Abstract
Objective:
To describe a spinal epidural abscess that originated from cellulitis after moxibustion.
Methods:
Case report.
Findings:
A 78-year-old woman with diabetes mellitus was diagnosed with tetraplegia due to a cervical spinal epidural abscess extending to the thoracic spinal epidural space. The abscess was caused by osteomyelitis and cellulitis of the right third finger, which had been cauterized repeatedly with moxa. After surgical decompression and drainage of the spinal epidural abscess and comprehensive rehabilitation, motor strength and functional level improved.
Conclusions:
This case illustrates the risk of spinal epidural abscess in persons with diabetes mellitus who present with focal cellulitis and osteomyelitis.
Keywords: Spinal cord injuries, Tetraplegia, Spinal epidural abscess, Moxa, Moxibustion, Diabetes mellitus, Cellulitis, Osteomyelitis, Oriental medicine, Group B Streptococcus
BACKGROUND
Spinal epidural abscesses account for 0.2 to 2 cases per 10,000 hospital admissions (1); among outpatients with back pain, its estimated prevalence is 0.00037 (2). The most common symptoms of spinal epidural abscess are back pain, radicular pain, weakness, and sensory deficit. Unfortunately, early symptoms are far from pathognomic, and, thus, prompt diagnosis during the early stage is difficult. Nevertheless, precise diagnosis and early surgical treatment, including emergency evacuation of the abscess, are critical because the seriousness of neurologic deficits depends on time elapsed from onset to surgery (3). The percentage of patients now achieving full recovery has plateaued at 41 to 47%, and mortality remains at 16% (1,4).
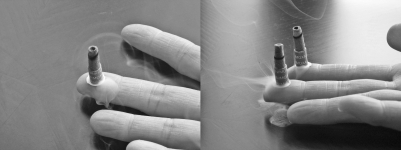
Moxibustion entails burning the moxa herb (mugwort) on or above the skin at acupuncture points to warm these points and alleviate symptoms. It has been widely used in Oriental medicine to treat chronic pain for thousands of years in China, Korea, and other Asian countries (5). Moxa is dried and ground to a fluff and, sometimes, processed into a stick. The herb may be applied indirectly using acupuncture needles or burned directly on the patient's skin as in this case (Figure 1).
Figure 1. Well-dried and finely compressed moxa on a finger tip. Cauterization is achieved by igniting the moxa. (This photograph is not of this patient's finger.).
Although widely considered safe, acupuncture and moxibustion may have some adverse effects, such as ecchymoses or hematoma, burn injuries, local discomfort, dizziness, nausea and vomiting, and contact dermatitis (6). In Korea, many people rely on Oriental medicine and folk remedies, such as moxa cautery, acupuncture, and bloodletting for the treatment of physical pain. As with any invasive procedures, these practices may cause infection. In particular, repeated moxibustion can cause burn injuries and subsequent skin infection. Superficial infection can spread to other sites, as in this case in which spinal epidural abscess was traced to a local infection.
CASE REPORT
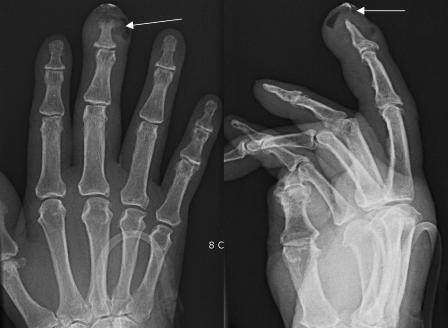
A 78-year-old woman presented at the emergency room with shoulder and neck pain and remittent fever of 7 days' duration. History was positive for diabetes mellitus, hypertension, low back pain, and osteoarthritis of both knees of several years' duration. She had cauterized her right third finger with moxa repeatedly over a period of 4 years and had experienced recurrent infections with intermittent pus discharge. On examination, the patient had a swollen, red right third fingertip with pustular discharge and was diagnosed as having cellulitis. Primary laboratory data showed an elevated white blood cell count of 13,900/mm3 and an erythrocyte sedimentation rate of 95 mm/h. She had no neurologic complaints and no symptoms or signs of upper respiratory, urinary tract, or other infection. Simple radiograph of the affected finger showed air density with diffuse soft-tissue swelling and cortical destruction at the distal phalange, which were compatible with cellulitis combined with osteomyelitis (Figure 2). After an incision and drainage of the finger, she was given intravenous antibiotics.
Figure 2. Abscess formation and osteomyelitis at the tip of the right middle finger (arrowed). Simple radiograph showing air density with diffuse swelling and cortical destruction at distal phalanges of the right middle finger.
