Abstract
Background/Objective:
To examine the lipoprotein profiles of men and women with paraplegia and tetraplegia. Impairment of the sympathetic nervous system (dependent on the level of injury) and the extent of physical capacity and activity were correlated with the lipid profile in men with spinal cord injury (SCI). Sex-related differences of the lipoprotein profiles could be found in nondisabled and premenopausal women with SCI mainly because of the different effects of sexual hormones.
Methods:
Lipoprotein profiles of 112 participants with SCI (32 premenopausal women, 80 men) were analyzed and correlated to sex, lesion level, and physical performance capacity.
Results:
Women with tetraplegia or paraplegia showed significantly higher levels of high-density lipoprotein and lower ratios of total cholesterol to high-density lipoprotein-cholesterol compared with men with corresponding lesion levels, without a difference in peak oxygen consumption. Concentrations of very-low-density lipoproteins were lower in women with paraplegia than in men with paraplegia; no differences were found in total cholesterol, low-density lipoprotein-cholesterol, and triglycerides. Sex-independent elevations in total cholesterol and low-density lipoprotein-cholesterol were associated with paraplegia, and sex-independent elevations in triglyceride levels were associated with tetraplegia.
Conclusions:
Persons with SCI showed sex-related differences in their lipoprotein profiles. Independent of physical fitness, the lipoprotein profile of premenopausal women with SCI did not exhibit the adverse lipoprotein characteristics observed in men with SCI, probably because of the influence of sexual hormones independent of lesion level.
Keywords: Lipid profile, Cardiovascular risk factors, Spinal cord injuries, Sex, Tetraplegia, Paraplegia, Sexual hormones
INTRODUCTION
Lipoprotein metabolism is influenced by a wide range of factors including anthropometric data, peripheral insulin resistance, catecholamines, physical activity, performance capacity, and sex. These factors have been investigated thoroughly in nondisabled individuals. The majority of the recent studies in men with spinal cord injury (SCI) have shown an unfavorable lipid profile caused by lower high-density lipoprotein-cholesterol (HDLC) or higher low-density lipoprotein-cholesterol (LDLC), independent of the injury level (1–6). This could be one reason for increased atherogenesis in persons with SCI (7–9). SCI causes a loss of muscular function and sensory perception, as well as impairment in the regulation of the autonomic nervous system. A spinal cord lesion causes an interruption of pathways from activating centers in the brain to the peripheral sympathetic nervous system. Results range from an extremely reduced sympathetic activity in persons with tetraplegia to chronic overactivity in patients with low paraplegia. Different catecholamine concentrations at rest and during exercise can be seen by level of injury (10). Catecholamines are known to influence lipid metabolism, eg, positive correlations have been found between catecholamines and total cholesterol (TC), LDLC, and HDLC (11–13). Furthermore, the impairment of sympathetic activity leads to pronounced cardiovascular and metabolic alterations resulting in higher physical inactivity and lower physical performance with negative effects on the lipid profile (2,14–18). In previous studies, differences were found between the lipoprotein profiles of men with SCI compared with those of male controls. Among men with SCI, tetraplegia was associated with higher very-low-density lipoprotein-cholesterol (VLDLC) and triglycerides (TG) and lower HDLC, and paraplegia was associated with higher TC and LDLC (19).
Various studies that showed sex-specific differences in the lipoprotein profile of nondisabled men compared with nondisabled premenopausal women, with the latter having higher HDLC because of the effects of estrogen (20,21). No differences in HDLC levels were found among women with paraplegia and tetraplegia and in nondisabled women, whereas significantly higher TG levels were shown in women with tetraplegia. Similarly, LDLC levels were significantly higher in women with paraplegia compared with nondisabled women (22). Few comparative data are available regarding the lipid profiles of women and men with SCI of different injury levels (1,23). Therefore, the aim of this study was to examine and to compare the lipoprotein profiles of men and women with paraplegia and tetraplegia.
METHODS
Subjects
This study involved 112 participants with long-term complete SCI, 32 of them being premenopausal women 23 to 40 years of age and 80 men 23 to 44 years of age. Participants were recruited from the community, and all were white. Individuals taking medication that influenced lipid metabolism or who had pulmonary or cardiovascular disease were excluded from the study. After giving written informed consent, they were examined according to the ASIA standards of 1996. The participants were divided into 8 women and 30 men with spinal lesions between C4 and C8 (tetraplegia) and 24 women and 50 men with spinal lesions at T1 and lower (paraplegia).
