Abstract
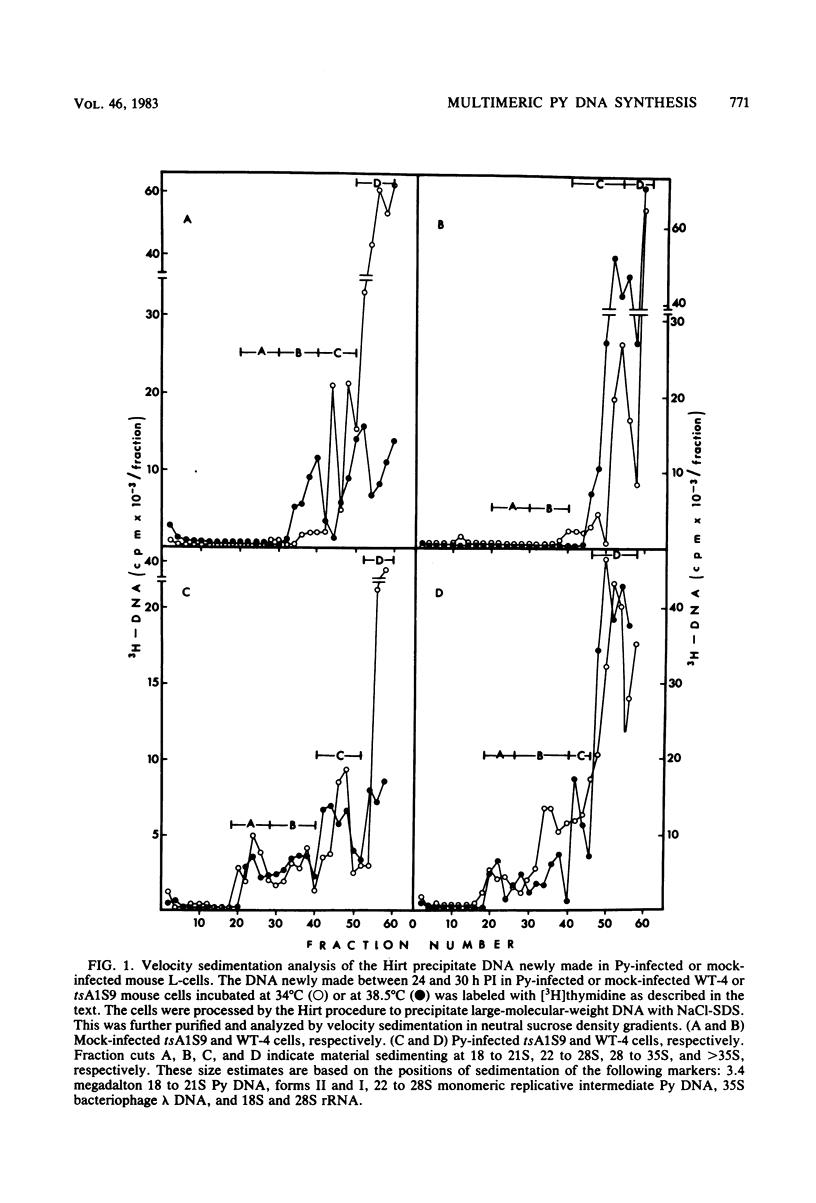
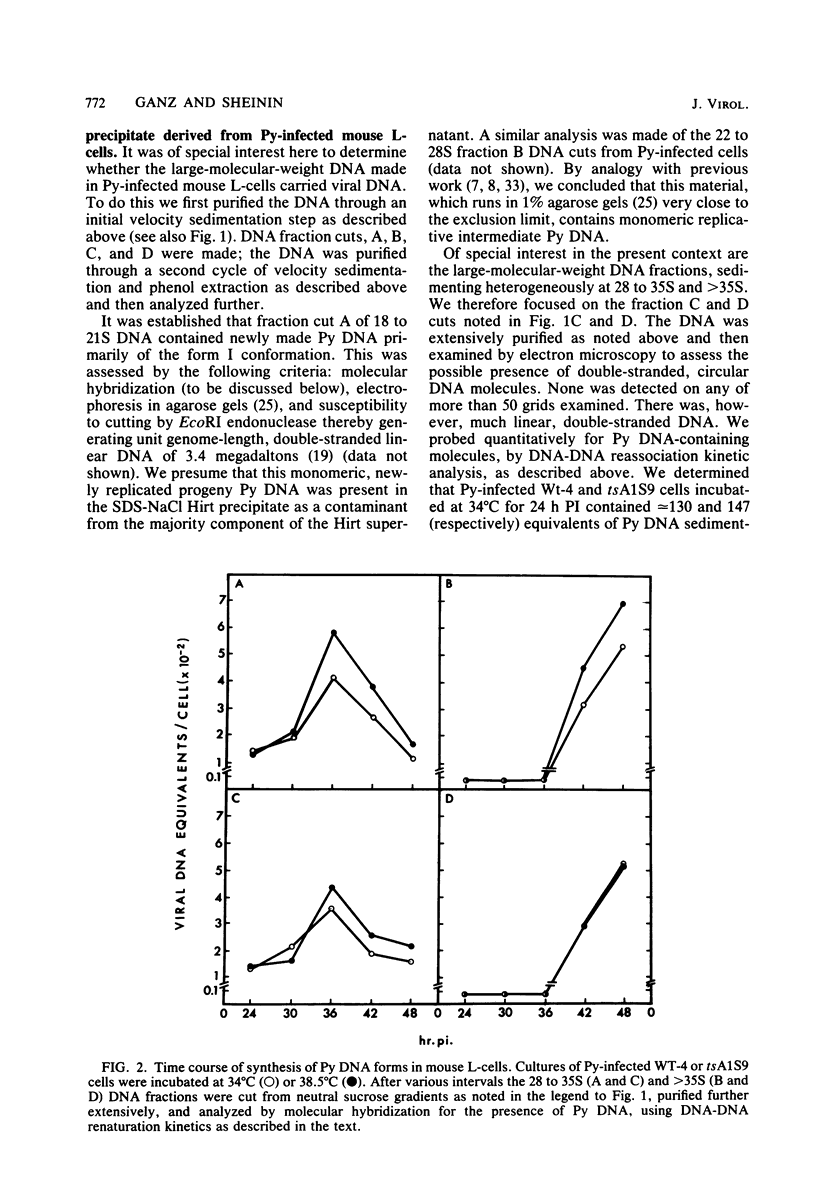
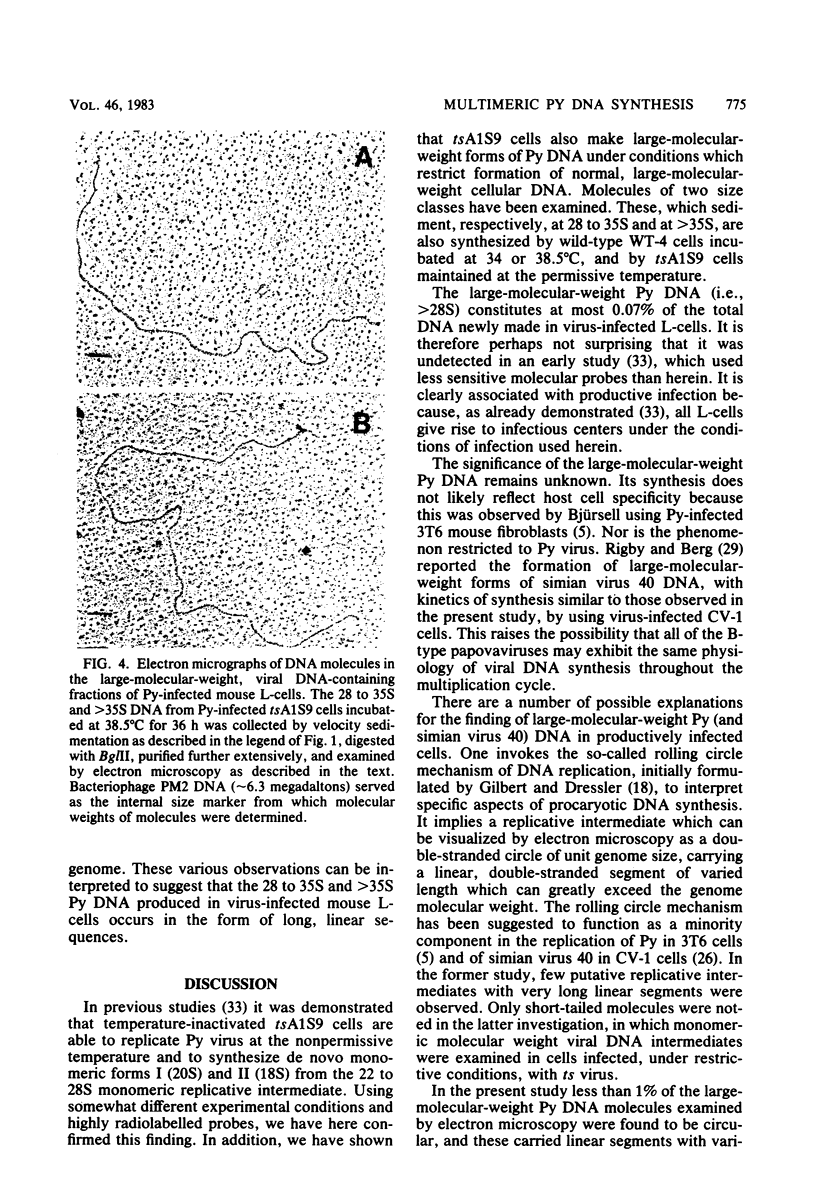
Several different forms of progeny viral DNA can be identified in polyoma virus (Py)-infected mouse L-cells. The majority comprise mature form I superhelical DNA and the circular, double-stranded "theta" replicating intermediates in which the progeny DNA strands never exceed the unit genome length of the template. There is formed, in addition, a minority fraction of multimeric, linear, double-stranded Py DNA molecules that sediment heterogeneously at 28 to 35S and greater than 35S. Restriction enzyme analysis of these large Py DNA molecules reveals them to be tandem arrays of multiple unit genome lengths, covalently linked head to tail. It is estimated that the 28 to 35S multimeric DNA has an average size of about 20 megadaltons, made up of 6 to 20 Py genome units. The greater than 35S Py DNA is, of course, larger. Kinetic analysis indicates that formation of the monomeric progeny viral DNA and the 28 to 35S multimeric Py DNA reaches a peak at about 35 to 36 h postinfection. Synthesis of the very large linear molecules of greater than 35S is first detected after this interval and continues thereafter. The de novo synthesis of all of these progeny Py DNA molecules proceeds apparently normally in Py-infected tsA1S9 mouse L-cells incubated at 38.5 degrees C under conditions which restrict normal cellular DNA replication. These findings suggest that the cellular DNA topoisomerase II activity, encoded in the tsA1S9 locus (R. W. Colwill and R. Sheinin, submitted for publication), is not required for de novo formation of any form of Py DNA. However, the total amount made and the rate of synthesis of the large molecular weight Py DNA are affected very late in temperature-inactivated tsA1S9 cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastia D., Sueoka N. Studies on the late replication of phage lambda: rolling-circle replication of the wild type and a partially suppressed strain, Oam29 Pam80. J Mol Biol. 1975 Oct 25;98(2):305–320. doi: 10.1016/s0022-2836(75)80120-3. