Abstract
Background:
Pressure ulcers are one of the most prevalent causes of morbidity in patients with spinal cord injury (SCI). For those requiring hospital-based management, conventional wound management may necessitate a prolonged institutional stay. This may subsequently increase the likelihood of comorbidities and increase the social, psychological, and financial burdens associated with wound management. Therefore, novel adjunct treatments that potentiate improved healing rates should be seriously considered.
Study Design:
Case reports.
Objective:
To observe the efficacy of the EpiFLO device as an adjunct treatment modality in chronic wound management.
Setting:
An SCI unit at a Veterans Affairs Medical Center.
Methods:
Three men with SCI, who each presented with a stage IV pressure ulcer in the pelvic region, were treated with the EpiFLO device as an adjunct therapy. In Case 1, the patient was monitored for 9 weeks, whereas in Cases 2 and 3, the patients were monitored for 5 weeks. Healing was determined on a weekly basis by wound dimensions and volume, which were compared before and after the intervention.
Results:
Comparison of pre- and posttreatment outcome measurements showed significant improvement with EpiFLO in each case.
Conclusion:
EpiFLO seems to have had a positive effect on the healing rate of chronic pressure ulcers in individuals with SCI.
Keywords: Pressure ulcer, Spinal cord injuries, Wound management, Hyperbaric oxygen therapy, Transdermal oxygen delivery system
INTRODUCTION
It has been established that oxygenation, along with other tissue characteristics, such as fibroblast activity, collagen synthesis, and amino acid production/adequacy, serves as a cellular envoy to promote wound healing (1). Niinikoski (2) identified the stimulation of leukocytic activities, the stimulation of fibroblast proliferation, increased collagen formation, and augmented neovascularization as being important manifestations of hyperbaric oxygenation. Similarly, ischemic soft tissues also benefit from hyperoxygenation through the improved preservation of energy metabolism. Historically, hyperbaric oxygen therapy (HBOT) was a treatment modality used in the treatment of CO2 poisoning and decompression sickness in deep sea divers. More recently, HBOT has been used as a treatment that improves oxygen supply to wounds and promotes wound healing (3). However, in the case of treating chronic wounds, the ultimate goal of HBOT is that of bacteriostasis. In support of this, Greensmith (4) stipulated that by enhancing bactericidal functions of leukocytes, wound necrosis may be prevented and wound healing accelerated.
Although the application of systemic HBOT differs from topical oxygenation, such as with the EpiFLO device (Ogenix Corporation, Cleveland, Ohio), the concept of tissue oxygenation therapy is similar. EpiFLO is a new, US Food and Drug Administration–approved, portable, self-sustained, transdermal oxygen delivery system for wound therapy. According to the manufacturer, EpiFLO extracts oxygen from the air, concentrates it to near 100%, and pumps the oxygen through a cannula to saturate the wound in oxygen (5).
The purpose of this study was not to compare HBOT to the EpiFLO device or to suggest equivalency between the 2 modalities, but rather to share the results of 3 case studies using the EpiFLO device (a similar concept) as a novel adjunct treatment modality in chronic wound management.
