Abstract
In this study, we have identified and evaluated the cardiovascular anomalies associated with Williams-Beuren syndrome in children.
In a retrospective, lineal, and observational study, we reviewed the files of children who were seen from 1980 through 2005 (25 years) after a clinical diagnosis of Williams-Beuren syndrome.
Forty children were diagnosed with this syndrome at the National Institute of Pediatrics in Mexico City. Of these, 32 (80%) were found to have congenital heart defects. The male-to-female ratio was 1.3:1 and ages ranged from 6 months to 15 years (mean, 4.4 years) at the time of diagnosis. All of the patients had morphologic and genetic characteristics typical of the syndrome.
We emphasize the cardiovascular aspects from a clinical point of view. Supravalvular aortic stenosis was our most frequent finding, in 18 of 32 patients (56%); gradient differences in these patients ranged from 14 to 81 mmHg. Five patients showed combined lesions, the most frequent being supravalvular aortic stenosis in combination with pulmonary artery brachial stenosis, or with atrial and ventricular defects. Patients with incomplete atrioventricular defect and bicuspid aortic valve, as were seen at our hospital, have not to our knowledge been reported in other studies.
One of the patients was scheduled for balloon dilation; another was scheduled for surgery; a 3rd patient was operated on twice for the placement of an aorto-aortic bridge; another underwent ventricular septal defect closure; and yet another underwent aortoplasty, this last dying shortly after surgery.
Key words: Aortic coarctation; aortic valve stenosis, supravalvular; child; face/abnormalities; elastin/genetics; facial expression; gene deletion; heart defects, congenital/diagnosis; hypercalcemia; mental retardation; Mexico/epidemiology; psychomotor performance; Williams syndrome
Williams-Beuren syndrome (W-BS) is an illness characterized by typical facies, growth delays, mild mental retardation, extroverted personality, and congenital heart defects (CHD)—such as supravalvar aortic stenosis (SAoS) and other forms of arterial problems—seen in 77% to 79% of all patients.1-3 The real frequency of this syndrome is unknown, although it is estimated to be 1 in 10,000–50,000 live births.4-6
This syndrome was named after J.C.P. Williams in 1961, although there were reports previously published by other authors.7 At the same time, Rashkind and colleagues8 suggested the association of idiopathic childhood hypercalcemia with cardiopathy. In 1962, Alois J. Beuren and associates9 pointed to the characteristic “elfin facies” and, later, to the possibility of an association with pulmonary artery (PA) branch stenosis.10
Inheritance of W-BS is, in most cases, sporadic.2,11 However, there is a 50% risk of transmission of deletions, and cases among family members suggest an autosomal dominant inheritance pattern. 5,11,12 Williams-Beuren is considered to be a syndrome with contiguous genes, but it is the gene for elastin, a protein in the aortic wall, that especially influences the aortic area. In 1993 and the years following, the elastin gene has been found in 95% of patients with W-BS in the same subunit (7q11.23) of the long arm of chromosome 7.13-17 As mentioned elsewhere,4,18 SAoS is an inherited vascular condition known as an “arteriopathy of elastin,” which can cause heart problems and death.