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Rixon F. J. Replication of herpesvirus DNA. IV: analysis of concatemers. Virology. 1979 Apr 15;94(1):61–70. doi: 10.1016/0042-6822(79)90438-0. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Tokazewski S. A. Replication of herpesvirus DNA. II. Sedimentation characteristics of newly synthesized DNA. Virology. 1977 Jun 15;79(2):292–301. doi: 10.1016/0042-6822(77)90356-7. [DOI] [PubMed] [Google Scholar]
- Birg F., Dulbecco R., Fried M., Kamen R. State and organization of polyoma virus DNA sequences in transformed rat cell lines. J Virol. 1979 Feb;29(2):633–648. doi: 10.1128/jvi.29.2.633-648.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell G. Effects of 2'-deoxy-2'-azidocytidine on polyoma virus DNA replication: evidence for rolling circle-type mechanism. J Virol. 1978 Apr;26(1):136–142. doi: 10.1128/jvi.26.1.136-142.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell G., Munck V., Therkelsen A. J. Polyoma DNA synthesis in isolated nuclei: evidence for defective replication forks. J Virol. 1979 Jun;30(3):929–932. doi: 10.1128/jvi.30.3.929-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton P. E., Sheinin R. Studies on the replication of polyoma DNA: physicochemical properties of viral DNA synthesized when protein synthesis is inhibited. Can J Biochem. 1973 Mar;51(3):305–317. doi: 10.1139/o73-036. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Branton P. E., Sheinin R. Characterization of abnormal DNA formed in polyoma virus-infected cells. Virology. 1970 Mar;40(3):768–772. doi: 10.1016/0042-6822(70)90227-8. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. II. Biochemical and electron microscopic analysis of simian virus 40 virion assembly. J Virol. 1979 Jul;31(1):199–208. doi: 10.1128/jvi.31.1.199-208.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consigli R. A., Center M. S. Recent advances in polyoma virus research. CRC Crit Rev Microbiol. 