Abstract
Malignant or complex benign tumors of the left heart can present a formidable challenge for complete resection, due to anatomic inaccessibility. Cardiac autotransplantation (cardiac explantation, ex-vivo tumor resection, reconstruction, and reimplantation) was introduced for complex benign primary left-heart cardiac tumors by Cooley and for malignant left-heart tumors by Reardon. Herein, we update our previously reported experience.
From April 1998 through July 2008, 20 patients underwent 21 cardiac autotransplantations for complex left-sided cardiac tumors that were nonresectable by traditional means. Demographics, tumor histology, operative data, and mortality rates were analyzed. Follow-up was complete in all patients.
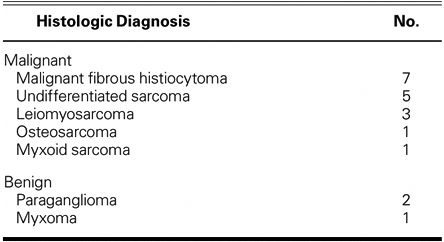
Of the 20 patients, 17 had malignant lesions, and 3 had benign disease. Two patients had left ventricular lesions and the rest had left atrial lesions. Histology showed 7 malignant fibrous histiocytomas, 5 undifferentiated sarcomas, 3 leiomyosarcomas, 1 malignant osteosarcoma, 1 myxoid sarcoma, 2 paragangliomas, and 1 myxoma. Fourteen patients had previous resection of their cardiac tumors, and 1 patient had repeat autotransplantation for recurrent disease. There were no operative deaths in patients undergoing autotransplantation alone (0/15), and 3 operative deaths in patients undergoing combined cardiac autotransplantation and pneumonectomy (3/6, 50%). All 3 patients with benign disease survived surgery and are alive without recurrent disease. Local recurrence occurred in 3/18 patients with malignant disease: 1 underwent successful repeat autotransplantation and 2 are receiving chemotherapy. The mean survival for all patients with sarcoma is 22 months.
Cardiac autotransplantation enables complete resection and accurate reconstruction in many primary malignant and complex benign left-heart tumors.
Key words: Heart neoplasms/mortality/surgery; heart transplantation/methods; replantation/methods; sarcoma/surgery; transplantation, autologous
Cardiac tumors occur either as secondary tumors metastatic to the heart or as cardiac primary tumors. Metastatic tumors are far more common than primary cardiac tumors, which have an incidence of only 0.1% to 0.3%,1 or of about 1 in every 500 cardiac surgeries. Most (75%) of these primary tumors are benign. Of the malignant tumors, most (75%) are sarcomas.2 The most surgically challenging primary cardiac tumors are those that involve the left atrium and the left ventricle. Accessibility and anatomic constraints make resection technically difficult, and the standard approaches yield a high incidence of early local recurrence.
Cardiac autotransplantation was developed to overcome the technical difficulties associated with left-sided tumor resection. Cardiac autotransplantation for large complex benign lesions was reported in the seminal case by Cooley in 1985.3 Our 1st cardiac autotransplantation to treat malignant left atrial disease was done in 1998,4 our 1st cardiac autotransplantation to treat malignant left ventricular disease was done in 2003,5 and our initial series of 12 autotransplantations was reported in 2006.6 Our series has now extended to 21 cardiac autotransplantations, enabling us to draw further inferences on the benefits and challenges of cardiac autotransplantation.
Patients and Methods
From April 1998 through July 2008, 20 patients underwent 21 autotransplantation operations by a single surgeon (MJR). Seventeen patients had malignant tumors and 3 had benign tumors (Table I). Fourteen patients underwent a redo resection of a local recurrence of a malignant left atrial tumor. One patient underwent repeat autotransplantation.
Table I. Histologic Diagnosis of Left-Sided Cardiac Tumors in 20 Patients
The operations were performed at The Methodist DeBakey Heart & Vascular Center (18 operations), the M.D. Anderson Cancer Center (2 operations), and in Poznan, Poland (1 operation). Demographics, tumor histology, operative data, and mortality rates were analyzed. Retrospective review was carried out with internal review board approval.
Surgical Technique
Our surgical technique has been presented in detail previously.7 All operations were conducted via a median sternotomy. Cardiopulmonary bypass was established by a variety of standard techniques. Venous cannulation, since it must enable complete right atrial removal, included direct cannulation of the inferior and superior venae cavae, femoral venous cannulation, and brachiocephalic vein cannulation. Arterial cannulation was performed in the distal ascending aorta except in the case of the repeat autotransplantation, in which the femoral artery was used for arterial inflow. Patients were cooled systemically to 28°C, and cardiac arrest was achieved by antegrade administration of cold potassium cardioplegic solution, 10 mL/kg. Cardiac explantation was performed by dividing the superior vena cava, the inferior vena cava, the great vessels, and the left atrium. The heart was then placed in iced saline, and the tumors were resected ex vivo. Cardiac reconstruction and valve replacement as necessary were performed ex vivo, and the heart was then reimplanted in the orthotopic position.