Currently, the cause of hypercalcemia is unknown. It is thought to be related to an absence of (or deficiency in) the secretion of C cells (calcitonin producers) in individuals with W-BS. Some of the manifestations of hypercalcemia then arise from the action of a neuropeptide that has physiologic effects in the cardiovascular and central nervous systems.19-21 It is thought that the excessive administration of vitamin D might produce sclerosis, aortic calcification, and head and facial changes.4,14,19,22 In pregnant rabbits, vitamin D administration can bring about fetal cardiopathies and changes in the blood vessels.20,23
Some studies suggest that SAoS is associated with histologic changes characteristic of prenatal abnormalities, such as hyperplasia of the intima and excessive collagen in the elastic sheet. There are also changes in the homeostasis of fetal calcium,24 but the exact mechanism for the pathogenesis of SAoS has not been well defined. Problems related to SAoS are the disorganization of aortic elastin fibers, myocardial infarction, arrhythmias, and death.25,26
In auscultation of the heart that has been affected by SAoS, the examining physician hears a systolic ejection murmur at the aortic focus, which radiates to the sternal notch and neck vessels, without a protosystolic noise, as in cases of valvular aortic stenosis. In addition, continuous murmurs are heard in both lung fields, secondary to PA branch stenosis.27
In children with W-BS and cardiopathy, SAoS is seen in 81% of cases. Conversely, only about 50% of SAoS patients have W-BS. In a study of 81 patients with SAoS, for example, 40 had W-BS.28
Other cardiovascular anomalies found in association with W-BS are ventricular septal defect (VSD), patent ductus arteriosus (PDA), stenosis of outlying arteries (renal, cerebral, carotid, coronary, brachiocephalic, subclavian, and mesenteric), coarctation of the aorta, mitral valve incompetence, tetralogy of Fallot (TOF), and vascular ring.19,27,29–35 There are also references in the literature to W-BS in association with total anomalous connection of lung vessels, arteriovenous shunt, interruption of the aortic arch, vein aplasia, and aortic aneurysm.6,11,36–38 It is not unusual to find an association of several heart lesions in the same W-BS patient.11,26,39 There are infrequent cardiovascular lesions such as mitral valve prolapse (15%) (secondary to an increase in collagen in the mitral valve), arterial systemic hypertension (8%) (usually seen in older patients), and aortic subvalvular stenosis.24,34,40,41 Williams-Beuren syndrome has often been associated with displacement of lung arteries or coronary arteries, with fibrosis and microfocal chronic ischemia in the subendocardium and the papillary muscles of the left ventricle, and with severe myocardial infarction causing death in elderly patients and in children.25,42,43 The statistics can be found in several sources.2,3,17,42,44
Detection of the genetic microdeletions is done by using the fluorescence in situ hybridization (FISH) method, which enables a diagnosis in 99% of the cases. Case studies focus on observing the progression of cardiac disease with annual checkups and on monitoring blood pressure.
The differential diagnosis of SAoS is made by weighing such diverse alternatives as Noonan, Coffin-Lowry, cardiofacial, Shone, congenital rubella, Kabuki, alcoholic fetopathy, and Smith-Magenis syndromes, in addition to storage disease and hypothyroidism.3,4,6
Treatment is directed towards correcting the CHD.4 The treatment for SAoS includes the dilation or amplification of the stenotic segment of aortic or coronary wall, which is sometimes repaired with pericardial patches.22,26,45 In cases of PA branch stenosis, there is the possibility of stent placement.27 In 58% of cases, surgery is required, depending on the severity of the obstruction at different levels. In addition to the severity of the arterial stenosis, advanced age is a negative factor.28
Although SAoS progresses with age, PA branch stenosis tends to improve spontaneously.11,42,46,47 Myocardialinfarctions and sudden death have been reported in childhood, secondary to lesions of coronary dysplasia in cases of W-BS, but they are unusual.18,25 Coronary artery stenosis, with or without SAoS, and severe obstruction to the biventricular outflow are factors that predispose patients to sudden death.48 When patients have associated hypertrophic obstructive cardiomyopathy or left ventricular pressure and volume overload, SAoS and mitral regurgitation occur at greater risk.49 In patients with SAoS, factors that contribute to high rates of sudden death during heart catheterization are a decrease in cardiac output, arrhythmia, and myocardialinfarction. Stenosis of the renal artery or coarctation ofthe aorta contributes to an increase in the frequency of systemic hypertension.11,18,47 Systemic hypertension is more frequent in adults than in children, in cases of W-BS.17
In W-BS, morbidity and mortality rates are high due to the related cardiovascular defects and their sequelae.20
Patients and Methods
The purpose of this study was to describe the spectrum of cardiovascular anomalies in patients with W-BS who were seen during 25 years at the National Institute of Pediatrics in Mexico City, and to document follow-up and prognosis.
In a retrospective, lineal, and observational study, we reviewed the files of children who had been clinically diagnosed as having W-BS from 1980 through June 2005 (25 years). The FISH method had also been used in diagnosis. We analyzed the following variables: sex, age at the time of diagnosis, inheritance type, degree and type of cardiopathy, diagnostic method, evolution, and treatment. The association with other cardiopathies (combined lesions) was also considered, as was the association with pulmonary hypertension, systemic hypertension, other syndromes, hypercalcemia, and psychomotor delay. We excluded from the study those cases that did not fulfill the clinical characteristics of W-BS, and those cardiopathies that were not correctly diagnosed by echocardiography or angiography. All cases of SAoS not associated with W-BS were removed from the study.