Causes for disability were car or motorcycle accidents in 62%, sports-related accidents in 8%, and accidents at work in 6% of the cases, the rest having less frequent causes, ie, inflammatory spinal diseases, residual lesions after operation for neoplasm, or abscess. All participants consumed a typical western diet. Persons on special diets were excluded from the study. The participants were not engaged in regular sports activities.
Body Characteristics and Exercise Test
Weight and length in supine position were measured after blood was drawn. All participants underwent a graded exercise test to the point of subjective exhaustion on a wheelchair ergometer (WCE; Ergotronic 9000; Sopor Sunrise Medical Company, Malsch, Germany) (17). Exercise was timed for 3 minutes at each level, starting with 20 W for participants with paraplegia and 10 W for participants with tetraplegia and rising by 10 W for participants with paraplegia and 5 W for those with tetraplegia, respectively. Oxygen uptake (Vo2) was continuously measured with an open-circuit spirometry (Oxycon; Jäger Company, Hoechberg, Germany).
Chemical Analysis
Venous blood was obtained between 8:00 am and 10:00 am after 12-hour fasting. The participants rested quietly in an upright seated position for 10 minutes before the blood sample was drawn. Possible stimuli for autonomic dysreflexia were ruled out by catheterization and bowel care. Lipid parameters such as serum cholesterol (TC) and TGs were determined by means of enzymatic tests on fresh serum (Boehringer Mannheim, Mannheim, Germany). HDLC, LDLC, and VLDLC lipoproteins were separated by quantitative electrophoresis (REP Analytik; Greiner Bio-One, Frickenhausen, Germany). Lipoprotein(a) [Lp(a)] concentrations were determined quantitatively by nephelometry [N Latex Lp(a) Reagent; Behring Diagnostics, Marburg, Germany].
Statistics
Average values and SDs were calculated. The groups were compared by means of the nonparametric Mann-Whitney U test. Multiple testing was considered by adjusting P values according to the Holm procedure (24).
RESULTS
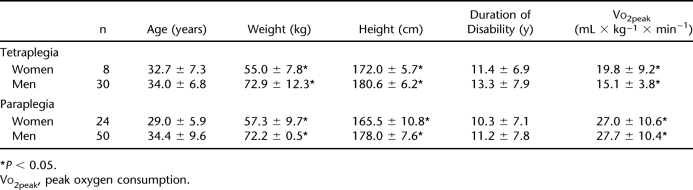
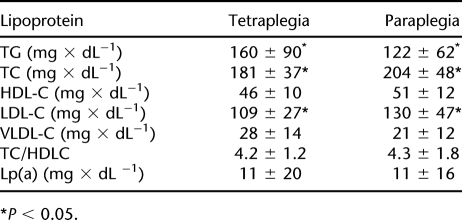
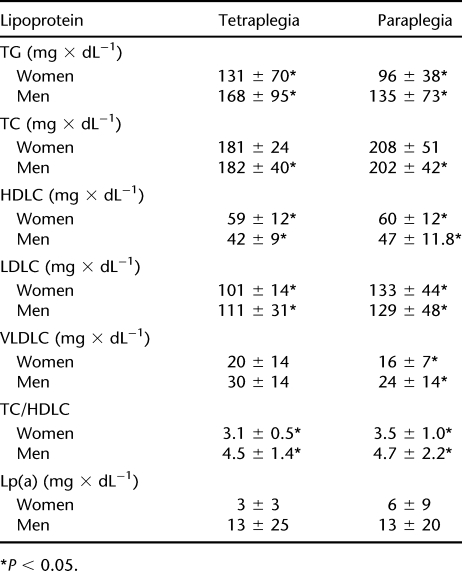
Women with SCI were significantly shorter and had lower body weight than men. Both women and men with tetraplegia showed a significantly lower Vo2peak than subjects with paraplegia (Table 1). In comparison with people with tetraplegia, individuals with paraplegia had higher TC levels caused by higher LDLC. Subjects with tetraplegia showed increased concentrations of TGs compared with individuals with paraplegia. Regarding Lp(a), no significant differences were observed between the groups (Table 2). Lipoprotein profiles are compared in Table 3. Levels of HDLC were significantly higher in women in both the paraplegic and the tetraplegic groups. Women with paraplegia and tetraplegia showed a significantly lower ratio of TC to HDLC than did their male counterparts. VLDLC levels were lower in women, which was statistically significant in subjects with paraplegia. Levels of LDLC did not differ by sex but were higher in subjects with paraplegia compared with persons with tetraplegia. TG levels did not differ significantly between men and women but were significantly higher in persons with tetraplegia than in subjects with paraplegia. Persons with paraplegia showed sex-independent higher TC levels than subjects with tetraplegia. Lp(a) levels were higher in men of both groups, but the difference in Lp(a) levels in women was not statistically significant.
