Abstract
Summary:
Due to the varied and numerous changes in spinal cord tissue following injury, successful treatment for repair may involve strategies combining neuroprotection (pharmacological prevention of some of the damaging intracellular cascades that lead to secondary tissue loss), axonal regeneration promotion (cell transplantation, genetic engineering to increase growth factors, neutralization of inhibitory factors, reduction in scar formation), and rehabilitation. Our goal has been to find effective combination strategies to improve outcome after injury to the adult rat thoracic spinal cord. Combination interventions tested have been implantation of Schwann cells (SCs) plus neuroprotective agents and growth factors administered in various ways, olfactory ensheathing cell (OEC) implantation, chondroitinase addition, or elevation of cyclic AMP. The most efficacious strategy in our hands for the acute complete transection/SC bridge model, including improvement in locomotion [Basso, Beattie, Bresnahan Scale (BBB)], is the combination of SCs, OECs, and chondroitinase administration (BBB 2.1 vs 6.6, 3 times more myelinated axons in the SC bridge, increased serotonergic axons in the bridge and beyond, and significant correlation between the number of bridge myelinated axons and functional improvement). We found the most successful combination strategy for a subacute spinal cord contusion injury (12.5–mm, 10–g weight, MASCIS impactor) to be SCs and elevation of cyclic AMP (BBB 10.4 vs 15, significant increases in white matter sparing, in myelinated axons in the implant, and in responding reticular formation and red and raphe nuclei, and a significant correlation between the number of serotonergic fibers and improvement in locomotion). Thus, in two injury paradigms, these combination strategies as well as others studied in our laboratory have been found to be more effective than SCs alone and suggest ways in which clinical application may be developed.
Keywords: Spinal cord injuries, Schwann cells, Olfactory ensheathing cells, Axonal regeneration, Chondroitinase, Cyclic adenosine monophosphate
INTRODUCTION
After spinal cord injury (SCI), many damaging tissue processes are initiated that lead to secondary tissue loss. Just a few of these changes are the release of excessive excitatory amino acids and activation of their receptors, excessive Ca2+ entry into cells, Ca2+-stimulated activation of proteolytic and other degradative enzymes, cytoskeletal protein breakdown, oxygen free-radical release leading to membrane damage and generation of a number of cytokines and chemokines (1). In addition to these changes that cause secondary tissue damage, substances inhibitory to repair appear, inflammation begins, a barrier to axon growth (a scar) forms, sustenance for surviving nerve cells is diminished, and particularly in contusion injury, large cysts form due to tissue deterioration. Thus, to repair the cord we must consider halting the spread of secondary tissue damage in order to save as many neurons as possible, curb inflammation, reduce scar formation, neutralize inhibitory factors, awaken nerve cells to regrow fibers, provide sustenance to surviving nerve cells, promote fiber growth across the area of injury, guide growth to appropriate areas, and enable the formation of connections. It is clear, then, at least at this point in time, that a combination strategy will be needed to address this panoply of requirements for repair.
In the early 1900s, one of Santiago Ramón y Cajal's students by the name of Jorge Francisco Tello y Muñóz placed pieces of degenerating peripheral nerve into a lesioned rabbit cerebral cortex (2). When the tissue was examined, they found that newly grown nerve fibers in the cortex converged onto the pieces of peripheral nerve. This led Ramón y Cajal to speculate that if the environment is suitable, then central neurons will regenerate axons. This speculation was substantiated later by the experiments of the Aguayo team (3,4). This team showed that, following the removal of a segment of spinal cord and the placement of a piece of peripheral nerve into the resulting gap, neurons in the stumps bordering the peripheral nerve extended axons into the nerve (3). Because by this time reliable neuroanatomical tracing methods had been developed, this growth was shown clearly to have occurred from the nearby central neurons.
Besides peripheral nerve, other tissues, cell types, and matrices have been placed into sites of injury in the spinal cord. Fetal central nervous tissue, olfactory ensheathing cells (OECs), activated macrophages, fibroblasts that have been engineered to generate growth factors, embryonic stem cells, bone marrow stromal cells sometimes transduced by viruses to secrete growth factors, progenitor cells, and collagen have been used (for examples, see references 5–8). Schwann cells (SCs) taken from peripheral nerve also have been used following SCI, and it is primarily our SC studies that will be described in this article (for review, see references 9–12) (Table 1).
Table 1.