A statistical analysis was done of age, in which we obtained mean values ± SD. An occurrence proportions test was done for each of these cardiopathies: SAoS, PA branch stenosis, SAoS with PA branch stenosis, coarctation of the aorta, aortic stenosis, VSD, PDA, and bicuspid aortic valve. In addition, we considered the types and frequencies of cardiopathies seen in the United States, Canada, Korea, Finland, and Spain, and we compared those statistics with ours. In order to maintain a per-experiment error rate of α E=0.05, the Bonferroni principle was used, wherein α E was divided by 5, in order to compare the frequency of each type of cardiomyopathy in Mexico. The comparison error was a C = α E/5 = 0.01.50
Results
Of the 40 patients diagnosed with W-BS, 23 were boys and 17 were girls, at a 1.3:1 ratio. Ages at diagnosis ranged from 6 months to 15 years, averaging 4.4 years ± 4.3 years. All the appearances of the anomaly were sporadic.
Twenty-three cases of W-BS were presenting clinically by means of elfin facial characteristics (Fig. 1), while 17 cases were diagnosed by means of the FISH method (Fig. 2). All W-BS patients presenting at our Institute after June 1997 were diagnosed using the FISH method.
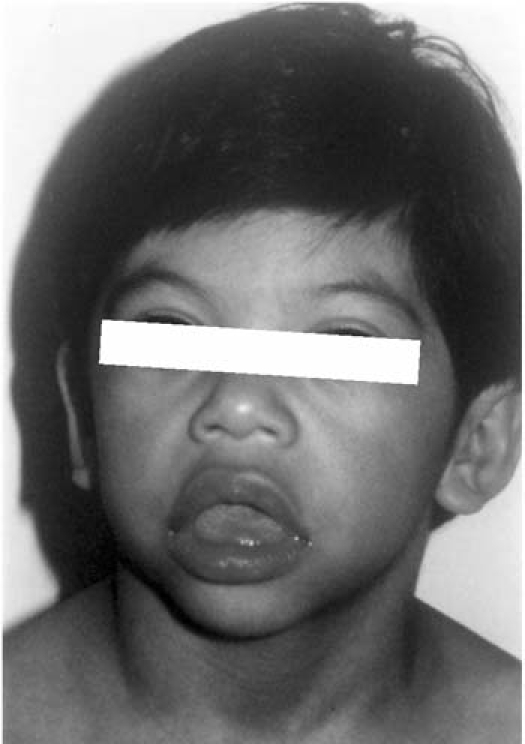
Fig. 1 Characteristic “elfin facies” in 1 of our patients, showing hypertelorism, wide nose and mouth opening, thick lips, micrognathia, and low-set earlobes.
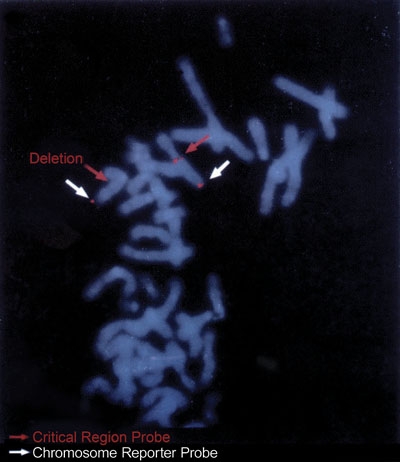
Fig. 2 The fluorescence in-situ hybridization (FISH) method shows the deletion of 7q11.23 in one of our patients.