METHODS
Three patients with spinal cord injury (SCI) who presented to the spinal cord unit with a stage IV pressure ulcer in their pelvic region were treated with the EpiFLO device as an adjunct therapy. Before the intervention, conventional wound care was provided according to the patient's wound characteristics. After exhausting many wound treatment modalities and the observance of no significant decrease in wound size, the EpiFLO device was added to the conventional dressing regimen. Linear measurements of the wound in each case were calculated weekly, using the formula length + width + depth in centimeters (6). With a disposable paper ruler, length and width were obtained by measuring the wound surface at the widest point, perpendicular to each other along the body axis. Depth was determined by inserting a cotton-tipped swab into the wound at its deepest point and measuring the amount of swab below the wound edge, also with a disposable paper ruler. The volume of each wound was calculated weekly using the formula length × width × depth in cubic centimeters (cm3). To enhance reliability, the same rater was used to obtain all wound measurements. Depending on the wound bed condition, dressings such as wet to dry, wet to moist, and calcium alginate were frequently used on the SCI unit. Platelet-derived growth factors were sometimes ordered for off-label use, in addition to the conventional dressing as noted in Case 2. In each case, as the wound status changed, dressing regimens were revised in an effort to gain a more desirable result in wound healing. Before application of the EpiFLO device, the wound and periwound area was cleansed with normal saline and dried of all visible moisture to facilitate an airtight seal of the final transparent dressing. The flexible rubber cannula of the EpiFLO device was placed on the wound bed, and the recommended dressing was applied. An airtight seal was created with the clear occlusive dressing, which allowed for easy monitoring of the wound and periwound tissue. The dressing would conform to body contour, which decreased the risk of displacement and minimized unnecessary dressing changes. In addition to the ease of application, this dressing was also easily removed, thus decreasing the risk for further damage to the intact skin beneath. During the final step of the application process, the concentrator device was secured to the patients' abdominal area with a cloth adhesive tape. This was the area of choice to alleviate the potential for pressure ulcer development, which could result from the patient resting on the device.
RESULTS
Case 1
A 60-year-old man with C7 ASIA A tetraplegia, according to the American Spinal Injury Association International standards for neurological classifications (7), presented with a chronic difficult-to-heal stage IV pressure ulcer of the left ischium, which had persisted for more than 3 months before admission. Throughout his stay on the SCI unit, several conventional treatment modalities were exhausted, but they failed to render desirable results. Attempted treatment modalities included wet-to-moist dressings, calcium alginate, hydrogel, and sharp and mechanical debridement. Despite the conventional treatment regimen, this wound was not showing a significant decrease in wound size, nor was it making any significant progress toward healing. Therefore, EpiFLO was applied as an adjunct treatment to his routine wound therapy.
This individual was assessed using linear measurements for a period of 9 weeks before the application of the device. For 9 weeks after the application of the device, these same assessments were continued and documented weekly. On admission, the wound dimensions (L + W + D) measured 21 cm linearly, with a volume measurement of 308.8 cm3. Nine weeks later, the baseline assessment before intervention showed little change in size, with the wound measuring 20.5 cm linearly and a volume measurement of 252 cm3. This was a 2% change in the linear measurement during the pretreatment phase of 9 weeks. The posttreatment measurements 9 weeks later were 10.2 cm linearly, with a volume measurement of 24 cm3. Linearly, this was a 49% improvement from baseline.
Case 2
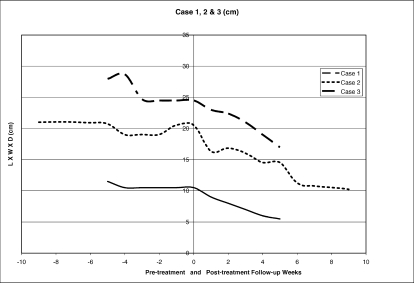
A 57-year-old man with T12 ASIA A paraplegia was admitted with a 6-month history of a chronic, difficult-to-heal, stage IV pressure ulcer of the right ischium. This ulcer was complicated by a fistula with purulent drainage. Shortly after admission, he was taken to surgery for excision of the fistula. Bone biopsy and debridement were performed. After surgery, this individual received the conventional inpatient wound care of moist dressing changes every shift, with the addition of daily becaplermin. Initially, small changes were noted in the wound size linearly, but these changes reached a plateau as shown in Figure 1. Because there had been no significant healing during the 5 weeks between the time of surgery and the baseline assessment 5 weeks later, EpiFLO was applied as an adjunct treatment to his routine wound therapy.
Figure 1. Pre- and posttreatment length + width + depth (cm).