Schwann Cell Transplantation Strategies Described in This Article
Everywhere in the peripheral nervous system, SCs surround axons, either by forming myelin around single axons or ensheathing thinner axons with their cytoplasm. When a peripheral nerve is cut or damaged, each nerve fiber degenerates quickly beyond the damage but the SCs remain within their tunnels of basal lamina and matrix. These tunnels, named bands of Büngner, survive and are the areas into which regenerating axons grow. It has been known for sometime that it is the SCs that promote the regeneration of axons in peripheral nerve. They produce growth factors and extracellular matrix components that aid in fostering this regeneration, they myelinate (13) and ensheathe axons in the central nervous system (CNS), and following demyelination, they are able to restore axonal conduction (10). They are readily accessible in that they can be obtained from a piece of peripheral nerve from rats or humans and then generated in very large numbers in tissue culture, and they can be genetically engineered to produce larger amounts of growth factors than they ordinarily do (14–17). An appealing aspect of SCs is the possibility of autologous transplantation; they can be obtained from a piece of peripheral nerve from a person with SCI, generated in very large numbers in culture, and then be available for transplantation into that person's area of SCI. The SCs to be transplanted can be added to fibrin or Matrigel (BD Biosciences, San Jose, CA) that then gels into a solid cable to be placed between the stumps of a transected spinal cord. We have placed these SC cables into polyvinylchloride/polyacrylonitrile polymer channels that hold the severed stumps of the spinal cord and the intervening cable in place (18).
The Complete Transection/SC Bridge Model
The first injury model that we studied was placement of an SC cable at the rostral stump or between the completely severed stumps of thoracic spinal cord from female Fischer rats (18,19). The advantage of complete transection is that the question of spared and sprouted fibers is eliminated, allowing identification of regenerated axons with certainty. Two million SCs or more, depending upon the length of the transection gap, were drawn into a polymer tube with Matrigel and kept in culture medium overnight while the Matrigel gelled. A gap was created by completely transecting the spinal cord at T8, stumps were inserted into each end of the channel, and the rats were maintained for at least 1 to 2 months. Upon removal of the cord with the SC bridge and polymer channel from the animal, it was seen that there was excellent union of the ends of the SC bridge with the stumps of the spinal cord (18). When sectioned and immunostained for axons, many axons were observed to extend from the spinal cord stump into the SC bridge. Those axons appeared thicker because they had been fasciculated by the SCs. In cross sections of the SC bridge, the appearance was that of peripheral nerve because the SCs had myelinated the axons that had grown into the bridge and also ensheathed nonmyelinated axons (18,19). In this thoracic transection model utilizing implanted SCs, axons grew onto the SC bridge from both stumps, with a mean of 2,000 myelinated axons and 8 times more nonmyelinated, ensheathed axons (18).
This regenerative response, while promising, was deficient in that there was scar formation at the interface with the spinal cord including accumulation of chondroitin sulfate proteoglycans (20), the response of brain stem neurons was minimal, and the regenerated axons did not leave the bridge to enter spinal cord territory. These observations led us in the 1990s to realize that a combination strategy will be required. It is worthy of note that Ramón y Cajal (2) speculated that to repair the CNS, adequate alimentation (growth factors) and specific orienting substances (guidance molecules) must be provided. This was undoubtedly the first suggestion of a combination strategy. Not only should a combination strategy be considered because of the multifarious interventions needed to counteract so many of the damaging processes initiated after SCI, but also, if SCs are to be used, additional interventions are needed for efficacious repair.
Combinatorial Strategies: A number of combination strategies have been tested in this complete transection model: (a) SCs with methylprednisolone (21), (b) SCs plus brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) infused around the SC bridge (22), (c) SCs transduced to secrete BDNF implanted into the lesion site and in a trail caudal to transection (14), (d) BDNF- and NT-3-AAV injected into the cord beyond the bridge (23), and implantation of SCs plus OECs (24) and SCs plus OECs plus chondroitinase (25). All these combination strategies led to improved outcomes compared with the implantation of SCs alone: more myelinated axons were present on the bridge, there was an increase in regenerated axons from brain stem neurons onto the bridge, and some regenerated axons exited the bridge to grow back into the cord. Also, an improvement in locomotion was seen in some studies. An increase in regenerated axons from the brain stem neurons onto the bridge indicated that a distance factor had been overcome to some extent. For example, in work from the Aguayo laboratory (26), a piece of peripheral nerve inserted into the lower cord induced only a few brain stem neurons to extend axons into the nerve. There was a significantly improved response, however, if the piece of nerve was inserted into the cord at low and, particularly, at high cervical levels.