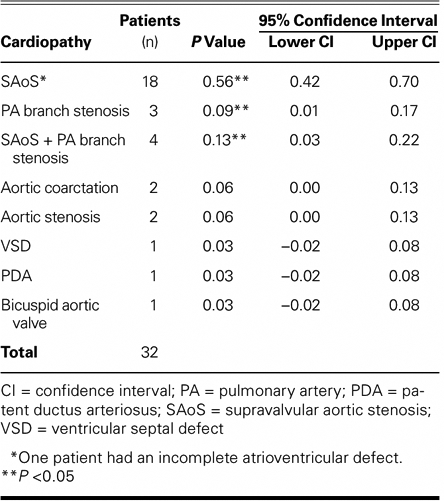
Of the 40 patients, 8 had healthy hearts and 32 (80%) had associated congenital cardiopathies (P = 0.80), which were diagnosed clinically and by electrocardiography, radiography, angiography, and echocardiography. Of the 32 patients who had cardiopathies, 18 had SAoS (Fig. 3); 1 of these patients also had muscular VSD, and another had an ostium primum ASD, this last an incomplete atrioventricular defect with left obstruction. Four patients had SAoS in association with PA branch stenosis: 3 of these 4 patients had ostium secundum ASD plus pulmonary stenosis (1 of the 3 had VSD with ostium secundum ASD), and the 4th had ASD of the infundibular type. The remaining 2 patients had aortic stenosis, both with coarctation of the aorta (1 of them with aortic hypoplasia as well); in addition, there was 1 patient with perimembranous VSD, 1 with PDA, and 1 with bicuspid aortic valve (Table I).
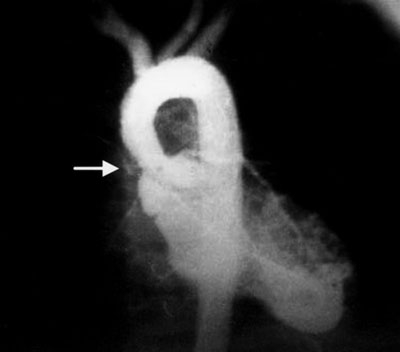
Fig. 3 Left ventricular arteriogram with contrast agent shows supravalvular aortic stenosis (arrow), the cardiopathy most frequently seen in Williams-Beuren syndrome.
Table I. Frequency of Cardiopathies in Williams-Beuren Syndrome
The pressure gradients of the patients with SAoS ranged from 14 to 81 mmHg. Several cases were of the ring type, and 2 were of the membranous type. One of the patients with coarctation of the aorta had pseudocoarctation, with a pressure gradient of 14 mmHg. The aortic hypoplasia at the thoracic level had a 25-mmHg gradient from the ascending to the descending aorta and was associated with severe systemic hypertension. Pressure gradients in the 3 patients with PA branch stenosis were 26, 50, and 70 mmHg (Fig. 4). A patient with ring-type aortic stenosis had a pressure gradient of 66 mmHg, and the patient with pulmonary stenosis had a gradient of 37 mmHg.
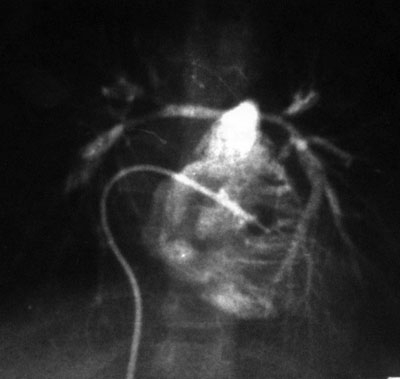
Fig. 4 Arterial angiogram (posteroanterior projection) with contrast media in the right ventricle shows important decrease in the lumina of the pulmonary branches.
The associated congenital heart defects SAoS plus VSD and PA branch stenosis plus ASD yielded systemic hypertension pressure gradients of 35 and 100 mmHg, respectively. Patients with SAoS plus VSD and with VSD alone had pulmonary hypertension with a pressure gradient of 40 mmHg.
The 8 patients with healthy hearts were evaluated clinically and electrocardiographically. The patient with PDA and one with a healthy heart were also diagnosed by echocardiography (to rule out diagnostic doubt). In the patient with aortic hypoplasia, echocardiography, catheterization, angiography, and an aortic biopsy were performed. The other 30 patients were evaluated by means of echocardiography and, in 2 cases, by heart catheterization as well. None of the 40 patients with W-BS was found to have another genetic syndrome. Psychomotor delay of varying severity was seen in all cases.
Treatment focused on improving heart failure with drugs such as digitalis and diuretic agents. Of the 40 patients with W-BS, only 2 were treated surgically: an aorto-aortic bridge was created to treat aortic hypoplasia on 2 occasions (same patient) and a VSD was closed with a Dacron patch in another patient; this last patient showed signs of complete atrioventricular block and requires a pacemaker, but today is evolving satisfactorily. A patient with SAoS is also scheduled for balloon aortoplasty, while another with SAoS underwent aortoplasty, fell into cardiac arrest, and died shortly afterwards.
