Abstract
Stroke is a major cause of neurologic morbidity in neonates and children. Since neonatal and pediatric stroke frequently present with seizures, the question of which anticonvulsant best blocks acute ischemic seizures and reduces injury is clinically relevant. The purpose of this study was to determine the extent to which gabapentin is neuroprotective and suppresses acute seizures in this model of ischemic injury in the immature brain. Postnatal day 12 CD1 mice underwent right common carotid artery ligation and immediately after ligation received a 0, 50, 100, 150, or 200 mg/kg dose of gabapentin intraperitoneally. Acute seizure activity was behaviorally scored and hemispheric brain atrophy measured. In vehicle treated mice, severity of acute seizures correlated with hemispheric brain atrophy four weeks later. Gabapentin significantly decreased acute seizures at 200 mg/kg and reduced brain atrophy at doses of 150 and 200 mg/kg but not at lower doses. These results suggest that gabapentin effectively reduces acute seizures and injury after ischemia in the immature brain. When analyzed by animal sex, the data suggest that gabapentin may more effectively reduce acute seizures and injury in male pups versus female pups.
Keywords: ischemia, seizures, mouse pup, carotid ligation, gabapentin, immature mouse brain ischemia, ischemic seizures, neuroprotection
One in four thousand term neonates are diagnosed with stroke (1) and approximately 8 in 100,000 children experience neurologic morbidity due to stroke (2). Stroke in neonates and children frequently results in cerebral palsy, learning disabilities, visual field deficits, and epilepsy (3). The postnatal day 12 (P12) CD1 mouse unilateral carotid ligation model used in this study has previously been shown to result in acute ischemic seizure activity and brain injury in the distribution of the middle cerebral artery (4–6). Importantly, in this model, the severity of acute seizures correlates with the severity of brain injury one week after ligation (4). This novel aspect of the immature mouse unilateral carotid ligation model mimics the clinical situation in that neonatal stroke presents acutely with seizures (7) and in pediatric stroke the occurrence of seizures is correlated with more severe neurologic outcome (8).
Developmental regional brain vulnerability to ischemic injury has been associated with the proximity of selected neuronal populations to developing glutamatergic circuits (9). Furthermore, developmentally regulated variations in α-amino-3-hydroxy-5-methyl-4-isoxazole-proprionic acid (AMPA) receptor subunits, N-methyl-D-aspartate (NMDA) receptor subunits, and in the activity of ion channels have all been implicated in excitotoxicity and ischemia-induced seizures (10–12). These developmentally regulated differences may contribute to an age-related period of susceptibility to seizures and brain injury from impaired brain perfusion. Since the immature brain responds very differently to ischemic insults (13), it is therefore crucial that relevant immature animal models be utilized for preclinical neuroprotection studies.
Immature brain neuroprotection has been the goal of much research aimed at interrupting the excitotoxic cascade and subsequent injury due to free radicals and inflammation (14). Researchers have learned that long-term protection must be shown after treatment to prove the drug prevents, rather than just delays, brain injury. So far however, only hypothermia after moderate neonatal brain asphyxia has been shown clinically to reduce injury (15). Since most neonates and young children with strokes present with seizures, the question of which anticonvulsant(s) both reduce the acute ischemic seizures and provide neuroprotection is clinically relevant. The goal of this study was to determine the extent of seizure suppression and neuroprotection afforded by the anticonvulsant gabapentin [1-(aminomethyl)-cyclohexan-acetic acid] to this model of ischemic injury in the immature brain.
METHODS
All materials and methods were approved by the Johns Hopkins University Animal Care and Use Committee.
Surgery
At P12, CD1 mice (Charles River Laboratories, Boston, Massachusetts) underwent right common carotid artery ligation surgery. Anesthesia was induced and maintained with 4% and 1.2% isoflurane anesthesia respectively. Immediately thereafter, pups from each litter were administered intraperitoneally either a 0 (vehicle), 50, 100, 150, or 200 mg/kg dose of gabapentin dissolved in saline solution. Pups were then placed into a 36°C incubator for seizure scoring.
