Rehabil 2006;87(11):1496–502. [DOI] [PubMed]
- 12.Zec RF, Burkett NR, Markwell SJ, Larsen DL. Normative data stratified for age, education, and gender on the Boston Naming Test. Clin Neuropsychol 2007;21(4):617–37. [DOI] [PubMed]
- 13.Yochim B, Baldo J, Nelson A, Delis DC. D-KEFS Trail Making Test performance in patients with lateral prefrontal cortex lesions. J Int Neuropsychol Soc 2007;13(4):704–9. [DOI] [PubMed]
- 14.Burnett CM, Duncan JM, Frazier OH, Sweeney MS, Vega JD, Radovancevic B. Improved multiorgan function after prolonged univentricular support. Ann Thorac Surg 1993;55(1): 65–71. [DOI] [PubMed]
- 15.Dasse KA, Frazier OH, Lesniak JM, Myers T, Burnett CM, Poirier VL. Clinical responses to ventricular assistance versus transplantation in a series of bridge to transplant patients. ASAIO J 1992;38(3):M622–6. [DOI] [PubMed]
- 16.Dowling RD, Park SJ, Pagani FD, Tector AJ, Naka Y, Icenogle TB, et al. HeartMate VE LVAS design enhancements and its impact on device reliability. Eur J Cardiothorac Surg 2004;25(6):958–63. [DOI] [PubMed]
- 17.Long JW, Kfoury AG, Slaughter MS, Silver M, Milano C, Rogers J, et al. Long-term destination therapy with the HeartMate XVE left ventricular assist device: improved outcomes since the REMATCH study. Congest Heart Fail 2005;11(3): 133–8. [DOI] [PubMed]
- 18.Scott-Burden T, Tock CL, Bosely JP, Clubb FJ Jr, Parnis SM, Schwarz JJ, et al. Nonthrombogenic, adhesive cellular lining for left ventricular assist devices. Circulation 1998;98(19 Suppl):II339–45. [PubMed]
- 19.Graham TR, Dasse K, Coumbe A, Salih V, Marrinan MT, Frazier OH, Lewis CT. Neo-intimal development on textured biomaterial surfaces during clinical use of an implantable left ventricular assist device. Eur J Cardiothorac Surg 1990;4(4): 182–90. [DOI] [PubMed]
- 20.Birks EJ, Tansley PD, Yacoub MH, Bowles CT, Hipkin M, Hardy J, et al. Incidence and clinical management of life-threatening left ventricular assist device failure. J Heart Lung Transplant 2004;23(8):964–9. [DOI] [PubMed]