Of the original 40 patients, 22 are still under our supervision, 7 abandoned treatment, 10 have been discharged from the hospital due to improvement, and 1 died of cardiogenic shock after an aortoplasty.
Discussion
Historically, diagnoses of W-BS at our center have been made on the basis of clinical criteria; only recently has W-BS been diagnosed using FISH.51 Since June 1997, cases of W-BS have been diagnosed by the FISH method. At our institute, the average age of these patients at diagnosis is the same (4.4 yr) as that reported in other studies (range, 4–6 yr at diagnosis).
The percentage of W-BS cases that were associated with congenital heart defects (80%) at the National Institute of Pediatrics is in the upper end of the range of percentages reported by other investigators (77%–81%),2,16,28 probably because our hospital is a referral center for children from the entire country. We are unaware of the frequency of W-BS among patients with SAoS; this is beyond the scope of our article. However, in a previous review52 of patients seen at this institute from 1971 through 1983, 10 of 15 SAoS patients were found to have associated W-BS (66%), a bit higher than the 50% figure that we found in a literature search.28
Our figures concur with the current medical literature in that the 2 most frequent cardiopathies we found in association with W-BS were SAoS (56%) and SAoS with PA branch stenosis (13%); this last figure takes into account the association of SAoS and PA branch stenosis in 4 patients (Table II).
Table II. Cardiopathies in Williams-Beuren Syndrome: Experience at the National Institute of Pediatrics Compared with Others Around the World
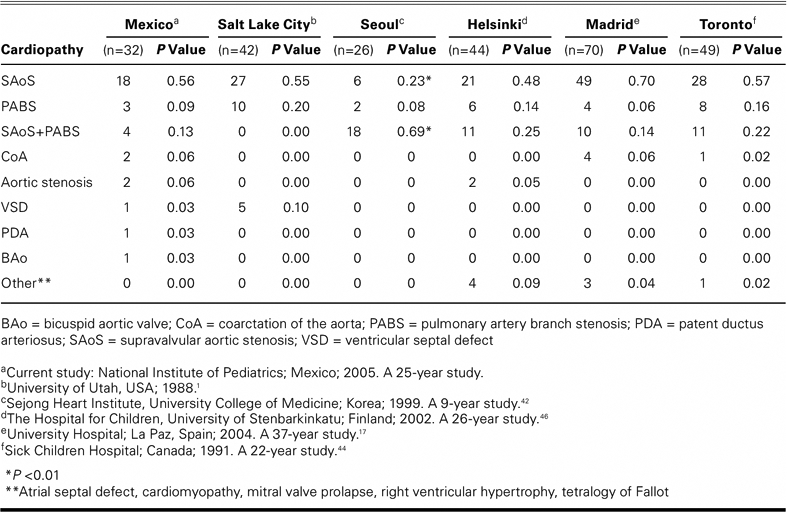
Our study highlights a case of PDA cardiopathy, which condition is uncommon in W-BS. We also present a case with bicuspid aortic valve and another with partial atrioventricular defect, neither of which, to the best of our knowledge, has been reported in the world literature in association with W-BS (Tables I and II).
In our institute, only 2 patients were found to have hypercalcemia. Systemic hypertension was seen in the 1 patient with aortic hypoplasia. Decreased vascular diameter may of course be a cause of systemic hypertension.
In our study, all patients had various degrees of mental retardation, as previously described.24 When SAoS occurs independently of W-BS—either sporadically or familially—such patients are likely to have normal intelligence.
Only 1 of our patients died. The overall good survival is due to the fact that the cardiopathy is benign in childhood, although it is progressive and complications are often seen in later stages of life.11,18,25
We compared our study results with those of other hospitals and found no significant differences in number except for the cases of SAoS and SAoS with PA branch stenosis at Seoul, the percentages of which were at substantial variance from ours (P < 0.01). Cases of PDA, bicuspid aortic valve, and atrioventricular defect were infrequent among our patients and were not seen at all among the other hospitals whose results we reviewed (Table II). A contrary situation occurred with other cardiopathies not seen in our group of patients but registered at the other hospitals: mitral valve prolapse, ASD, cardiomyopathy, TOF, and right ventricular hypoplasia (Table II). We considered our patient with aortic hypoplasia to be in the aortic coarctation group because aortic hypoplasia is a type of left obstruction, which is also uncommon.