Table 1.
Anthropometric Data and Peak Oxygen Consumption by Sex and Level of Lesion
Table 2.
Lipoprotein Levels by Level of Lesion
Table 3.
Lipoprotein Levels by Sex and Level of Lesion
DISCUSSION
Persons with SCI are particularly prone to an adverse risk profile for atherogenesis. Among other risk factors, this might be attributed to an unfavorable lipid profile (1,4,5,7,25–28). In former studies of male subjects with SCI, we were able to show a correlation between the lipoprotein profile and the level of injury. Lipoprotein concentrations in male subjects with tetraplegia compared with nondisabled persons were characterized by elevated concentrations of VLDLC and TGs and reduced concentrations of HDLC. Despite normal LDLC and TC levels, men with tetraplegia might be at increased risk for developing cardiovascular complications. The risk profile of men with paraplegia was characterized by elevations of TC and LDLC compared with nondisabled control subjects (18). These findings are in accordance with the literature (1,5,29–31). The adverse lipid profile in men with SCI compared with control subjects results from physical inactivity with low Vo2max levels depending on the injury level (15,16,28). Inactivity is associated with higher insulin resistance, increased occurrence of glucose intolerance and diabetes mellitus, and reduced activity of the lipoprotein lipase. The lipid profile of men with SCI seems to be similar to that of patients with metabolic syndrome (7,32) and can be positively influenced by dieting and physical activity (8,22,33–35). Men with SCI should have their cardiovascular risk evaluated by the lipoprotein profile. Interpretation of the lipoprotein profile should particularly consider HDLC, VLDLC, and TGs in men with tetraplegia and LDLC in men with paraplegia. Therapies should be guided by current guidelines, ie, increasing physical activity, dietary and pharmaceutical interventions, and avoiding additional risk factors (3,22,34–41). Lp(a) levels did not differ significantly between men with paraplegia and tetraplegia despite different daily physical activity and Vo2max levels. Therefore, Lp(a) levels depend more on genetic determination than on physical performance capacity or activity (29,42–44).
Women with SCI, however, showed different results. Although they exhibit reduced physical performance capacity and a decreased level of physical activity—each depending on the level of injury—their HDLC levels did not differ significantly from those of female controls (21). These findings are supported by Bauman et al (1). Nevertheless, moderate elevation of TGs in women with tetraplegia was shown (45).
Comparing men and women with SCI, we found sex-dependent differences in the lipoprotein profiles. According to Bauman et al (1) and Szlachcic et al (23), female subjects showed significantly higher levels of HDLC. It has been shown earlier that estrogens increase the levels of HDLC (46). Because the SCI does not influence the ovulatory menstrual cycle, it can be assumed that the levels of sexual hormones are not affected either (47). Therefore, it could be speculated that differences in HDLC levels between men and women with SCI are influenced by estrogen. In addition, previous studies have shown a significant correlation of body mass index (BMI) and several lipid parameters, although BMI is of limited use in the SCI population (1,5). Furthermore, we found significantly lower levels of VLDLC in women with paraplegia. The lower VLDLC and the tendentiously lower TGs may be an indication of a higher insulin sensitivity of women with SCI. However, physical performance capacity (Vo2peak) was not significantly different between women and men with SCI.
Levels of TC, LDLC, and TGs did not show any sex-specific differences. Nevertheless, LDLC concentrations were significantly higher in men and women with paraplegia. This seems to be a consequence of differences in impairment of the sympathetic nervous system between subjects with high and low levels of injury. Catecholamines are known to influence lipid metabolism, eg, a positive correlation with LDLC and epinephrine has been found (11,12). As described earlier, individuals with paraplegia showed an augmented sympathetic activity resulting in higher catecholamine concentrations in comparison with tetraplegic and control persons (10). The limitations of this study include small sample sizes, absence of several anthropometric data, and additional risk factors of coronary heart disease.
The elevation of the mean Lp(a) concentrations of men with SCI compared with women with SCI did not reach statistical significance. Further studies are necessary to examine whether these findings were because of the small number of participants (48–50).