1978;6(3):263–299. doi: 10.3109/10408417809090624. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Robbins A. K., Nicklin P. M. Location of the origin and terminus of replication in polyoma virus DNA. J Gen Virol. 1974 Oct;25(1):133–142. doi: 10.1099/0022-1317-25-1-133. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Syrett C., Wilde A. The replication of polyoma DNA. J Gen Virol. 1973 Dec;21(3):515–521. doi: 10.1099/0022-1317-21-3-515. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Ganz P. R., Pearlman R. E. Purification from Tetrahymena thermophila of DNA polymerase and a protein which modifies its activity. Eur J Biochem. 1980 Dec;113(1):159–173. doi: 10.1111/j.1432-1033.1980.tb06151.x. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Evidence for semiconservative replication of circular polyoma DNA. Proc Natl Acad Sci U S A. 1966 Apr;55(4):997–1004. doi: 10.1073/pnas.55.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ladin B. F., Blankenship M. L., Ben-Porat T. Replication of herpesvirus DNA. V. Maturation of concatemeric DNA of pseudorabies virus to genome length is related to capsid formation. J Virol. 1980 Mar;33(3):1151–1164. doi: 10.1128/jvi.33.3.1151-1164.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lania L., Boast S., Fried M. Excision of polyoma virus genomes from chromosomal DNA by homologous recombination. Nature. 1982 Jan 28;295(5847):349–350. doi: 10.1038/295349a0. [DOI] [PubMed] [Google Scholar]
- Martin R. F. Analysis of polyoma virus DNA replicative intermediates by agarose gel electrophoresis. J Virol. 1977 Sep;23(3):827–832. doi: 10.1128/jvi.23.3.827-832.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P. The initiation of SV40 DNA synthesis is not unique to the replication origin. Cell. 1980 Jun;20(2):381–391. doi: 10.1016/0092-8674(80)90624-8. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P. From deoxynucleotides to DNA synthesis. Fed Proc. 1978 Jan;37(1):9–14. [PubMed] [Google Scholar]