Conclusions
Congenital heart defects were found in 32 (80%) of our W-BS patients. The defect that we saw most frequently in association with W-BS was SAoS, which we found in more than half of our cases (18 of 32 cases, or 56%). When SAoS is found in patients with W-BS, there may be other heart lesions as well. Indeed, the cardiopathy that we found second most frequently associated with W-BS was SAoS with PA branch stenosis, found in 4 of 32 cases (13%).
In our study, we found 1 case of PDA and another of aortic hypoplasia, both of which are uncommon in infants with W-BS. We also found a case of bicuspid aortic valve and a case of partial atrioventricular defect, neither of which has been reported in other studies.
Pulmonary hypertension, systemic hypertension, and hypercalcemia are not often manifest in children, and the childhood prognosis for these is benign; the complications are seen in later decades.
The clinical and echocardiographic diagnosis in patients who have W-BS is of the utmost importance, because this is a degenerative and progressive illness.
Acknowledgments
We thank Marlene Llopiz and Trish Arzani for their help in preparing the manuscript.
Footnotes
Address for reprints: Jesús De Rubens Figueroa, MD, Departamento de Cardiología, Instituto Nacional de Pediatría, Insurgentes Sur 3700 – C. Col. Insurgentes Cuicuilco, CP 04530 México, D.F. México
E-mail: derubens@hotmail.com
References
- 1.Morris CA, Demsey SA, Leonard CO, Dilts C, Blackburn BL. Natural history of Williams syndrome: physical characteristics. J Pediatr 1988;113(2):318–26. [DOI] [PubMed]
- 2.Jones KL, Smith DW. The Williams elfin facies syndrome. A new perspective. J Pediatr 1975;86(5):718–23. [DOI] [PubMed]
- 3.Preus M. The Williams syndrome: objective definition and diagnosis. Clin Genet 1984;25(5):422–8. [DOI] [PubMed]
- 4.Greenberg F. Williams syndrome. Pediatrics 1989;84(5): 922–3. [PubMed]
- 5.Cassidy SB, Allanson JE. Williams syndrome. In: Management of genetic syndromes. New York: Wiley-Liss; 2001. p. 517–32.
- 6.Taybi H, Lachman RS. In: Radiology of syndromes, metabolic disorders, and skeletal dysplasias. 4th ed. St. Louis: Mosby; 1996. p. 528–30.
- 7.Williams JC, Barratt-Boyes BG, Lowe JB. Supravalvular aortic stenosis. Circulation 1961;24:1311–8. [DOI] [PubMed]
- 8.Rashkind WJ, Golinko R, Arcasoy M. Cardiac findings in idiopathic hypercalcemia of infancy. J Pediatr 1961;58: 464–9. [DOI] [PubMed]
- 9.Beuren AJ, Apitz J, Harmjanz D. Supravalvular aortic stenosis in association with mental retardation and a certain facial appearance. Circulation 1962;26:1235–40. [DOI] [PubMed]
- 10.Beuren AJ, Schulze C, Eberle P, Harmjanz D, Apitz J. The syndrome of supravalvular aortic stenosis, peripheral pulmonary stenosis, mental retardation and similar facial appearance. Am J Cardiol 1964;13:471–83. [DOI] [PubMed]
- 11.Gorlin RJ, Cohen MM, Levin LS. In: Syndromes of the head and neck. 3rd ed. New York: Oxford University Press; 1990. p. 143–8.