We have used OECs in some of the combination therapies. These cells have sparked the interest of CNS regeneration investigators because of their location in the body. These cells are found in the olfactory mucosa in the nose, in olfactory nerves spanning the area between the periphery and the CNS, and in the olfactory bulb. It is in these areas that neurite growth continues throughout life because of the death and birth of sensory neurons in the olfactory mucosa, necessitating continuing axonal growth from the mucosa to the olfactory bulb. There have been numerous studies using these cells for implantation into the CNS (for reviews, see references 10,12,27–31). They have been found to reduce scar formation, diminish cavitation following contusion injury, promote SC migration into the area of injury, support axon regeneration, and in some cases, myelinate axons. In our work, we found that they enabled axons to leave the SC bridge to grow into the spinal cord (24). They also have been found to improve function (for reviews, see references 10,12,27–31). From the SC standpoint, these cells are not as readily accessible, large numbers are not as able to be generated in culture, and in our hands, they do not survive well in the contusion lesion milieu (32).
A number of the combination intervention studies that we have performed involved implanting OECs on either side of an SC implant (24,25,32). In the study by Ramón-Cueto and colleagues (24), findings included the following:
5HT–positive fibers severed at the rostral stump were present beyond the complete spinal cord transaction/SC bridge in the spinal cord. These fibers appeared to prefer the OEC-fibroblast environment on the outside of the polymer channel over the OEC-SC environment inside the channel.
Following an injection of the tracer HRP-WGA (Vector Labs, Burlingame, CA) rostral to the SC bridge, labeled fibers extended through the bridge and into the cord caudal to the SC bridge. It was striking that none of these were seen in the transected SC-bridged animals lacking OEC implantation.
There were labeled neurons in the lumbar cord suggestive of regrowth of fibers at the caudal interface, through the SC bridge, and across the rostral interface to pick up the tracer at level C7. If this was the case, then there was axonal regeneration of at least 2.5 cm.
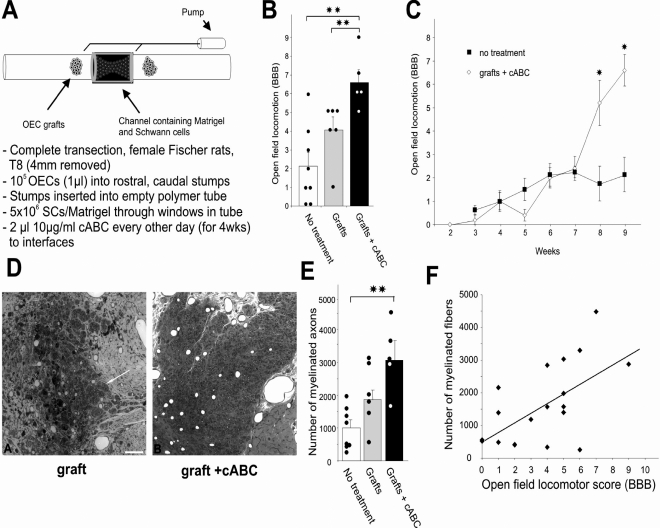
Another combination strategy, built upon the transected cord/SC bridge/OEC implantation study (24), included administering an enzyme, chondroitinase ABC (cABC), at the rostral and caudal interfaces (25). The goal was to provide a permissive substratum for axonal growth (SC bridge), enable exit of regenerated axons from the bridge into the cord (OEC implantation at either end of the bridge), and reduce the chondroitin sulfate proteoglycans at the SC bridge/cord interfaces. Two microliters of 10 μg/mL enzyme were delivered every other day to both interfaces for 4 weeks (Figure 1). Appropriate immunostaining showed that chondroitin sulfate proteoglycan was indeed reduced. Locomotion was improved, and the BBB test revealed significant improvement both between the grafted and the control (Matrigel only) animals and also between the grafted and the grafted-plus-enzyme-treated animals (2.1 ± 0.7 vs 6.6 ± 0.7; revealing extensive movement of an additional joint in the hind limb). The significant difference began at 7 weeks. A significant improvement also was seen in forelimb/hind limb movement coupling. Serotonergic fibers on the bridge and caudal to it were significantly increased with the grafting and enzyme treatment. The number of myelinated axons in the bridge was tripled in the grafted-plus-enzyme-treated animals compared with control animals. A significant correlation was found between the number of myelinated axons in the bridge and functional recovery. A follow-up study (33) demonstrated that not only are raphespinal fibers found caudal to the SC bridge, but also that axons from additional brain stem areas (vestibular nuclei and reticular formation) reach regions caudal to the bridge.