This individual was assessed using linear measurements for a period of 5 weeks before the application of the device. For 5 weeks after the application of the EpiFLO device, these same assessments were performed weekly. On admission, the wound dimensions (L + W + D) measured 11.5 cm linearly, with a volume measurement of 50 cm3. Five weeks later, the baseline assessment before intervention showed little change in size, with the wound measuring 10.5 cm linearly and a volume measurement of 30 cm3. This was a 9% change in the linear measurement during the pretreatment phase. The post-treatment measurements 5 weeks after baseline were 5.5 cm linearly, with a volume measurement of 5 cm3. Linearly, this was a 48% improvement from baseline.
Case 3
A 66-year-old man with T10 ASIA A paraplegia was admitted with a history of a difficult-to-heal, necrotic, stage IV, sacral pressure ulcer. He was referred to the SCI unit from an outside surgical center for consultation and treatment of his sacral ulcer that was noted on day 6 after vascular surgery. After admission, the wound required sharp debridement and received the conventional inpatient wound care of wet-to-dry dressing changes every 6 hours with the addition of daily papain. Initially, the wound size was increased because of the debridement of necrotic tissue. Subsequently, small changes in wound sizes were noted linearly, but these changes subsided and reached a plateau as shown in Figure 1. Because there had been no significant healing during the 5 weeks between the time of admission and the baseline assessment, EpiFLO was applied as an adjunct treatment to his routine wound therapy.
This individual was assessed using linear measurements for a period of 5 weeks before the application of the device. For 5 weeks after the application of the EpiFLO device, these same assessments were performed. On admission, the wound dimensions (L + W + D) measured 27.9 cm linearly, with a volume measurement of 759 cm3. Five weeks later, the baseline assessment before intervention showed little change in size, with the wound measuring 24.5 cm linearly and a volume measurement of 385 cm3. This was a 12% change in the linear measurement during the pretreatment phase of 5 weeks. The posttreatment measurements 5 weeks later were 17 cm linearly, with a volume measurement of 131 cm3. Linearly, this was a 31% improvement in from baseline.
DISCUSSION
Chronic and difficult-to-heal pressure ulcers are a common and serious medical complication among the population with SCI. In a study by Garber and Rintala (8), 39% of all patients on the SCI roster were reported to have received medical attention for this complication. These same authors reported that the average number of hospitalization days for wound management was 150 days. Over a period of 3 years, the approximate cost for wound care to their study population of 57 patients was $8,550,000. This equates to an average cost of $150,000 per patient hospitalized for wound care. Garber and Rintala (8) further stated that the average cost to heal one complex full-thickness pressure ulcer is estimated to be around $70,000 dollars annually. Similarly, the Veteran Administration Office of Inspector General (9) projected the annual cost for the treatment of pressure ulcers to be between $1.3 and $3.6 billion dollars. The direct cost to treat pressure ulcers is therefore very expensive. However, when the indirect cost such as loss of income and productivity resulting from hospitalization and the psychosocial cost are factored in, the total cost may become even more inflated. Wound care while hospitalized is not only costly but may place the patient at risk for cross-contamination, which generally results in infections (10). Thus, the longer the wound is present, the greater the risk for infections. Although there are many treatment options available for the treatment of pressure ulcers, they are often unsuccessful, and pressure ulcers continue to be a lifelong challenge to the patient and their health care providers.
Within the SCI population, limitations in mobility often result in pressure ulcers that are large, chronic, and debilitating. The size of the wound may also limit the number of options available for effective treatment. Williams and Armstrong (11) suggested that most patients referred to HBOT have larger wounds. These same researchers supported the fact that wounds in the SCI population are most appropriate for this modality, because they are also generally large. Despite the compatibility of HBOT with large wounds, barriers still exist. Initially, the cost of this treatment can be very expensive. According to some sources, HBOT sessions may cost the patients $150 to $1,000 dollars in fees per session, while anywhere from 50- to 100-hour-long sessions may be required, depending on the patients and their wound characteristics (12). Availability and portability are additional barriers to the utilization of HBOT for individuals with SCI. Most importantly, the reality is that, for a patient with SCI, access to HBOT is often times impossible. Therefore, a portable and less costly concentrated topical oxygen delivery system could prove to be beneficial in the treatment of difficult-to-heal pressure ulcers within this population.