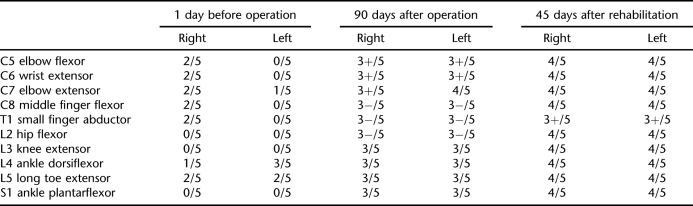
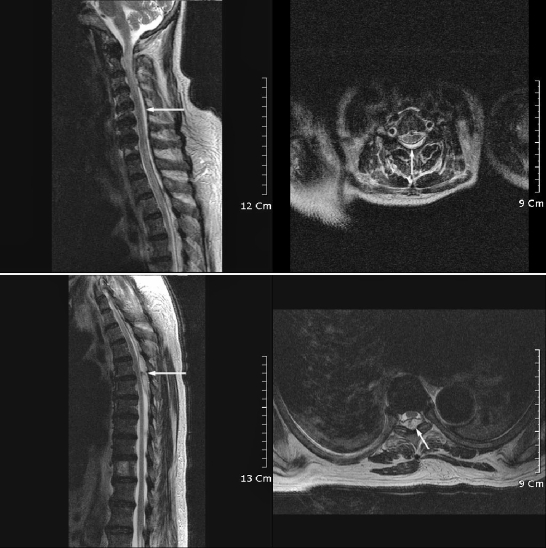
Streptococcus agalactiae was cultured from pus obtained from her fingertip. Three days after admission, the patient experienced a severe headache, high fever (39°C), and motor weakness and sensory loss of the upper and lower extremities. Manual muscle testing showed an inability to move against gravity in all extremities (Table 1). Lumbar puncture was performed to rule out meningitis. Cerebrospinal fluid showed an elevated white blood cell count of 2,400/mm3. Magnetic resonance imaging of the spine was performed, and a T2-weighted sagittal image showed an epidural collection of high intensity from C2 to T7, compatible with an epidural abscess, and high signal intensity in the spinal cord at C2-C3 (Figure 3).
Table 1.
Serial Change of Muscle Strength of the Patient
Figure 3. Magnetic resonance image of the epidural abscess of the cervical and thoracic spine (T2-weighted image). Spinal magnetic resonance image showing fluid collection (arrowed) with high signal intensity at the posterior body extending from the second cervical segment to the seventh thoracic segment.
The patient underwent immediate decompressive laminectomy at T5-T6. Pus was encountered during the operation, and a swab was taken. Suction of pus and irrigation were performed, and a drainage tube was inserted. After surgery, intravenous vancomycin hydrochloride (2.0 g/d) and ceftriaxone sodium (4.0 g/d) were administered for 2 and 4 weeks, respectively, whereupon intravenous levofloxacin (500 mg/day) was given for 3 weeks followed by oral levofloxacin (300 mg/day).
Ninety days later, the patient was transferred to the physical medicine and rehabilitation department for comprehensive rehabilitation. At the time of transfer, manual muscle testing showed improvement vs findings before decompressive laminectomy (Table 1). However, muscle strength was insufficient for independent functional use. Sensory examination showed reduced sensitivity to light touch and pain below the C4 dermatome level but intact perianal sensation.
Bulbocavernous reflex was positive by finger examination. The patient could not sit or stand by herself and needed maximal assistance for even the basic activities of daily life. To strengthen her muscles, active assisted range of motion exercises and functional electrical stimulation were started, and standing exercises were performed using a tilt table. With subsequent improvements in muscle strength, the training programs were changed to manually resisted or progressive resistance exercise programs, and functional mattress commenced. Activities of daily living training were initiated and progressed with motor recovery.
Thirty days after transfer to the physical medicine and rehabilitation department (120 days after operation), the patient started gait training. Forty-five days after transfer (135 days after operation), manual muscle testing showed 4/5 for all nerve roots except T1, which showed 3+/5 (Table 1); 170 days after the operation for epidural abscess, follow-up magnetic resonance imaging was performed, and the T2-weighted image showed no fluid collection or abscess in the epidural space; the patient was then discharged. At that time, she was able to ambulate with a walker and needed only a moderate level of assistance to perform the activities of daily living.