Temperature measurements
In a separate experiment, we recorded rectal temperatures in 20 P12 CD1 mouse pups (10 sham surgery and 10 ligates); half of each group received vehicle injections and half received gabapentin 200mg/kg i.p (n=5/group). Rectal temperatures were measured before surgery while pups were in home cage with dam, immediately after surgery, two hours after surgery in 36°C incubator, and four hours after surgery in 36°C incubator. This experiment was done in a separate group of mice from the gabapentin neuroprotection experiment in order to not interrupt seizure rating in the main study.
Seizure scoring
Seizure activity was scored by an investigator blinded to ligation status, according to a seizure rating scale as previously reported (16). Every 5 minutes, the score corresponding to the highest level of seizure activity observed during that time period was recorded. Briefly, seizure behavior was scored as follows: 0 = normal behavior; 1 = immobility; 2 = rigid posture; 3 = repetitive scratching, circling, or head bobbing; 4 = forelimb clonus, rearing, and falling; 5 = mice that exhibited level four behaviors repeatedly; and 6 = severe tonic-clonic behavior. After four hours, the mice were returned to the dam and each of their seizure scores was individually summed to produce a total seizure score.
Perfusion
At P40, mice were anesthetized with 90 mg/kg chloral hydrate, perfused transcardially with ice-cold 4% paraformaldehyde, post-fixed for 12 hours in the same fixative, cryoprotected, and snap frozen.
Atrophy Measurement
Using MCID 7.0 Elite (InterFocus Imaging Ltd., Cambridge UK) cross-sectional hemispheric areas of 50 µm-thick, Nissl-stained sections equally spaced and spanning rostral striatum to caudal hippocampus were measured (n=10–12 sections per animal). Extent of hemispheric brain atrophy was calculated according to: (1-(ipsilateral/contralateral))*100. MCID measurements were performed by an experimenter blinded to the dose of gabapentin administered. Presence or absence of brain injury was determined by microscopic inspection of sections at 4X and 10X according to the presence or absence of an infarct, atrophic malformed structures, or cellular loss in the hippocampus.
Statistical Analysis
Statistical analyses were run in SPSS for Windows (SPSS Inc., Chicago, Illinois, USA). Only data from injured animals that survived until perfusion were included in the analysis (n = 47). For all analyses, statistical significance was set at (p < 0.05).
Median seizure score and hemispheric brain atrophy analysis
In order to make the number of injured animals that survived in the vehicle treated group comparable to that of the other dose groups, 10 out of the 16 animals from the control group were randomly selected by a computer program in SPSS. Median percent hemispheric brain atrophy (non-parametric analyses were used because groups were not normally distributed) and median seizure scores were calculated for each dose of gabapentin. Mann-Whitney U tests were done to determine statistical significance compared to vehicle treated injured mice. Spearman’s rho correlations were used to determine the strength and significance in correlations between median seizure score and median percent hemispheric brain atrophy.
Epoch analysis of seizure severity
For this analysis, treatment cohorts were grouped into no/low dose gabapentin (0, 50, 100 mg/kg) and high dose gabapentin (150 and 200 mg/kg). A computer generated random selection was done to have equal animal numbers in both groups (n = 22). Average number of seizure-free epochs/animal (i.e., score 0) was calculated for each group and compared by student’s t-test. Average (per animal) and total numbers of epochs given scores of 1, 2, 3, 4, 5, or 6 were also calculated and compared. The number of epochs scored as “0” before the onset of seizures was also calculated and compared as an estimation of the time to behavioral seizure onset after surgery. The seizure epoch analysis was done this way in order to have sufficient numbers of epochs for each seizure score and because brain injury was similar within the two groups.