Figure 1. Combination strategy of SC implantation as a bridge spanning a complete gap in the spinal cord, injection of OECs into the spinal cord stumps on either side of the bridge, and administration of cABC at both rostral and caudal bridge/cord interfaces. (A) The order in which the experimental steps were performed. (B) Illustration of the significant increase in the BBB scores between the control animals (Matrigel only) and the grafted and grafted-and-enzyme-treated groups. The dots represent scores of single animals. **The double asterisk indicates significance at P < 0.01. (C) The BBB scores of control and cABC-treated animals start to differ significantly at 8 weeks after injury. *The asterisk indicates significance at P < 0.05. (D) Illustration of the greater density in the bridge tissue due to a greater number of SC-myelinated axons after digestion of chondroitin sulfate proteoglycans with cABC; the number of SC-myelinated axons is tripled in the grafted-and-enzyme-treated group compared with the control group (E) (scale bar, 150 μm; 12 weeks postinjury). **The double asterisk indicates significance at P < 0.01. (F) There was a significant correlation (r2 = 0.63) between functional recovery and the number of myelinated axons. *The asterisk indicates significance at P < 0.05. **The double asterisk indicates significance at P < 0.01. From Fouad et al (25).
A new paradigm that was investigated in the rat thoracic complete transection/SC bridge model is intervention at the neuronal soma as well as at the injury site (34). Our novel objective was to stimulate the growth factor MAP kinase pathway without administering growth factors. Constitutively activated MEK and ERK genes were introduced into the reticular formation by means of adeno-associated (AAV) viral vectors. Viruses carrying GFP genes were injected with and without constitutively activated MEK and ERK. The GFP label and myc tag enabled detection of a high transduction rate. Viruses were injected into both sides of the brain 2 weeks before transection and the placement of the SC bridge. By 7 weeks, the BBB scores of the MEK– and ERK–treated animals began to diverge from those of the GFP-only animals, ending with a difference of 6.5 ± 0.3 or 7.0 ± 0.3 vs 4.8 ± 0.5 by 9 weeks after implantation. Green axons were counted at the rostral cord/bridge interface, in the bridge, near the caudal interface, and beyond. A mean of more than 250 green axons was found in the center of the bridge and a smaller number was found beyond the bridge when the animals received AAV/GFP plus AAV/MEK or AAV/ERK viruses; no green axons were observed in the bridge in the AAV/GFP–injected rats.
The Contusion/SC Implantation Model
SCs also have been transplanted into contusion injuries induced in adult thoracic rat spinal cord. The rat contusion injury model is highly relevant to injury in the human spinal cord; large cavities are found in both species following contusion. Following moderate/severe contusion injury (12.5 mm, 10 g weight, MACSIS impactor) and SC injection into the contusion site at 7 days, the expected large cavities are not visible or are substantially reduced weeks later (32,35–37). SCs significantly improve tissue sparing compared with injection of culture medium only into the contusion site and lead to higher numbers of myelinated axons in the implant (35). With the injection of medium, there is a mean of more than 2,000 myelinated axons in the implant, undoubtedly due to the migration of endogenous SCs into the lesion site. With the injection of SCs, the mean is increased to more than 5,000 myelinated axons. When retrograde labeling is performed by injecting tracer beyond the caudal interface of the graft with the cord, neuronal somata of the fibers that reach the tracer can be counted to detect the numbers of fibers present caudal to the lesion. There is a significant increase in labeled neuronal somata in both the spinal cord and brain stem, indicating that there are more fibers below the lesion site after SC implantation compared with injection of medium. Because there may be some spinal cord tissue retained around the cord periphery after contusion injury, a clear distinction between spared, sprouted, and regenerated fibers cannot be made. Thus, the increase in fibers that we observed caudal to the lesion may be due to sparing, sprouting from spared fibers, or regrowth through or around the implantation site into the caudal spinal cord. There is a modest improvement in the BBB score. In sum, in this thoracic contusion model following SC injection, there is a significant obliteration of or reduction in cyst formation, increased tissue sparing, support of axonal growth into the graft, significant improvement in axon sparing and/or growth of spinal and supraspinal axons beyond the graft, and modest improvement in locomotor function (35).