- Rigby P. W., Berg P. Does simian virus 40 DNA integrate into cellular DNA during productive infection? J Virol. 1978 Nov;28(2):475–489. doi: 10.1128/jvi.28.2.475-489.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- STANNERS C. P. STUDIES ON THE TRANSFORMATION OF HAMSTER EMBRYO CELLS IN CULTURE BY POLYOMA VIRUS. II. SELECTIVE TECHNIQUES FOR THE DETECTION OF TRANSFORMED CELLS. Virology. 1963 Nov;21:464–476. doi: 10.1016/0042-6822(63)90207-1. [DOI] [PubMed] [Google Scholar]
- Sheinin Polyoma and cell DNA synthesis in mouse L cells temperature sensitive for the replication of cell DNA. J Virol. 1976 Mar;17(3):692–704. doi: 10.1128/jvi.17.3.692-704.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin R., Darragh P., Dubsky M. Mitochondrial DNA synthesis in mouse L cells temperature sensitive in nuclear DNA replication. Can J Biochem. 1977 May;55(5):543–547. doi: 10.1139/o77-077. [DOI] [PubMed] [Google Scholar]
- Sheinin R., Guttman S. Semi-conservative and non-conservative replication of DNA in temperature-sensitive mouse L-cells. Biochim Biophys Acta. 1977 Nov 2;479(1):105–118. doi: 10.1016/0005-2787(77)90130-7. [DOI] [PubMed] [Google Scholar]
- Sheinin R. Preliminary characterization of the temperature-sensitive defect in DNA replication in a mutant mouse L cell. Cell. 1976 Jan;7(1):49–57. doi: 10.1016/0092-8674(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Syvanen M. In vitro genetic recombination of bacteriophage lambda. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2496–2499. doi: 10.1073/pnas.71.6.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türler H. Interaction of polyoma and mouse DNAs. IV. Time course and extent of integration of polyoma DNA into mouse DNA during lytic infection. J Virol. 1977 Aug;23(2):272–285. doi: 10.1128/jvi.23.2.272-285.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türler H. Interactions of Polyoma and Mouse DNAs III. Mechanism of Polyoma Pseudovirion Formation. J Virol. 1975 May;15(5):1158–1167. doi: 10.1128/jvi.15.5.1158-1167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]