Adjuvant Chemotherapy
Adjuvant chemotherapy with doxorubicin hydrochloride (75 mg/m2) and ifosfamide (106 mg/m2), in 4 to 5 doses, was recommended to all patients who had cardiac sarcomas. Two patients refused such therapy and died at 2 and 3 months postoperatively. All other operative survivors completed the chemotherapy regimen. Two patients with recurrent local disease are currently receiving chemotherapy.
Statistical Analysis
Survival curves were calculated via the Kaplan-Meier method. Data analysis was performed by use of the Statistical Package for Social Sciences version 13.0 (SPSS Inc.; Chicago, Ill).
Results
Twenty patients underwent 21 cardiac autotransplantations. There were 10 men and 10 women. All 3 benign cases occurred in men, who ranged in age from 26 to 63 years (average, 44.6 yr). For malignant tumors, the age range was 20 to 57 years (average, 39.5 yr). The most prominent symptom at presentation was congestive heart failure, which often was rapidly progressive and associated with hypotension. Chest pain, cough, and fever also occurred in this group. Only 1 patient was asymptomatic; that patient's left ventricular tumor has been described in detail.5
Operative Death and Survival
There were 3 in-hospital deaths (Table II). All hospital deaths occurred in patients who required pneumonectomy in addition to cardiac autotransplantation. All 3 developed severe postoperative coagulopathy that necessitated multiple transfusions. Each developed unilateral pulmonary edema in the remaining lung, increasing hypoxia, and right-heart failure that led to death. There was no hospital death in patients who underwent autotransplantation alone (Table II). As of July 2008, all 3 patients with benign disease were alive without recurrence at 41 to 46 months (mean, 43.6 mo). In the patients with malignant disease, 8/17 (47%) were alive at 3 to 50 months (mean, 22 mo). The survival curve for all patients in our series who underwent autotransplantation for cardiac sarcoma is shown in Figure 1. The survival curves for all our cardiac sarcoma patients who underwent cardiac autotransplantation—with and without pneumonectomy—are shown in Figure 2.
Table II. Demographics, Illnesses, and Outcomes of Autotransplantation Patients
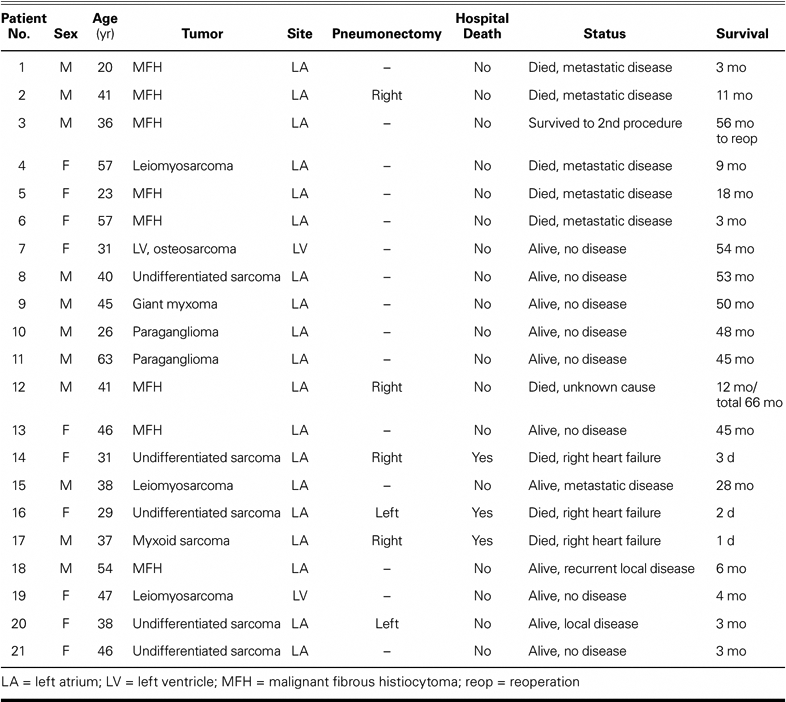
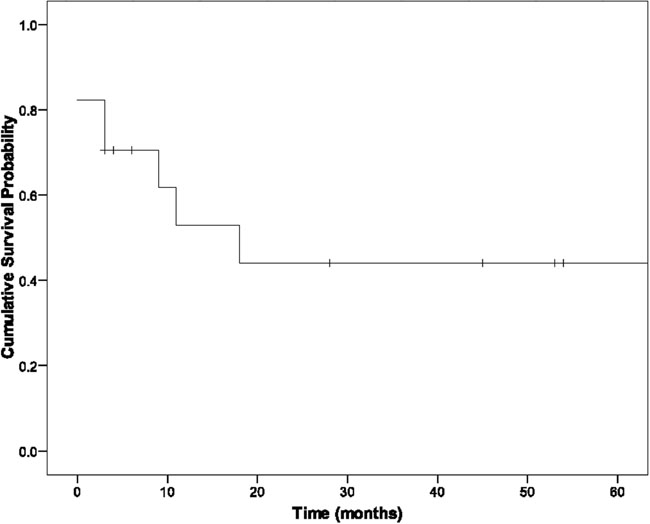
Fig. 1 Survival curve for all patients with sarcoma who underwent autotransplantation.