Combinatorial Strategies: Combination strategies also have been tested in this contusion model, one of them being the transduction of SCs ex vivo with the D15A gene introduced by either lentiviral (LV) or adenoviral (AdV) vectors (17). D15A is a modified NT-3 molecule that activates both trk B and C receptors, thus leading to both BDNF and NT-3 activity (38); some SCs were infected with GFP gene–containing viral vectors as controls. The D15A secreted from the transduced SCs was found to be bioactive in culture, and D15A was detected in the spinal cord after transplantation. The extent of the implant, clearly seen due to the GFP label in the SCs, was increased fivefold compared with the GFP-only SCs, and the SCs were increased fivefold as well. There was a significant increase in myelinated axon number when D15A SCs were implanted compared with GFP SCs; for AdV/GFP/D15A SCs, the mean exceeded 18,000, whereas the LV/GFP/D15A SCs resulted in more than 26,000 myelinated axons in the graft. The myelinated axon counts for nontransduced SCs or the control AdV/GFP SCs or LV/GFP SCs were similar to the earlier study (35), with means ranging from 4,700 to 5,100 myelinated axons. Using a method we devised to estimate total numbers of axons, that is, myelinated and nonmyelinated axons, we found that with the AdV/GFP/D15A SCs, the estimated total number was 45,000, and with the LV/GFP/D15A SCs, it was 75,000 axons. In summary, D15A present in the SC graft led to a significant increase in myelinated axons and total axons and also a significant increase in serotonergic, DβH, and CGRP fiber growth in the implant. Locomotion was not improved, as assessed by BBB (Basso, Beattie, Bresnahan) scoring, probably because few fibers left the implant. Thus, the goal of the experiment, to increase fiber growth into the implant, was accomplished, but an additional strategy is needed to promote this growth out of the implant.
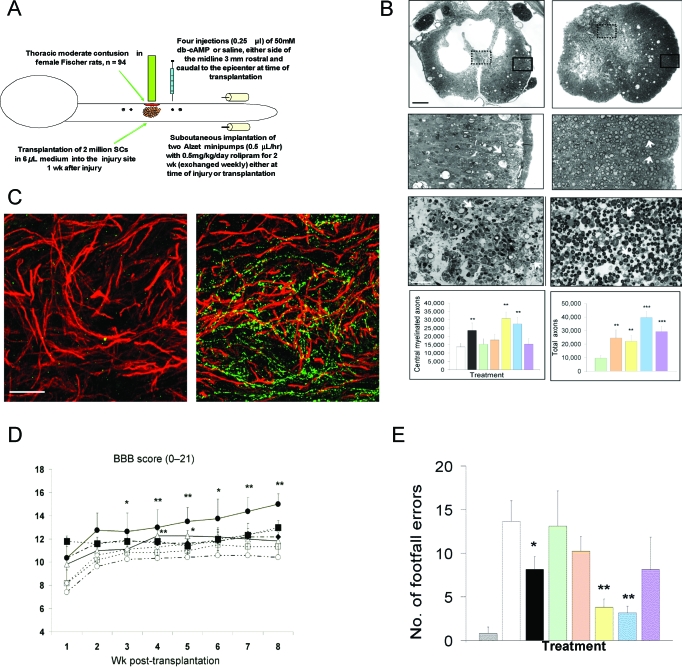
Cyclic AMP (cAMP) levels are key for the ability of a nerve cell to extend processes on inhibitory substances such as myelin in culture. Neurons removed from perinatal animals extend neurites on myelin, unlike neurons from older animals; this switch to inhibition of neurite regeneration coincides with a dramatic drop in endogenous levels of cAMP (39). This inhibition in older neurons can be prevented by increasing cAMP levels. This information led us to study a strategy combining a contusion injury with SC implantation and elevation of cAMP levels. At the time of contusion, a simultaneous (or 1-week delayed) subcutaneous infusion of a phosphodiesterase-4 inhibitor, rolipram, was initiated for 2 weeks and, 1 week later, SCs were implanted and an analog of cAMP, dibutyryl cAMP (db-cAMP), was injected in 4 places above and below the SC implant (36) (Figure 2). The experimental groups were (1) contusion only, (2) contusion plus rolipram immediately initiated, (3) SCs, (4) SCs plus db-cAMP, (5) SCs plus rolipram immediately initiated, (6) SCs plus rolipram immediately initiated plus db-cAMP, and (7) SCs plus rolipram initiated at 1 week plus db-cAMP. Levels of cAMP fall after injury, but if rolipram is initiated immediately, that drop is prevented and the db-cAMP injection increases levels to above those found normally. Thus, we were assured that levels of cAMP were raised by the administration of rolipram and injection of db-cAMP.