CONCLUSION
Sex-related differences were noted in the lipoprotein profiles of persons with SCI. Premenopausal women with SCI, independent of physical fitness, exhibited a lipoprotein profile that was without the adverse lipoprotein characteristics observed in men with SCI. Longitudinal studies may be considered with additional participants to determine whether the lipid profile in women with SCI confers less risk than that of men with SCI on the development and progression of vascular disease.
REFERENCES
- Bauman A, Adkins RH, Spungen AM, et al. Is immobilization associated with an abnormal lipoprotein profile? Observation from diverse cohort. Spinal Cord. 1999;37:485–493. doi: 10.1038/sj.sc.3100862. [DOI] [PubMed] [Google Scholar]
- Brenes G, Dearwater S, Shapera R, LaPorte RE, Collins E. High density lipoprotein cholesterol concentrations in physically active and sedentary spinal cord injured patients. Arch Phys Med Rehabil. 1998;67:445–450. [PubMed] [Google Scholar]
- Heldenberg D, Rubinstein A, Levtov O, Werbin B, Tamir I. Serum lipids and lipoprotein concentrations in young quadriplegic patients. Atherosclerosis. 1981;39:163–167. doi: 10.1016/0021-9150(81)90065-4. [DOI] [PubMed] [Google Scholar]
- Lee MY, Myers J, Hayes A, et al. C-reactive protein, metabolic syndrome, and insulin resistance in individuals with spinal cord injury. J Spinal Cord Med. 2005;28:20–25. doi: 10.1080/10790268.2005.11753794. [DOI] [PubMed] [Google Scholar]
- Maki KC, Briones ER, Langbein WE, et al. Associations between serum lipids and indicators of adiposity in men with spinal cord injury. Paraplegia. 1995;33:102–109. doi: 10.1038/sc.1995.24. [DOI] [PubMed] [Google Scholar]
- Shetty KR, Sutton CH, Rudman IW, Rudman D. Lipid and lipoprotein abnormalities in young quadriplegic men. Am J Med Sci. 1992;303:213–216. doi: 10.1097/00000441-199204000-00001. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24:266–277. doi: 10.1080/10790268.2001.11753584. [DOI] [PubMed] [Google Scholar]
- Janssen TWJ, van Oers CAJM, van Kamp GJ, TenVoorde BJ, van der Woude LHV, Hollander AP. Coronary heart disease risk indicators, aerobic power, and physical activity in men with spinal cord injuries. Arch Phys Med Rehabil. 1997;78:697–705. doi: 10.1016/s0003-9993(97)90076-9. [DOI] [PubMed] [Google Scholar]
- Yekutiel M, Brooks ME, Ohry A, Yarom J, Carel R. The prevalence of hypertension, ischemic heart disease and diabetes in traumatic spinal cord injured patients and amputees. Paraplegia. 1989;27:58–62. doi: 10.1038/sc.1989.9. [DOI] [PubMed] [Google Scholar]
- Schmid A, Huonker M, Stahl F. Free plasma catecholamines in spinal cord injured persons with different injury levels at rest and during exercise. J Autonom Nerv Syst. 1998;68:96–100. doi: 10.1016/s0165-1838(97)00127-6. [DOI] [PubMed] [Google Scholar]
- Arner P, Wahrenberg H, Lönnqvist F, Angelin B. Adipocyte ß-Adrenoceptor sensitivity influences plasma lipid levels. Arteriosclerosis. 1993;13:967–972. doi: 10.1161/01.atv.13.7.967. [DOI] [PubMed] [Google Scholar]
- Ward KD, Sparrow D, Landsberg L, Young JB, Vokonas PS, Weiss ST. The relationship of epinephrine excretion to serum lipid levels: the normative aging study. Metabolism. 1994;43:509–513. doi: 10.1016/0026-0495(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Smith U. Adrenergic control of lipid metabolism. Acta Med Scand. 1983;672((suppl)):41–47. doi: 10.1111/j.0954-6820.1983.tb01612.x. [DOI] [PubMed] [Google Scholar]
- Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation. 2002;105:310–315. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- Hopman MTE. Circulatory responses during arm exercise in individuals with paraplegia. Int J Sports Med. 1994;15:126–131. doi: 10.1055/s-2007-1021033. [DOI] [PubMed] [Google Scholar]