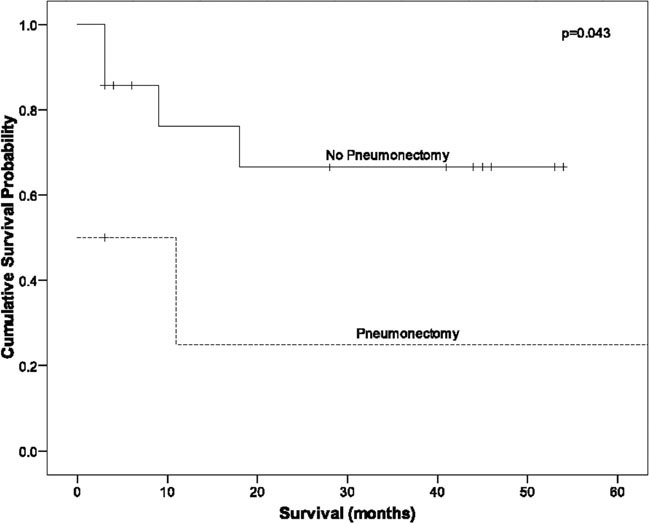
Fig. 2 Survival curves for all sarcoma patients who underwent autotransplantation with and without pneumonectomy.
Discussion
The advent of cardiopulmonary bypass enabled the 1st resection of an intracardiac primary cardiac tumor, by Crafoord in 1954.8,9 Echocardiography enabled better visualization of the cardiac chambers and more accurate diagnosis. However, primary cardiac tumors are so rare that only 20 primary cardiac tumors (excluding myxoma) were seen from 1961 through 1983 at The Texas Heart Institute, during which time 52,500 cardiac operations were performed. This rarity leaves few institutions and even fewer surgeons with a sufficient volume of cases from which to draw conclusions for treatment of this difficult disease.10 At the Methodist Hospital, we saw only 85 patients with primary cardiac tumors, including myxoma, from 1975 through 2002, after which our cardiac-tumor program began to develop more rapidly.11
Because cardiac sarcomas represent only about 1% of all soft-tissue sarcomas, most of our treatment approaches to them are extensions of our approaches to soft-tissue sarcomas in general.12 However, chemotherapy has not proved effective in the initial treatment of most nonpediatric left-heart sarcomas, for patients often present in advanced heart failure; and radiation therapy is difficult in all but pulmonary artery sarcomas, due to the cardiac toxicity of radiation.13 Surgical resection remains the primary mode of treatment for most left-heart tumors.
Cardiac sarcomas treated with chemotherapy alone have a dismal prognosis—a median survival time of only 6 months.14 In the surgical series reported by Putnam and co-authors,15 the median survival time for patients with complete resection was 24 months, compared with a median survival of 10 months for the entire group.15 Complete surgical resection, then, appears to double the survival time over palliative resection.
Most cardiac tumors are benign and can be cured by surgical resection. Large complex left atrial tumors may, however, present a considerable impediment to complete resection due to the posterior location of the left atrium and difficult accessibility. Cardiac autotransplantation was introduced by Cooley to enable surgical access to the posterior heart for the resection of such tumors.3 In our series, we found that the Cooley approach to complex benign left atrial tumors enables excellent exposure for complete resection; indeed, all of our patients with benign-disease resections are alive and well, without recurrent disease.