Sex-related analyses
To evaluate this data for sex-related differences in the effects of gabapentin, median percent hemispheric brain atrophy and median seizure score data were combined into two groups for each gender: no/low dose gabapentin (0, 50, and 100 mg/kg) and high dose gabapentin (150 and 200 mg/kg). In order to compare median percent hemispheric brain atrophy or median seizure scores between the genders, the sample size for both female and male mice in the low dose group was standardized at n = 15 by randomly selecting the data for 15 animals using SPSS software. The sample size in the 150 and 200 mg/kg group was not changed, as an equal number of mice already existed in this group, for both genders. Mann-Whitney U tests were used to compare median percent hemispheric brain atrophy and median seizure score between the no/low (0, 50, or 100 mg/kg) dose group and the high (150 or 200 mg/kg) dose group for males and females separately. Mann-Whitney U tests were used to compare median percent hemispheric brain atrophy in each group between genders (i.e. median seizure scores for males in the no/low dose group vs. for females in the no/low dose group). Spearman’s rho correlations were used to determine the strength in the correlation between median percent hemispheric brain atrophy and median seizure scores in each group within genders.
RESULTS
Temperature responses
Mean rectal temperatures and anesthesia times were not significantly different between the four groups in the temperature study: range of baseline mean temperatures was 33.3–33.8°C; right after the surgical procedure range of mean temperatures was 32.4–32.7 °C; two hours after surgery range of mean temperatures was 34.4–34.6°C; and four hours after surgery range was 34.4–34.7°C. Therefore, anesthesia during surgery lowered temperatures by the same amount for all four groups and gabapentin did not alter temperatures. A posthoc multiple comparisons test showed no significant differences in mean rectal temperatures at any of the four timepoints between the 4 groups.
Gabapentin neuroprotection study
Overall mortality was 10% for all groups. No statistically significant difference in mortality was found in any of the groups. Anesthesia times were not significantly different between any of the treatment groups.
Seizure Score – both sexes
Regardless of sample size used for the control group (n = 10 randomly selected or total n=16), seizure scores after 150 mg/kg (n = 13, 6 male, 7 female) and 200 mg/kg (n = 9, 5 male and 4 female) doses of gabapentin were reduced compared to vehicle treated mice, with significance reached at 200 mg/kg and a trend at 150 mg/kg gabapentin. Seizure scores were not significantly different after 50 (n = 9, 6 male and 3 female) or 100 mg/kg (n = 10, 3 male and 7 female) doses of gabapentin. Median seizure score for vehicle treated mice (n = 10, 6 male and 4 female) was 15.5 (range = 0 – 65), 12 for 50 mg/kg dose of gabapentin (range = 0 – 37; NS); 10 for 100 mg/kg dose (range = 0 – 97; NS); 10 for 150 mg/kg (range = 0 – 23, p = 0.093), and 3 for 200 mg/kg dose of gabapentin (range = 0 – 24; p < 0.01) (see Figure 1A). Notably, none of the gabapentin doses produced any appreciable acute toxicity with regards to the quality of non-seizure movement. Therefore, we were easily able to rate seizure behavior in all the groups.
Figure 1. Acute seizures after injury.