Figure 2. Combination approach of SC implantation in a contusion site with elevation of cAMP. (A) Indicates the steps in the experimental protocol. (B) The left column presents photomicrographs of injured control spinal cord (injected with culture medium only) from which counts of spared oligodendrocyte-myelinated axons were obtained as an indicator of white matter sparing. The column on the right shows images of implants from the acute rolipram/SC implant/db-cAMP group; the typical cavitation is no longer seen, and more central myelinated axons are visible than on the left (scale bars, 250μm, 50 μm). The grafts at the bottom show that there were significantly more central myelinated axons in rolipram groups and that the estimated highest total number of axons was observed in the acute rolipram/SC implant/db-cAMP group. *The asterisk indicates significance at P < 0.05, **The double asterisk indicates significance at P < 0.01. (C) Illustration of immunostaining for SCs (red) and for 5HT-positive fibers (green); the fibers are prominent in the implant from an acute rolipram/SC implant/ db-cAMP–treated animal (right) but are absent in the SC-implanted-only animal (left). (D) The highest BBB scores were observed for the acute rolipram/SC implant/db-cAMP group. *The asterisk indicates significance at P < 0.05, **The double asterisk indicates significance at P < 0.01. (E) Significantly fewer footfall errors on a gridwalk were found in the rolipram groups. Uninjured control (gray); injured-only control (white); acute rolipram (black); SC-only implant (green); SC implant/db-cAMP (tan); acute rolipram/SC implant (yellow); acute rolipram/SC implant/db-cAMP (blue); and delayed rolipram/SC implant/db-cAMP (violet). Eleven weeks post injury. *The asterisk indicates significance at P < 0.05. **The double asterisk indicates significance at P < 0.01. From Pearse et al. (36).
Tissue sparing was assessed by counting central myelinated fibers in areas that had remained intact in the periphery of the cord after the injury (36). In the groups that received rolipram (group 2), rolipram plus SCs (group 5), or rolipram immediately plus SCs and db-cAMP (group 6), sparing was improved significantly (doubling of central myelinated axon number). SC-myelinated axons in the implants were increased 2.5-fold (groups 3 vs 6); the estimated total number of axons was 40,000 in group 6 compared with 10,000 in group 3. Retrograde tracer introduced caudal to the lesion/implant labeled the neuronal somata of those fibers that reached that level. Counting the labeled neuronal somata in the reticular formation, raphe nuclei and red nuclei revealed a significant increase in fibers from those areas beyond the lesion/implant in all groups receiving rolipram. Thus, there were increased numbers of fibers present below the area of the lesion/implant from at least these 3 brain stem nuclei. In the case of raphe nuclei, there was an additional significant increase in labeled neuronal somata in the acute rolipram/SC/db-cAMP group compared with animals receiving rolipram/SCs. The implants in the acute rolipram/SC/db-cAMP animals also contained a significantly higher number of serotonergic fibers, and serotonergic fibers extended beyond the implant as well. BBB testing revealed a significant improvement from 10.4 plus or minus 1.2 in the control animals to 15.0 plus or minus 0.9 (indicative of fore limb/hind limb coordination) in the animals receiving the acute triple treatment. A significant correlation was found between the BBB score and the number of serotonergic fibers in the graft and also caudal to the graft. Footprint analysis revealed less foot exo-rotation and improved base of support in the animals receiving rolipram. The gridwalk analysis showed significantly fewer foot missteps, particularly in the rolipram/SC and the acute triple treatment groups. In conclusion, we were able to raise the levels of cAMP in the spinal cord (as well as in the brain stem) after injury. There was significant improvement in lateral white matter sparing, number of myelinated axons in the grafts, serotonergic fiber growth into SC grafts and beyond, number of axons (including from supraspinal neurons) beyond the lesion/implant, and functional hind limb recovery (estimated to be up to 70% improvement). The best results were observed with the full triple treatment started acutely.