DISCUSSION
The incidence and prevalence of spinal epidural abscess appear to be increasing, presumably because of an aging population, the increasing abuse of intravenous drugs, and the growing number of immunocompromised patients (7). Risk factors for epidural abscess include trauma, immunodeficiency (eg, human immunodeficiency viral infection, alcoholism, diabetes mellitus, distal site infection, spine surgery, bloodstream infection, intravenous drug abuse). However, in up to one third of patients, sources of infection cannot be readily identified (8,9). In many cases, multiple risk factors and potential infection sources are present. Diabetes mellitus is the most common of these, and its association with spinal epidural abscess may be explained by reduced immunocompetence in individuals with diabetes (10). Spinal epidural abscess can be either primary, due to hematogenous spread of infection to the epidural space, or secondary, from an extension into the epidural space from a contiguous process, typically vertebral osteomyelitis or diskitis (3). Skin abscesses, furuncles, and paronychia are often identified as sources of infection. Minor, local skin infections can spread hematogenously, leading to the seeding of an epidural abscess.
In this case, the patient had 2 risk factors, namely, diabetes mellitus and chronic local skin infection. Because we were able to rule out another source of infection, we believe that her spinal epidural abscess originated from osteomyelitis and cellulitis of the finger secondary to burn injury caused by repeated moxibustion. Risks of moxibustion have not been well studied; one study reported that moxibustion had never been reported to cause a serious complication (6). Moxibustion, however, can cause burn injuries, which can become infected secondarily. Infection risk and systemic spread are more likely among individuals with diabetes mellitus and other types of immunodeficiencies. Risk is likely to be lower when moxibustion is performed by a licensed practitioner of Oriental medicine than self administered, as in this case.
The triad of fever, back pain, and progressive neurologic deficit has long been the essential sign of a spinal epidural abscess (3). Signs and symptoms are often vague during the early stage, and, in fact, the classic triad is present in only 13% of patients at the time of diagnosis, which contributes to diagnostic delay in 75% of patients (11).
Staphylococcus aureus is isolated from 57 to 75% of patients with spinal epidural abscess; other gram-positive cocci, such as, Staphylococcus epidermidis, Streptococcus pneumoniae, and viridans group streptococci, account for 10% of cases (8,11). In this case, no organism was isolated directly from the epidural abscess, probably because of suppression by prior antibiotic treatments for cellulitis and osteomyelitis. Based on cultures of the exudate from the infected fingertip, we presumed that the cellulitis and osteomyelitis were caused by S agalactiae (a group B Streptococcus). Group B streptococci may colonize the vaginal and gastrointestinal tracts in healthy women and can easily cause infections in neonates and pregnant women. However, it is extremely rare for an epidural abscess to be caused by group B streptococci. Three of the 4 previously reported cases were related to acupuncture injury (13), vesicoureteral reflux (14), and pregnancy (15), and the remaining case occurred in a healthy elderly individual (16). In terms of group B streptococcal bacteremia in men and nonpregnant women, the most common underlying disease is diabetes mellitus, as in the present case, and the most common infectious sources are urinary tract infections, pneumonia, and soft-tissue infections (14,17).
Once a diagnosis has been made, immediate decompressive laminectomy with abscess drainage and debridement is necessary. Postoperatively, intravenous antibiotic therapy is then administered for 4 to 6 weeks followed by oral antibiotics for 2 to 4 more weeks. Diagnostic delays of longer than 3 days increase the risk of mortality, and the prognosis is dismal for those with paralysis lasting more than 36 to 72 hours (18). A comparison of outcomes with individuals with traumatic spinal cord injury showed a poorer long-term outlook for individuals with myelopathy secondary to spinal epidural abscess (19).
Diagnosis in the described case was slightly delayed. Nevertheless, after prompt surgical treatment and rehabilitative training (although the patient did not recover to the level of independence), she showed significant improvement in strength and mobility. In particular, after therapeutic exercises focused on functional electrical stimulation, strengthening, and functional mattress and gait training, she showed improved muscle strength by 1 grade in all muscle groups after 45 days. This patient had not received physical therapy before transfer to the rehabilitation department. Early rehabilitation management is necessary to achieve maximal recovery.
CONCLUSIONS
Immunocompromised patients and those with diabetes mellitus are at risk for complications of skin infections. Systemic spread, usually by hematogenous routes, can result in serious infections. In this case, recurrent use of moxibustion for pain relief resulted in an infection of the distal finger that progressed to localized cellulitis and osteomyelitis that was linked to subsequent spinal epidural abscess. In Korea, it is not uncommon for people to use traditional folk remedies, such as moxa cautery, acupuncture, and bloodletting, for the treatment of physical pain. Appropriate care is required to prevent infection in individuals at high risk for infectious complication, especially those with diabetes mellitus who may have peripheral polyneuropathy and impaired immunity. Patient education and prompt intervention can reduce the risk of serious infection and its sequelae. Although uncommon, spinal epidural abscess has high rates of mortality and morbidity, especially when diagnosis is delayed. Moreover, functional improvements and long-term outcomes are not as good as for individuals with traumatic spinal cord injury (19).