- Janssen TWJ, van Oers CAJM, van der Woude LHV, Hollander AP. Physical strain in daily life of wheelchair users with spinal cord injuries. Med Sci Sports Exerc. 1994;26:661–670. doi: 10.1249/00005768-199406000-00002. [DOI] [PubMed] [Google Scholar]
- Mollinger LA, Spurr GB, El Ghati AZ, et al. Daily energy expenditure and basal metabolic rates of patients with spinal cord injury. Arch Phys Med Rehabil. 1995;66:420–426. [PubMed] [Google Scholar]
- Schmid A, Huonker M, Barturen JM, et al. Catecholamines, heart rate and oxygen uptake in spinal cord injured persons during wheelchair exercise. J Appl Physiol. 1998;85:635–641. doi: 10.1152/jappl.1998.85.2.635. [DOI] [PubMed] [Google Scholar]
- Schmid A, Halle M, Stützle C, et al. Lipoproteins and free plasma catecholamines in spinal cord injured men with different injury levels. Clin Physiol. 2000;20:304–310. doi: 10.1046/j.1365-2281.2000.00263.x. [DOI] [PubMed] [Google Scholar]
- Gardner CD, Tribble DL, Young DR, Ahn D, Fortmann SP. Population frequency distributions of HDL, HDL(2), and HDL(3) cholesterol and apolipoproteins A-I and B in healthy men and women and associations with age, gender, hormonal status, and sex hormone use: the Stanford Five City Project. Prev Med. 2001;31:335–345. doi: 10.1006/pmed.2000.0715. [DOI] [PubMed] [Google Scholar]
- Roeters van Lennep JE, Westerveld HT, Erkelens DW, van der Wall EE. Risk factors for coronary heart disease: implications of gender. Cardiovasc Res. 2002;53:538–549. doi: 10.1016/s0008-6363(01)00388-1. [DOI] [PubMed] [Google Scholar]
- Storch MJ, König D, Bültermann D, et al. Lipid profile in spinal cord injured women with different injury levels. Prev Med. 2005;40:321–325. doi: 10.1016/j.ypmed.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Szlachcic Y, Adkins RH, Adal T, Yee F, Bauman W, Waters RL. The effect of dietary intervention on lipid profiles in individuals with spinal cord injury. J Spinal Cord Med. 2001;24:26–29. doi: 10.1080/10790268.2001.11753551. [DOI] [PubMed] [Google Scholar]
- Holm S. Simple sequentially reflective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Keil U. Coronary artery disease: the role of lipids, hypertension and smoking. Basic Res Cardiol. 2000;95:152–158. doi: 10.1007/s003950070010. [DOI] [PubMed] [Google Scholar]
- Krum H, Howes LG, Brown DJ, et al. Risk factors for cardiovascular disease in chronic spinal cord injury patients. Paraplegia. 1992;30:381–388. doi: 10.1038/sc.1992.87. [DOI] [PubMed] [Google Scholar]
- Ozgurtas T, Alaca R, Gulec M, Kutluay T. Do spinal cord injuries adversely affect serum lipoprotein profiles. Mil Med. 2003;168:545–547. [PubMed] [Google Scholar]
- Vidal J, Javierre C, Curia FJ, Garrido E, Lizarraga MA, Segura R. Long-term evolution of blood lipid profiles and glycemic levels in patients after spinal cord injury. Spinal Cord. 2003;41:178–181. doi: 10.1038/sj.sc.3101390. [DOI] [PubMed] [Google Scholar]
- Dearwater SR, LaPorte RE, Robertson RJ, Brenes G, Adams LL, Becker D. Activity in the spinal cord-injured patient: an epidemiologic analysis of metabolic parameters. Med Sci Sports Exerc. 1986;18:541–544. [PubMed] [Google Scholar]
- Halle M, Berg A, Baumstark MW, Keul J. LDL subfraktions and coronary heart disease—an overview. Z Kardiol. 1998;87:317–330. doi: 10.1007/s003920050187. [DOI] [PubMed] [Google Scholar]
- Hoffmeister H, Mensink GB, Stolzenberg H. National trends in risk factors for cardiovascular disease in Germany. Prev Med. 1994;23:197–205. doi: 10.1006/pmed.1994.1027. [DOI] [PubMed] [Google Scholar]
- Zhong YG, Levy E, Bauman WA. The relationships among uric acid, plasma insulin, and serum lipoprotein levels in subjects with spinal cord injury. Horm Metab Res. 1995;27:283–286. doi: 10.1055/s-2007-979960. [DOI] [PubMed] [Google Scholar]
- Berg A, Frey I, Baumstark MW, Halle M, Keul J. Physical activity and lipoprotein lipid disorders. Sports Med. 1994;17:6–21. doi: 10.2165/00007256-199417010-00002. [DOI] [PubMed] [Google Scholar]