Patients with malignant left-heart cardiac tumors have a dismal prognosis if untreated, due to local extension and metastatic disease. Many patients with left-heart malignant tumors die of hypotension, congestive heart failure, stroke, heart block, and tumor embolization. These tumors present a surgical challenge, because incomplete resection is followed by rapid local tumor recurrence.16,17 Typically, left atrial tumors are approached through the interatrial groove, and left ventricular tumors are approached through a transaortic valve, transmitral valve, or the ventricle itself. The interatrial groove approach is adequate for most benign left atrial tumors but is usually inadequate for malignant left atrial tumors, due to the posterior and inaccessible location of the chamber and to the proximity of vital cardiac structures. The autotransplantation approach has given us excellent visualization, which has enabled resection of the complete left atrium to achieve clear tumor margins. For left ventricular tumors, we have found the transaortic valve approach satisfactory for benign disease but insufficient for the larger malignant tumors that we have encountered.18 Ventriculotomy is not appealing due to the need to injure good muscle in entering the ventricle. The transmitral valve approach, with the heart explanted, has provided excellent exposure for complete intraventricular tumor resection, reconstruction of the interventricular septum, and mitral valve replacement.
Although cardiac transplantation has been performed in the treatment of patients who have benign and malignant tumors, drawbacks include the lack of organ availability, problems with immunosuppression and graft arteriopathy in the long term, and the unknown effects of immunosuppression on malignant-tumor growth. Cardiac transplantation in cardiac-tumor patients has a poor survival rate, with a mean survival time of 12 months reported by Gowdamarajan and Michler.19
Our 21 autotransplantations among 20 patients is the largest series of autotransplantations reported in the medical literature to date. Our operative mortality rate was 14% (3/21). Among autotransplantations without pneumonectomy (15/21), we had no operative deaths.
All 3 deaths occurred in patients who received concurrent pneumonectomy, which is a new finding since our prior report on autotransplantation. In an experienced surgical center, cardiac autotransplantation is a viable option for patients who have otherwise nonresectable cardiac tumors. Concurrent pneumonectomy, performed to achieve negative margins, is a poor prognostic factor, so great care must be taken in selecting these patients.
Median survival among patients who undergo standard resection for primary cardiac sarcomas has been reported to be 11 months.15 Median survival among patients who underwent cardiac autotransplantation for sarcoma in our series (17/20) was 22 months.
Conclusion
Survival rates among patients who have cardiac sarcoma are improved with complete resection. Autotransplantation is a viable means of obtaining complete resection, given the low operative mortality rate that can be achieved in experienced hands. However, concurrent pneumonectomy substantially increases the risk of death that is associated with cardiac autotransplantation.
Celebrating the Legacy
In this issue of the Texas Heart Institute Journal, we present a manuscript that grew out of my delivery at the 15th International Symposium of the Denton A. Cooley Cardiovascular Surgical Society in October of 2007. It is always a pleasure to present one's work, and even more so when done at one's educational home and in the presence of one's teachers. This year the chance to present meant even more to me, because the theme of the meeting was “Celebrating the Legacy.”
Selecting this facet of my professional work to discuss was a simple choice. In 1984, as Dr. Cooley's resident, I had the opportunity to assist on a case that involved a large left atrial pheochromocytoma deemed unresectable in Italy but the cause of heart failure and, without resection, imminent death in a young man. To our surprise at surgery, Dr. Cooley removed the entire heart and asked the scrub nurse to hang on to it, as he would like it back later. He proceeded to remove the left atrial pheochromocytoma that occupied most of the posterior left atrium, rebuild the atrium with pericardium, and then suture the heart back into place—thereby establishing cardiac autotransplantation as a new approach to the treatment of complex left heart tumors.1
My interest in complex cardiac tumors began at that moment. The subsequent work of our team and specifically our current series of cardiac autotransplants, that we present here are a direct outgrowth of that inspiration handed to me by Dr. Cooley that day and reflect a very personal legacy for which I thank Dr. Cooley.
Michael J. Reardon, MD
Professor and Chief of Cardiac Surgery, Department of Cardiovascular Surgery, Methodist DeBakey Heart & Vascular Center, Houston, Texas
Footnotes
Address for reprints: Michael J. Reardon, MD, Department of Cardiovascular Surgery, Methodist DeBakey Heart & Vascular Center, 6560 Fannin St., #1006, Houston, TX 77030
E-mail: MReardon@tmhs.org
Presented at the 15th International Symposium of the Denton A. Cooley Cardiovascular Surgical Society, Houston, Texas, 25–27 October 2007
References
- 1.Straus R, Merliss R. Primary tumor of the heart. Arch Pathol 1945;39:74–8.