REFERENCES
- Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 2000;23:175–204. doi: 10.1007/pl00011954. [DOI] [PubMed] [Google Scholar]
- Maslen DR, Jones SR, Crislip MA, Bracis R, Dworkin RJ, Flemming JE. Spinal epidural abscess: optimizing patient care. Arch Intern Med. 1993;153:1713–1721. [PubMed] [Google Scholar]
- Mackenzie AR, Laing RB, Smith CC, Kaar GF, Smith FW. Spinal epidural abscess: the importance of early diagnosis and treatment. J Neurol Neurosurg Psychiatr. 1998;65:209–212. doi: 10.1136/jnnp.65.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry WT, Jr, Hoh BL, Amin-Hanjani S, Eskandar EN. Spinal epidural abscess: clinical presentation, management, and outcome. Surg Neurol. 2005;63:364–371. doi: 10.1016/j.surneu.2004.08.081. [DOI] [PubMed] [Google Scholar]
- Sakakibara R, Murakami E, Katagiri A, et al. Moxibustion, an alternative therapy, ameliorated disturbed circadian rhythm of plasma arginine vasopressin and urine output in multiple system atrophy. Intern Med. 2007;46((13)):1015–1018. doi: 10.2169/internalmedicine.46.6450. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Tsukayama H, Tanno Y, Nishijo K. Adverse events in acupuncture and moxibustion treatment: a six-year survey at a national clinical in Japan. J Altern Complement Med. 1999;5((3)):229–236. doi: 10.1089/acm.1999.5.229. [DOI] [PubMed] [Google Scholar]
- Danner RL, Hartman BJ. Update of spinal epidural abscess: 35 cases and review of the literature. Rev Infect Dis. 1987;9:265–274. doi: 10.1093/clinids/9.2.265. [DOI] [PubMed] [Google Scholar]
- Chung SY, Chen CH, Yu WL. Spinal epidural abscess caused by group B Streptococcus in a diabetic woman presenting with febrile low back pain. Jpn J Infect Dis. 2005;58:177–179. [PubMed] [Google Scholar]
- Yin KS, Wang C, Lucero Y. Myelopathy secondary to spinal epidural abscess. J Spinal Cord Med. 1998;21:348–354. doi: 10.1080/10790268.1998.11719543. [DOI] [PubMed] [Google Scholar]
- Broner FA, Garland DE, Zigler JE. Spinal infections in the immunocompromised host. Orthop Clin North Am. 1996;27:37–46. [PubMed] [Google Scholar]
- Grewal S, Hocking G, Wildsmith JA. Epidural abscesses. Br J Anaesth. 2006;96:292–302. doi: 10.1093/bja/ael006. epub 2006 Jan 23. [DOI] [PubMed] [Google Scholar]
- Wheeler D, Keiser P, Rigamonti D, Keay S. Medical management of spinal epidural abscesses: case report and review. Clin Infect Dis. 1992;15:22–27. doi: 10.1093/clinids/15.1.22. [DOI] [PubMed] [Google Scholar]
- Lee SY, Chee SP. Group B Streptococcus endogenous endophthalmitis: case reports and review of the literature. Ophthalmology. 2002;109:1879–1886. doi: 10.1016/s0161-6420(02)01225-3. [DOI] [PubMed] [Google Scholar]
- Auletta JJ, John CC. Spinal epidural abscess in children: a 15-year experience and review of the literature. Clin Infect Dis. 2001;32:9–16. doi: 10.1086/317527. [DOI] [PubMed] [Google Scholar]
- Meis JF, Kullberg BJ, Kremer HP, Verweij PE. Cervical spinal epidural abscess due to group B Streptococcus in a previously healthy elderly male. Comment. Scand J Infect Dis. 2000;32:577. doi: 10.1080/003655400458974. [DOI] [PubMed] [Google Scholar]
- Jenkin G, Woolley IJ, Brown GV, Richards MJ. Postpartum epidural abscess due to group B. Streptococcus. Clin Infect Dis. 1997;25:1249. doi: 10.1086/516961. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Yuan HA. Neurosurgical care of spinal epidural, subdural, and intramedullary abscesses and arachnoiditis. Orthop Clin North Am. 1996;27:125–136. [PubMed] [Google Scholar]
- Mampalam TJ, Rosegay H, Andrews BT, Rosenblum ML, Pitts LH. Nonoperative treatment of spinal epidural infections. J Neurosurg. 1989;71:208–210. doi: 10.3171/jns.1989.71.2.0208. [DOI] [PubMed] [Google Scholar]
- Merrell CA, McKinley W. Infectious-related versus traumatic spinal cord injury: epidemiology, injury characteristics, and rehabilitation outcomes [abstract] J Spinal Cord Med. 2006;29((4)):455. [Google Scholar]