- 12.Ino T, Nishimoto K, Iwahara M, Akimoto K, Boku H, Kaneko K, et al. Progressive vascular lesions in Williams-Beuren syndrome. Pediatr Cardiol 1988;9(1):55–8. [DOI] [PubMed]
- 13.Curran ME, Atkinson DL, Ewart AK, Morris CA, Leppert MF, Keating MT. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell 1993; 73(1):159–68. [DOI] [PubMed]
- 14.Ewart AK, Jin W, Atkinson D, Morris CA, Keating MT. Supravalvular aortic stenosis associated with a deletion disrupting the elastin gene. J Clin Invest 1994;93(3):1071–7. [DOI] [PMC free article] [PubMed]
- 15.Ewart AK, Morris CA, Atkinson D, Jin W, Sternes K, Spallone P, et al. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat Genet 1993;5(1): 11–6. [DOI] [PubMed]
- 16.Lashkari A, Smith AK, Graham JM Jr. Williams-Beuren syndrome: an update and review for the primary physician. Clin Pediatr (Phila) 1999;38(4):189–208. [DOI] [PubMed]
- 17.Pascual-Castroviejo I, Pascual-Pascual SI, Moreno Granado F, Garcia-Guereta L, Gracia-Bouthelier R, Navarro Torres M, et al. Williams-Beuren syndrome: presentation of 82 cases [in Spanish]. An Pediatr (Barc) 2004;60(6):530–6. [DOI] [PubMed]
- 18.Bird LM, Billman GF, Lacro RV, Spicer RL, Jariwala LK, Hoyme HE, et al. Sudden death in Williams syndrome: report of ten cases. J Pediatr 1996;129(6):926–31. [DOI] [PubMed]
- 19.Black JA, Carter RE. Association between aortic stenosis and facies of severe infantile hypercalcaemia. Lancet 1963;2: 745–9. [DOI] [PubMed]
- 20.Jones KL. Williams syndrome: an historical perspective of its evolution, natural history, and etiology. Am J Med Genet Suppl 1990;6:89–96. [DOI] [PubMed]
- 21.Sanchez Cascos A. Genetica cardiovascular. Madrid: Jarpyo Editores; 1985. p. 111–2.
- 22.Doty DB, Polansky DB, Jenson CB. Supravalvular aortic stenosis. Repair by extended aortoplasty. J Thorac Cardiovasc Surg 1977;74(3):362–71. [PubMed]
- 23.Friedman WF, Roberts WC. Vitamin D and the supravalvar aortic stenosis syndrome. The transplacental effects of vitamin D on the aorta of the rabbit. Circulation 1966;34(1):77–86. [DOI] [PubMed]
- 24.Hallidie-Smith KA, Karas S. Cardiac anomalies in Williams-Beuren syndrome. Arch Dis Child 1988;63(7):809–13. [DOI] [PMC free article] [PubMed]
- 25.Conway EE Jr, Noonan J, Marion RW, Steeg CN. Myocardial infarction leading to sudden death in the Williams syndrome: report of three cases. J Pediatr 1990;117(4):593–5. [DOI] [PubMed]
- 26.Nakanishi T, Iwasaki Y, Momma K, Imai Y. Supravalvular aortic stenosis, pulmonary artery stenosis, and coronary artery stenosis in twins. Pediatr Cardiol 1996;17(2):125–8. [DOI] [PubMed]
- 27.Espino-Vela J. Estenosis supravalvular aortica. In: Cardiologia pediatrica. 3rd ed. Mexico City: Francisco Mendez Oteo; 1994. p. 284–5, 610.
- 28.Kitchiner D, Jackson M, Walsh K, Peart I, Arnold R. Prognosis of supravalve aortic stenosis in 81 patients in Liverpool (1960–1993). Heart 1996;75(4):396–402. [DOI] [PMC free article] [PubMed]
- 29.Arrington C, Tristani-Firouzi M, Puchalski M. Rapid progression of long-segment coarctation in a patient with Williams' syndrome. Cardiol Young 2005;15(3):312–4. [DOI] [PubMed]
- 30.Ingelfinger JR, Newburger JW. Spectrum of renal anomalies in patients with Williams syndrome. J Pediatr 1991;119(5): 771–3. [DOI] [PubMed]
- 31.Kaplan P, Levinson M, Kaplan BS. Cerebral artery stenoses in Williams syndrome cause strokes in childhood. J Pediatr 1995;126(6):943–5. [DOI] [PubMed]
- 32.Maisuls H, Alday LE, Thuer O. Cardiovascular findings in the Williams-Beuren syndrome. Am Heart J 1987;114(4 Pt 1):897–9. [DOI] [PubMed]
- 33.Ottesen OE, Antia AU, Rowe RD. Peripheral vascular anomalies associated with the supravalvular aortic stenosis syndrome. Radiology 1966;86(3):430–5. [DOI] [PubMed]
- 34.Pernot C, Worms AM, Marcon F, Admant P. Williams-Beuren facies with mental retardation and tetralogy of Fallot [in French]. Pediatrie 1984;39(1):53–8. [PubMed]
- 35.Soper R, Chaloupka JC, Fayad PB, Greally JM, Shaywitz BA, Awad IA, Pober BR. Ischemic stroke and intracranial multifocal cerebral arteriopathy in Williams syndrome. J Pediatr 1995;126(6):945–8. [DOI] [PubMed]
- 36.Char F. Williams facies with portal vein aplasia and mental retardation. Birth Defects 1972;8:262–3.