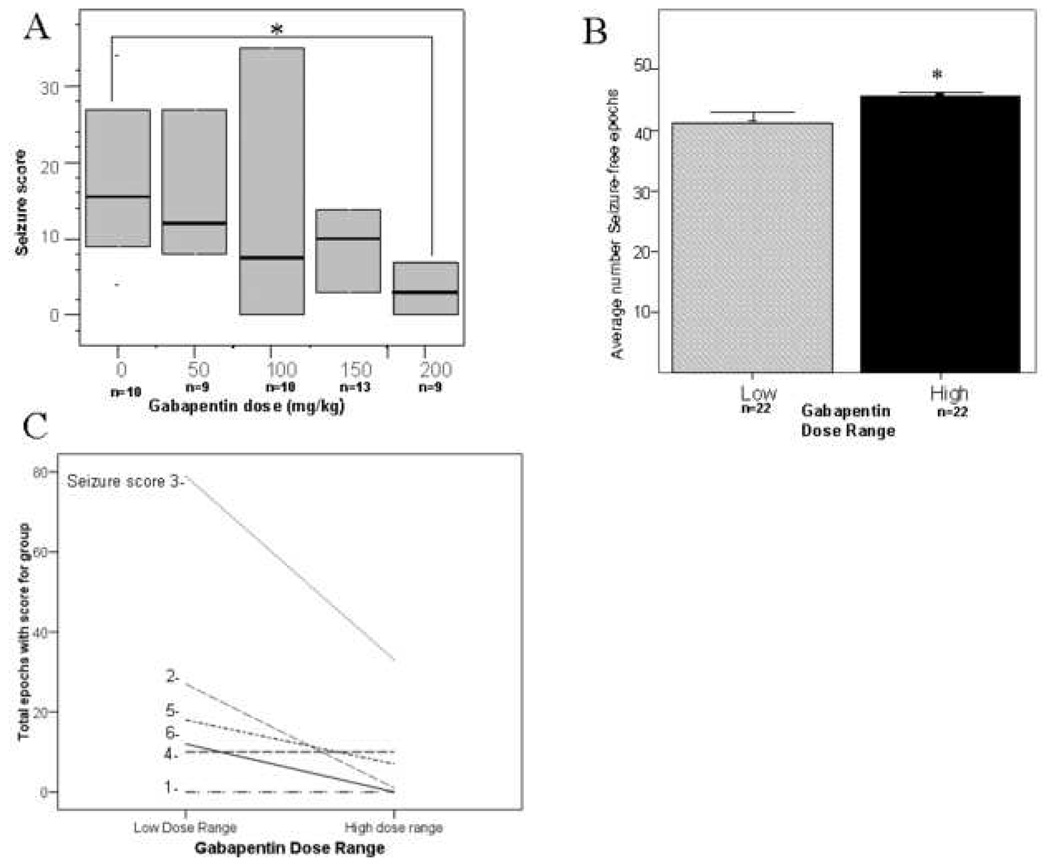
Panel A. Boxplot of seizure scores from injured CD1 mice administered vehicle or gabapentin injection immediately after ligation at P12. Dark lines indicate median seizure score and boxes indicate interquartile range. Median seizure score was significantly decreased after 200 mg/kg gabapentin compared with vehicle injected animals. Panel B. Average number of seizure free epochs. The average number of seizure free epochs was significantly greater in the high dose (150 or 200 mg/kg) gabapentin treated animals compared with the vehicle/low dose treated animals (0, 50 or 100 mg/kg dose). Panel C. The total number of epochs with seizure scores of 2, 3, 5 or 6 decreased in the animals treated with high dose gabapentin.
Analysis of seizure scores and epochs of zero/low dose and high dose gabapentin treated animals found that the number of seizure-free epochs was increased in the high-dose gabapentin treated animals (45.7 +/− 0.5 SEM) compared with the zero/low dose treated group (41 +/− 1.6 SEM, p<0.01; Figure 1B). The average number of epochs per animal and total epochs given scores was decreased in the high dose group for all seizure scores except score of 4 that was unchanged between the groups (Figures 1C). This decrease was significant for scores of 3 and 6. None of the high-dose treated animals received any scores of 6; the highest possible seizure score (Figure 1C). The number of epochs scored “0” before the onset of seizures, and therefore latency to acute seizures, was not significantly different between the zero/low dose and high dose groups.