In sum, in our work with SCs, there is no doubt that a combinatorial approach improves outcome following SCI. Studies in other laboratories are reaching similar conclusions. To cite one example, Lu et al (40) observed that the combined preconditioning stimulus of injecting db-cAMP into sensory neuronal ganglia before lesioning sensory axons in the cervical spinal cord and then injecting NT-3 into a graft of bone marrow stromal cells and into the spinal cord beyond the lesion/graft site resulted in axonal regeneration not only into, but also beyond, the lesion; growth beyond the lesion did not occur with cAMP or NT-3 administration alone. Because there has been substantial progress in testing promising strategies for spinal cord repair, the question is what combination therapy will be most efficacious. An initial intervention to curb the secondary loss of tissue will be key. In addition, subsequent interventions may be cellular bridges to span cavities in the spinal cord, with possible genetic engineering of the cells to generate additional growth factors, modification of the inflammatory response and scarring, and neutralization of factors inhibitory to axonal growth. The third, and equally important therapeutic strategy, will be rehabilitation to maximize the effect of the earlier regimens.
Acknowledgments
Our work continues to be driven by the vision of Dr. Richard P. Bunge. Many persons have contributed to the work described; their contributions are acknowledged in the individual publications. We greatly appreciate their hard work and dedication. The core facilities in The Miami Project to Cure Paralysis have greatly facilitated the work reported here. This work has been funded by The Miami Project to Cure Paralysis, the Christopher and Dana Reeve Foundation International Research Consortium, continuous National Institutes of Health funding over many years, the Buoniconti Fund, and the Hollfelder and Heumann Foundations. References 25 and 34 report work resulting from collaboration within the Christopher and Dana Reeve Foundation International Research Consortium. The author is the Christine E. Lynn Distinguished Professor of Neuroscience. Word processing by Jenissia Veloz-Jeanty is gratefully acknowledged.
Footnotes
This article describes the data presented in the Donald Munro Lecture 2007. It is neither a full report of the work performed in my laboratory nor is it a presentation that alludes to outstanding and relevant work accomplished in other laboratories.
REFERENCES
- Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Chapter VII: Study of regenerative processes of the cerebrum. In: May RM, De Felipe J, Jones EG, editors. Cajal's Degeneration and Regeneration of the Nervous System. trans. New York, NY: Oxford University Press; 1991. pp. 734–750. [Google Scholar]
- Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Reier PJ. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx. 2004;1:424–451. doi: 10.1602/neurorx.1.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon LDF, Bunge MB. From animal models to humans: strategies for promoting CNS axon regeneration and recovery of limb function after spinal cord injury. J Neurol Phys Ther. 2005;29:55–69. doi: 10.1097/01.npt.0000282512.16964.94. [DOI] [PubMed] [Google Scholar]
- Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Barakat DJ. Cellular repair strategies for spinal cord injury. Expert Opin Biol Ther. 2006;6:639–652. doi: 10.1517/14712598.6.7.639. [DOI] [PubMed] [Google Scholar]
- Bunge MB. Bridging areas of injury in the spinal cord. Neuroscientist. 2001;7:325–339. doi: 10.1177/107385840100700409. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Wood PM. Transplantation of Schwann cells and olfactory ensheathing cells to promote regeneration in the CNS. In: Selzer ME, Clarke S, Cohen LG, Duncan PW, Gage FH, editors. Textbook of Neural Repair and Rehabilitation. Cambridge, MA: Cambridge University Press; 2006. pp. 513–531. [Google Scholar]
- Oudega M, Xu XM. Schwann cell transplantation for repair of the adult spinal cord. J Neurotrauma. 2006;23:453–467. doi: 10.1089/neu.2006.23.453. [DOI] [PubMed] [Google Scholar]
- Oudega M. Schwann cell and olfactory ensheathing cell implantation for repair of the contused spinal cord. Acta Physiol. (OXF) 2007;189:181–189. doi: 10.1111/j.1748-1716.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- Gilmore SA, Sims TJ. Patterns of Schwann cell myelination of axons within the spinal cord. J Chem Neuroanat. 1993;6:191–199. doi: 10.1016/0891-0618(93)90041-2. [DOI] [PubMed] [Google Scholar]