The EpiFLO device seemed to have been an efficacious adjunct treatment modality for chronic and difficult to heal pressure ulcers among the SCI population. This device shares the same concept as HBOT, although the delivery mechanism differs. The EpiFLO device measured 2 × 1.4 × 0.9 in3, was silent, was disposable, and came in 2 parts. No special storage and minimal training for its use was required, because operation of the device involved only a flip of an on and off switch. Weighing 2 oz, the device concentrated oxygen from room air and deliver it at a rate of 3 mL/min to the wound bed, 24 hours daily by a cannula. The cannula was sterile, 43-in long, and could be cut to a suitable length per patient preference for placement of the concentrator and wound location. While wearing this device, ambulation and mobility was not compromised, and continuous oxygenation could be provided to the wound bed. As an adjunct treatment to conventional treatment modalities, the manufacturers of EpiFLO believe that the device was most effective when changed once a week. The device was attached to the patient in less than 60 seconds and required no additional equipment such as an oxygen tank. Furthermore, EpiFLO did not dry out the wound. In each case, the wound size and volume appeared to have more rapidly decreased during the use of the EpiFLO device; therefore, a reduced hospital stay for wound management could possible be achieved.
According to Kalliainen et al (13), advantages of topical oxygen therapy include low cost, the elimination of systemic oxygen toxicity, and increased access for in-home use. The latter advantage potentially allows a greater population of patients to garner the benefits of topical oxygen therapy. Additional benefits may include a decrease in local tissue ischemia and the promotion of granulation, angiogenesis, and epithelialization. These benefits are supported by Said et al (14), in their animal study of 4 full-thickness punch wounds, on which a similar device was used.
With ischemia being hypothesized as an important factor in pressure ulcer development, it seems reasonable that a therapy that supplies the compromised tissue with concentrated oxygen would be extremely beneficial. In light of the tremendous cost of wound care, any treatment modality that potentiates a decrease in wound chronicity and wound healing time would be advantageous. Increased healing rates may equate to a reduction of the duration of hospitalization, thus potentially lowering the direct and indirect cost of wound care. When considering the potential for increased healing rates, the EpiFLO device may very well be a cost-effective adjunct therapy. According to the manufacturers, the cost for the device is about $700 weekly, in comparison to the cost of HBOT, which could range from $7,500 to $100,000, depending on the number of sessions needed (10).
Although the results of these 3 cases shed a positive light on the effectiveness of the device in decreasing wound size, the significance of these results may have been dampened by the limited number of cases observed. In addition, because this was not a controlled trial, many confounding variables may have existed. Therefore, we examined several of the most important variables. All the wounds were ruled out for osteomyelitis. None of the participants received any medication such as oxandrolone or testosterone that would enhance wound healing. We also took into account hemoglobin levels for the assessment of anemia and the patients' albumin levels for the assessment of nutritional status. In each case, the hemoglobin values were found to be subnormal (range, 9.2–10.4 g/dL), but they all were stable throughout the study period. Regarding nutritional status, the albumin levels throughout the study period were 3.7 to 3.8g/dL for Cases 1 and 2 and 8 g/dL for Case 3. However, the albumin level for Case 2 decreased from 3.8 g/dL at the beginning of the study to 2.8 g/dL during the treatment phase. Despite the deterioration in nutritional status in Case 2, the progression of healing continued. In terms of limitations, there were occasional breaks in the airtight seal of the dressings. Overall, there were no significant changes in the patients' medications, dressing orders, or hematologic or nutritional status, which would enhance their wound healing. The only positive change in each case was the application of the EpiFLO device. Therefore, we can safely imply that these changes in wound size were caused by application of the device.