Hemispheric Brain Atrophy –both sexes
Injury was seen in the ipsilateral hemisphere. Regardless of sample size considered in the control group (n = 16 or n = 10), significantly reduced median percent hemispheric brain atrophy was seen with gabapentin administered at 150 mg/kg and 200 mg/kg but not at lower doses. Median hemispheric brain atrophy was 48% for control animals (n = 10, range= 31 – 77%), 39% for animals that received a 50 mg/kg dose of gabapentin (range = 13 – 60%, NS), 44% for animals that received a 100 mg/kg dose (range = 0 – 76%, NS), 25% for those that received a 150 mg/kg dose (range = 3 – 55%, p < 0.01), and 25% for those that received a 200 mg/kg dose of gabapentin (range = 2 – 77%, p < 0.05; see Figure 2).
Figure 2. Hemispheric brain atrophy after ligation.
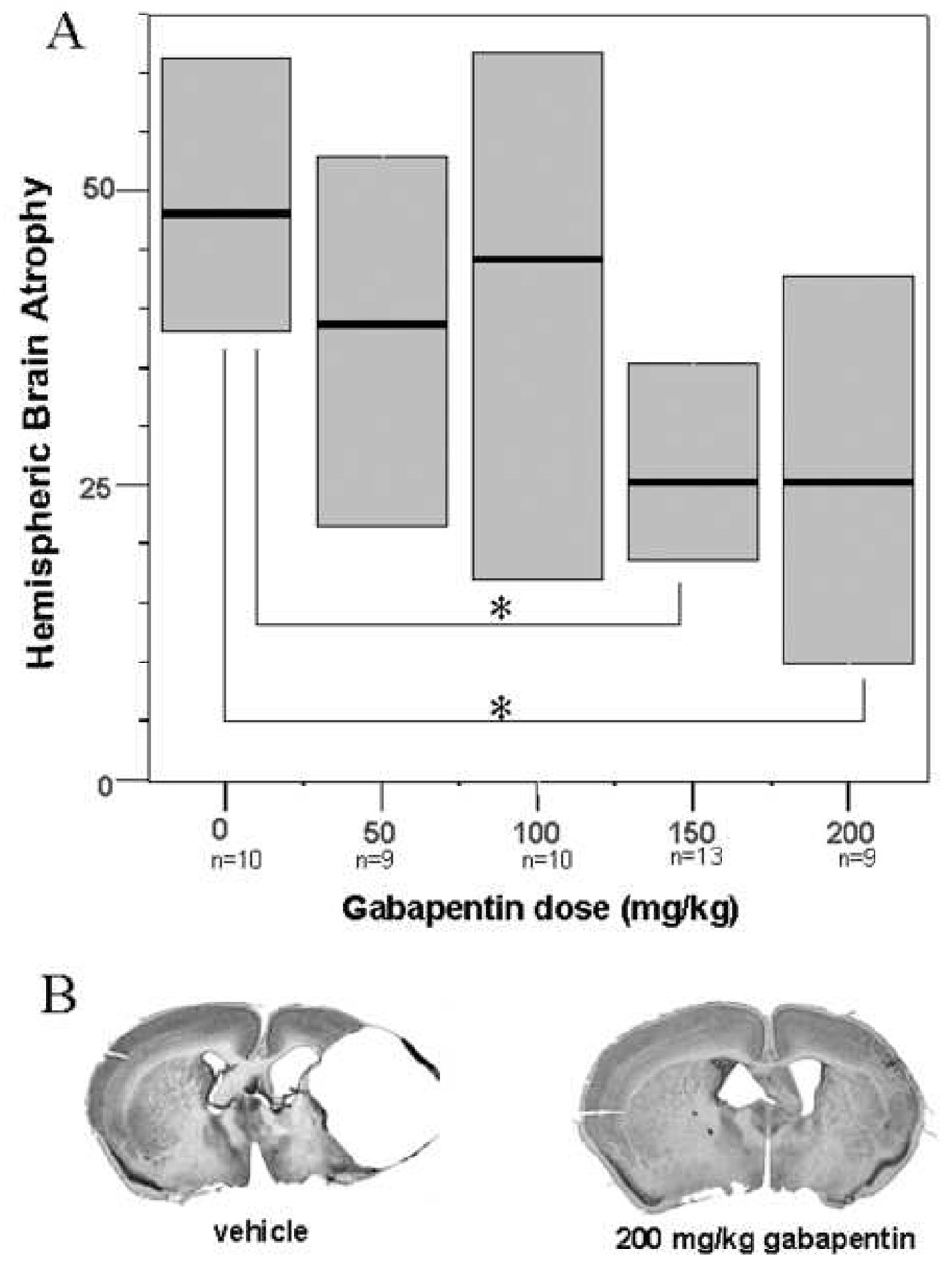
Panel A. Boxplots of hemispheric brain atrophy at P40 after ligation at P12. Dark lines indicate median hemispheric brain atrophy and boxes interquartile range. Median hemispheric brain atrophy was significantly reduced after 150 mg/kg or 200 mg/kg gabapentin injections (p<0.05) compared with vehicle treated animals. Panel B. Cresyl violet stained sections from brains closest to the median brain injury in vehicle and 200 mg/kg gabapentin treatment groups demonstrating greatly reduced ipsilateral hemispheric injury.