- 2.Reardon MJ, Malaisrie SC, Walkes JC, Vaporciyan AA, Rice DC, Smythe WR, et al. Cardiac autotransplantation for primary cardiac tumors. Ann Thorac Surg 2006;82(2):645–50. [DOI] [PubMed]
- 3.Cooley DA, Reardon MJ, Frazier OH, Angelini P. Human cardiac explantation and autotransplantation: application in a patient with a large cardiac pheochromocytoma. Tex Heart Inst J 1985;12(2):171–6. [PMC free article] [PubMed]
- 4.Reardon MJ, DeFelice CA, Sheinbaum R, Baldwin JC. Cardiac autotransplant for surgical treatment of a malignant neoplasm. Ann Thorac Surg 1999;67(6):1793–5. [DOI] [PubMed]
- 5.Reardon MJ, Walkes JC, Defelice CA, Wojciechowski Z. Cardiac autotransplantation for surgical resection of a primary malignant left ventricular tumor. Tex Heart Inst J 2006;33 (4):495–7. [PMC free article] [PubMed]
- 6.Walkes JC, Bavare C, Blackmon S, Reardon MJ. Transaortic resection of an apical left ventricular fibroelastoma facilitated by a thoracoscope. J Thorac Cardiovasc Surg 2007;134(3): 793–4. [DOI] [PubMed]
- 7.Conklin LD, Reardon MJ. Autotransplantation of the heart for primary cardiac malignancy: development and surgical technique. Tex Heart Inst J 2002;29(2):105–8. [PMC free article] [PubMed]
- 8.Crafoord CL. Discussion on mitral stenosis and insufficiency. In: Lam CR, editor. Proceedings of the International Symposium on Cardiovascular Surgery, Henry Ford Hospital, Detroit, Michigan, March 1955. Philadelphia: WB Saunders Company; 1955. p. 202.
- 9.Chitwood WR Jr. Clarence Crafoord and the first successful resection of a cardiac myxoma. Ann Thorac Surg 1992;54(5): 997–8. [DOI] [PubMed]
- 10.Reece IJ, Cooley DA, Frazier OH, Hallman GL, Powers PL, Montero CG. Cardiac tumors. Clinical spectrum and prognosis of lesions other than classical benign myxoma in 20 patients. J Thorac Cardiovasc Surg 1984;88(3):439–46. [PubMed]
- 11.Bakaeen FG, Reardon MJ, Coselli JS, Miller CC, Howell JF, Lawrie GM, et al. Surgical outcome in 85 patients with primary cardiac tumors. Am J Surg 2003;186(6):641–7. [DOI] [PubMed]
- 12.Mayer F, Aebert H, Rudert M, Konigsrainer A, Horger M, Kanz L, et al. Primary malignant sarcomas of the heart and great vessels in adult patients–a single-center experience. Oncologist 2007;12(9):1134–42. [DOI] [PubMed]
- 13.Reardon MJ, Walkes JC, Benjamin R. Therapy insight: malignant primary cardiac tumors. Nat Clin Pract Cardiovasc Med 2006;3(10):548–53. [DOI] [PubMed]
- 14.Herrmann MA, Shankerman RA, Edwards WD, Shub C, Schaff HV. Primary cardiac angiosarcoma: a clinicopathologic study of six cases. J Thorac Cardiovasc Surg 1992;103 (4):655–64. [PubMed]
- 15.Putnam JB Jr, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DA. Primary cardiac sarcomas. Ann Thorac Surg 1991;51(6):906–10. [DOI] [PubMed]
- 16.Okita Y, Miki S, Ueda Y, Tahata T, Sakai T, Matsuyama K. Recurrent malignant fibrous histiocytoma of the left atrium with extracardiac extension. Am Heart J 1994;127(6): 1624–8. [DOI] [PubMed]
- 17.Gabelman C, Al-Sadir J, Lamberti J, Fozzard HA, Laufer E, Replogle RL, Myerowitz PD. Surgical treatment of recurrent primary malignant tumor of the left atrium. J Thorac Cardiovasc Surg 1979;77(6):914–21. [PubMed]
- 18.Walkes JC, Bavare C, Blackmon S, Reardon MJ. Transaortic resection of an apical left ventricular fibroelastoma facilitated by a thoracoscope. J Thorac Cardiovasc Surg 2007;134(3): 793–4. [DOI] [PubMed]
- 19.Gowdamarajan A, Michler RE. Therapy for primary cardiac tumors: is there a role for heart transplantation? Curr Opin Cardiol 2000;15(2):121–5. [DOI] [PubMed]
References
- 1.Cooley DA, Reardon MJ, Frazier OH, Angelini P. Human cardiac explantation and autotransplantation: application in a patient with a large cardiac pheochromocytoma. Tex Heart Inst J 1985 Jun;12(2):171–6. [PMC free article] [PubMed]