- Dallmeijer AJ, van der Woude LH, van Kamp GJ, Hollander AP. Changes in lipid, lipoprotein and apolipoprotein profiles in persons with spinal cord injuries during the first 2 years post-injury. Spinal Cord. 1999;37:96–102. doi: 10.1038/sj.sc.3100776. [DOI] [PubMed] [Google Scholar]
- Okada M, Ishida R. Direct measurement of low-density-lipoprotein cholesterol is more effective than total cholesterol for the purpose of lipoprotein screening. Prev Med. 2001;32:224–229. doi: 10.1006/pmed.2000.0805. [DOI] [PubMed] [Google Scholar]
- Cullen P, von Eckardstein A, Assmann G. Diagnosis and management of new cardiovascular risk factors. Eur Heart J. 1996;19 Supp O:O13–O19. [PubMed] [Google Scholar]
- Mathes P. Cholesterin: Serum-Werte oder Gesamtrisiko als Entscheidungshilfe für medikamentöse Prophylaxe. Z Kardiol. 2004;93:16–20. doi: 10.1007/s00392-004-1203-9. [DOI] [PubMed] [Google Scholar]
- Nash MS, Jacobs PL, Mendez AJ, Goldberg RB. Circuit resistance training improves the atherogenic lipid profiles of persons with chronic paraplegia. J Spinal Cord Med. 2001;24:2–9. doi: 10.1080/10790268.2001.11753548. [DOI] [PubMed] [Google Scholar]
- Nash MS, Johnson BM, Jacobs PL. Combined hyperlipidemia in a single subject with tetraplegia: ineffective risk reduction after atorvastatin monotherapy. J Spinal Cord Med. 2004;27:484–487. doi: 10.1080/10790268.2004.11754559. [DOI] [PubMed] [Google Scholar]
- Natarajan S, Nietert PJ. National trends in screening, prevalence, and treatment of cardiovascular risk factors. Prev Med. 2003;36:389–397. doi: 10.1016/s0091-7435(02)00057-9. [DOI] [PubMed] [Google Scholar]
- Rensing UFE, Roskamm H, Betz P, et al. Lipid intervention and coronary heart disease in men less than 56 years of age. The Coronary Intervention Study: CIS. Z Kardiol. 1999;88:270–282. doi: 10.1007/s003920050286. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Adkins RH, Spungen AM, et al. Individuals with extreme inactivity do not have abnormal serum lipoprotein(a) levels. Horm Metab. 1998;30:601–603. doi: 10.1055/s-2007-978941. [DOI] [PubMed] [Google Scholar]
- Henrikkson P, Angelin B, Berglund L. Hormonal regulation of serum LP(a) levels. Opposite effects after estrogen treatment and orchidectomy in males with prostatic carcinoma. J Clin Invest. 1992;89:1166–1171. doi: 10.1172/JCI115699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle M, Berg A, von Stein T, Baumstark MW, König D, Keul J. Lipoprotein(a) in endurance athletes, power athletes, and sedentary controls. Med Sci Sports Exerc. 1996;28:962–966. doi: 10.1097/00005768-199608000-00004. [DOI] [PubMed] [Google Scholar]
- Dayspring TD. Coronary heart disease in woman: triglycerides and lipoprotein biology. J Gend Spec Med. 2002;5:27–33. [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Farese RV. Disorders of lipid metabolism. In: Wilson JD, editor. Williams Textbook of Endocrinology. Philadelphia, PA: Saunders; 1996. pp. 1099–1153. [Google Scholar]
- Reame NE. A prospective study of the menstrual cycle and spinal cord injury. Am J Phys Med Rehab. 1992;71:15–21. doi: 10.1097/00002060-199202000-00005. [DOI] [PubMed] [Google Scholar]
- Armstrong VW, Cremer P, Eberle E, et al. The association between serum Lp(a) concentrations and angiographically assessed coronary atherosclerosis. Atherosclerosis. 1986;62:249–257. doi: 10.1016/0021-9150(86)90099-7. [DOI] [PubMed] [Google Scholar]
- Scanu AM. Lipoprotein(a). A genetic risk factor for premature coronary heart disease. JAMA. 1992;267:3326–3329. doi: 10.1001/jama.267.24.3326. [DOI] [PubMed] [Google Scholar]
- Selby JV, Austin MA, Sandholzer C, et al. Environmental and behavioral influences on plasma lipoprotein(a) concentration in women twins. Prev Med. 1994;23:345–353. doi: 10.1006/pmed.1994.1048. [DOI] [PubMed] [Google Scholar]