CONCLUSION
Although the etiology of pressure ulcer development is multifactorial, ischemia is a major cause. Within the SCI population, EpiFLO seemed to have had a positive association with the healing rate of these 3 difficult-to-heal wounds. The potential for a deceased length of stay in the hospital and treatment efficacy supports the use of EpiFLO as an adjunct treatment modality within the SCI population. There is a paucity of research in the area of portable topical oxygen therapy and its cost effectiveness. Therefore, a randomized controlled study is needed to validate these clinical observations.
Acknowledgments
The authors thank Monique Washington, MS, RN, and Dr Kath Bogie for assistance with graphics and Theresa Spencer, MSN, NP, for study initiation.
Footnotes
The EpiFLO device (Model #01-100-100000) was supplied by the manufacturer, Ogenix Corporation, 23230 Chargin Boulevard, Bldg. 3, Ste. 950, Cleveland, OH 44122.
REFERENCES
- MacKay D, Miller AL. Nutritional support for wound healing. Altern Med Rev. 2003;8((4)):359–377. [PubMed] [Google Scholar]
- Niinikoski JH. Clinical hyperbaric oxygen therapy, wound perfusion and transcutaneous oximetry. World J Surg. 2004;28((3)):307–311. doi: 10.1007/s00268-003-7401-1. [DOI] [PubMed] [Google Scholar]
- Kranke P, Bennett M, Roeckl-Wiedmann I, Debus S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004. CD004123. [DOI] [PubMed]
- Greensmith JE. Hyperbaric oxygen therapy in extremity trauma. J Am Acad Orthop Surg. 2004;12((6)):376–384. doi: 10.5435/00124635-200411000-00002. [DOI] [PubMed] [Google Scholar]
- Ogenix. EpiFLOSD transdermal sustained O2 delivery oxygen concentrator: healing in the palm of your hand. Available at: http://www.ogenix.net/overview.htm. Accessed November 8, 2006.
- Bogie KM, Banks P, Washington M, Stubblefield AM, Ho CH. Is volume a true gold standard for wound size measurement? [abstract] Arch Phys Med. Rehabil. 2005. e16.
- American Spinal Cord Injury Association/International Medical Society of Paraplegia. International Standards for Neurological and Functional Classification of Spinal Cord Injury Patients. Chicago, IL: American Spinal Cord Injury Association/International Medical Society of Paraplegia; 2000. [Google Scholar]
- Garber SL, Rintala DH. Pressure ulcers in veterans with spinal cord injury: a retrospective study. J Rehabil Res Devel. 2003;40((5)):433–442. doi: 10.1682/jrrd.2003.09.0433. [DOI] [PubMed] [Google Scholar]
- Department of Veterans Affairs Office of Inspector General's (OIG) Healthcare inspection. Management of patients with pressure ulcers in Veterans Health Administration Facilities, December 2006. Available at: www.va.gov/oig/54/reports/VAOIG-05-00295-109.pdf. Accessed December 26, 2006.
- Kessler L, Bilbault P, Ortega F, et al. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: a prospective randomized study. Diabetes Care. 2003;6((8)):2378–2382. doi: 10.2337/diacare.26.8.2378. [DOI] [PubMed] [Google Scholar]
- Williams RL, Armstrong DG. Wound healing: new modalities for a new millennium. Review. Clin Podiatr Med Surg. 1998;15((1)):117–128. [PubMed] [Google Scholar]
- Cranton EM. If hyperbaric oxygen therapy is so good, why is it not more widely accepted. 2001. Available at: http://www.drcranton.com/hbo/widelyaccepted.htm. Accessed June 12, 2007.
- Kalliainen LK, Gordillo GM, Schlanger R Sen CK. Topical oxygen as an adjunct to wound healing: a clinical case series. Pathophysiology. 2003;9:81–87. doi: 10.1016/s0928-4680(02)00079-2. [DOI] [PubMed] [Google Scholar]
- Said HK, Hijjawi J, Roy N, Mogford J. Transdermal sustained-delivery oxygen improves epithelial healing in a rabbit ear wound model. Arch Surg. 2005;140:998–1004. doi: 10.1001/archsurg.140.10.998. [DOI] [PubMed] [Google Scholar]