- 37.Harris LC, Nghiem QX. Idiopathic hypercalcemia of infancy with interruption of the aortic arch. J Pediatr 1968;73(1): 84–8. [DOI] [PubMed]
- 38.Levy EP. Infantile hypercalcemia facies and mental retardation associated with atrial septal defect and anomalous pulmonary venous return. Birth Defects 1972;8:73–4.
- 39.Becker AE, Becker MJ, Edwards JE. Mitral valvular abnormalities associated with supravalvular aortic stenosis. Observations in 3 cases. Am J Cardiol 1972;29(1):90–4. [DOI] [PubMed]
- 40.Bzduch V, Masura J. Supravalvar aortic stenosis–a constant feature of Williams-Beuren syndrome. Pediatr Cardiol 1993;14 (1):66. [DOI] [PubMed]
- 41.Narin N, Ozyurek R, Bakiler AR, Parlar A, Arcasoy M, Koprubasi F. Williams syndrome and subaortic stenosis. Clin Genet 1993;44(4):223. [DOI] [PubMed]
- 42.Kim YM, Yoo SJ, Choi JY, Kim SH, Bae EJ, Lee YT. Natural course of supravalvar aortic stenosis and peripheral pulmonary arterial stenosis in Williams' syndrome. Cardiol Young 1999;9(1):37–41. [DOI] [PubMed]
- 43.van Son JA, Edwards WD, Danielson GK. Pathology of coronary arteries, myocardium, and great arteries in supravalvular aortic stenosis. Report of five cases with implications for surgical treatment. J Thorac Cardiovasc Surg 1994;108(1):21–8. [PubMed]
- 44.Zalzstein E, Moes CA, Musewe NN, Freedom RM. Spectrum of cardiovascular anomalies in Williams-Beuren syndrome. Pediatr Cardiol 1991;12(4):219–23. [DOI] [PubMed]
- 45.Actis Dato GM, La Torre M, Caimmi P, Actis Dato A Jr, Centofanti P, Ottino GM, Di Summa M. Williams-Beuren syndrome. Long-term results of surgical treatments in six patients. J Cardiovasc Surg (Torino) 1997;38(2):125–9. [PubMed]
- 46.Eronen M, Peippo M, Hiippala A, Raatikka M, Arvio M, Johansson R, Kahkonen M. Cardiovascular manifestations in 75 patients with Williams syndrome. J Med Genet 2002; 39(8):554–8. [DOI] [PMC free article] [PubMed]
- 47.Wessel A, Pankau R, Kececioglu D, Ruschewski W, Bursch JH. Three decades of follow-up of aortic and pulmonary vascular lesions in the Williams-Beuren syndrome. Am J Med Genet 1994;52(3):297–301. [DOI] [PubMed]
- 48.van Pelt NC, Wilson NJ, Lear G. Severe coronary artery disease in the absence of supravalvular stenosis in a patient with Williams syndrome. Pediatr Cardiol 2005;26(5):665–7. [DOI] [PubMed]
- 49.Bruno E, Rossi N, Thuer O, Cordoba R, Alday LE. Cardiovascular findings, and clinical course, in patients with Williams syndrome. Cardiol Young 2003;13(6):532–6. [PubMed]
- 50.Devore LJ. Probabilidad y estadística para ingenieria y ciencias. 4th ed. Mexico: International Thomson Editores, ITP; 1998. p. 362–3.
- 51.Committee on Genetics. American Academy of Pediatrics: Health care supervision for children with Williams syndrome [published erratum appears in Pediatrics 109(2):329]. Pediatrics 2001;107(5):1192–204. [PubMed]
- 52.Espinoza RG, Corona E, Espino JV. Estenosis aortica supravalvular. Acta Pediatrica de Mexico 1984;5:3–11.