- Menei P, Montero-Menei C, Whittemore SR, Bunge RP, Bunge MB. Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur J Neurosci. 1998;10:607–621. doi: 10.1046/j.1460-9568.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Weidner N, McCormack M, Miller I, Powell H, Connor J. Grafts of genetically modified Schwann cells for the spinal cord; survival, axon growth, and myelination. Cell Transplant. 1998;7:187–196. doi: 10.1177/096368979800700213. [DOI] [PubMed] [Google Scholar]
- Weidner N, Blesch A, Grill RJ, Tuszynski MH. Nerve growth factor–hypersecreting Schwann cell grafts augment and guide spinal cord axonal growth and remyelinate central nervous system axons in a phenotypically appropriate manner that correlates with expression of L1. J Comp Neurol. 1999;413:495–506. doi: 10.1002/(sici)1096-9861(19991101)413:4<495::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Golden KL, Pearse DD, Blits B, et al. Transduced Schwann cells promote axon growth and myelination after spinal cord injury. Exp Neurol. 2007;207:203–217. doi: 10.1016/j.expneurol.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Chen A, Guénard V, Kleitman N, Bunge MB. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and cuadal stumps of transected adult rat spinal cord. J Neurocytol. 1997;26:1–16. doi: 10.1023/a:1018557923309. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guénard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell–seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- Plant GW, Bates ML, Bunge MB. Inhibitory proteoglycan immunoreactivity is higher at the caudal than the rostral Schwann cell graft-transected spinal cord interface. Mol Cell Neurosci. 2001;17:471–487. doi: 10.1006/mcne.2000.0948. [DOI] [PubMed] [Google Scholar]
- Chen A, Xu XM, Kleitman N, Bunge MB. Methylprednisolone administration improves axonal regeneration into Schwann cell grafts in transected adult rat thoracic spinal cord. Exp Neurol. 1996;138:261–276. doi: 10.1006/exnr.1996.0065. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guénard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Blits B, Oudega M, Boer GJ, Bunge MB, Verhaagen J. Adeno-associated viral vector-mediated neurotrophin gene transfer in the injured adult rat spinal cord improves hindlimb function. Neuroscience. 2003;118:271–281. doi: 10.1016/s0306-4522(02)00970-3. [DOI] [PubMed] [Google Scholar]
- Ramón-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PM, Issa VM, Aguayo AJ. Regeneration of long spinal axons in the rat. J Neurocytol. 1984;13:165–182. doi: 10.1007/BF01148324. [DOI] [PubMed] [Google Scholar]
- Santos-Benito FF, Ramón-Cueto A. Olfactory ensheathing glia transplantation: a therapy to promote repair in the mammalian central nervous system. Anat Rec B New Anat. 2003;271:77–85. doi: 10.1002/ar.b.10015. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A. Olfactory ensheathing cells and spinal cord repair. Keio J Med. 2005;54:8–14. doi: 10.2302/kjm.54.8. [DOI] [PubMed] [Google Scholar]
- Ruitenberg MJ, Vukovic J, Sarich J, Busfield SJ, Plant GW. Olfactory ensheathing cells: characteristics, genetic engineering, and therapeutic potential. J Neurotrauma. 2006;23:468–478. doi: 10.1089/neu.2006.23.468. [DOI] [PubMed] [Google Scholar]
- Barnett SC, Riddell JS. Olfactory ensheathing cell transplantation as a strategy for spinal cord repair—what can it achieve. Nat Clin Pract Neurol. 2007;3:152–161. doi: 10.1038/ncpneuro0447. [DOI] [PubMed] [Google Scholar]
- Raisman G, Li Y. Repair of neural pathways by olfactory ensheathing cells. Nat Rev Neurosci. 2007;8:312–319. doi: 10.1038/nrn2099. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Sanchez AR, Pereira FC, et al. Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: survival, migration, axon association and functional recovery. Glia. 2007;55:976–1000. doi: 10.1002/glia.20490. [DOI] [PubMed] [Google Scholar]
- Vavrek R, Pearse DD, Fouad K. Neuronal populations capable of regeneration following a combined treatment in rats with spinal cord transection. J Neurotrauma. 2007;24:1667–1673. doi: 10.1089/neu.2007.0290. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Blits B, Joseph G, et al. Constitutive activation of the MAPK pathway promotes regeneration after complete spinal cord transection [abstract] The 36th Annual Meeting of the Society for Neuroscience. 2006.
- Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci. 2002;22:6670–6681. doi: 10.1523/JNEUROSCI.22-15-06670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Hill CE, Moon LDF, Wood PM, Bunge MB. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement and strategies to enhance survival. Glia. 2006;53:338–343. doi: 10.1002/glia.20287. [DOI] [PubMed] [Google Scholar]
- Urfer R, Tsoulfas P, Soppet D, Escandon E, Parada LF, Presta LG. The binding epitopes of neurotrophin-3 to its receptors trkC and gp75 and the design of a multifunctional human neurotrophin. EMBO J. 1994;13:5896–5909. doi: 10.1002/j.1460-2075.1994.tb06935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]