Correlation between Seizures and Atrophy – both sexes
Seizure activity was significantly correlated with median percent hemispheric brain atrophy in the vehicle treated animals (correlation coefficient = 0.794; p < 0.01; Figure 3A), a trend for a correlation was present at 50 mg/kg (correlation coefficient = 0.619; p = 0.08), and these variables were highly correlated at a dose of 100 mg/kg of gabapentin (Spearman’s rho = 0.944, p < 0.001. This is similar to the correlation between seizures and brain injury previously described in this model seven days after ligation (4). However, the correlation was lost at higher doses (Spearman’s rho after 150 mg/kg = 0.141, NS and after 200 mg/kg gabapentin = 0.105, NS; see Figure 3B).
Figure 3. Scatterplots showing the correlation between acute seizure scores and hemispheric brain atrophy at P40.
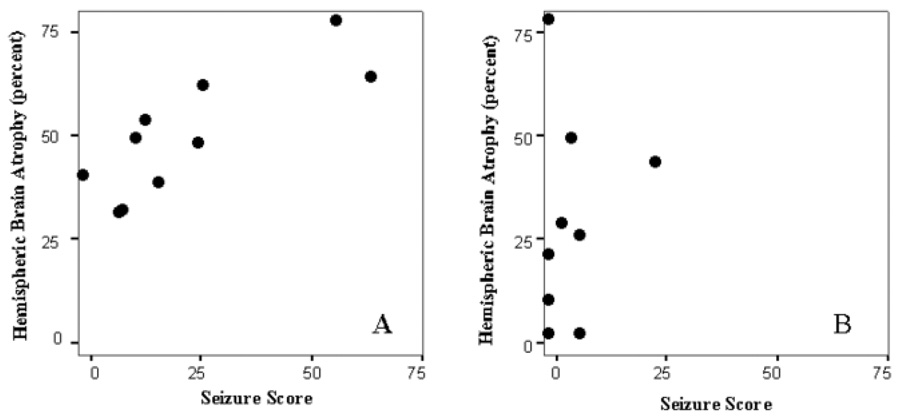
Panel A. Seizure scores highly and significantly correlated with hemispheric brain injury in vehicle treated animals. Panel B. Seizure scores did not correlate with hemispheric brain atrophy in mice treated with 200 mg/kg gabapentin.
Animal Sex and Seizure Score
Male mice that received higher doses of gabapentin (150 or 200 mg/kg, n = 11) showed a significant reduction in median seizure score relative to males that received lower doses (0, 50, or 100 mg/kg gabapentin) irrespective of whether all males or a randomly selected group of males was used for the male low dose group (n = 15, median seizure score in low dose treated male pups = 16, range 0 – 65 versus median seizure score = 5, range 0 – 23 in high-dose treated male pups, p < 0.05, see Figure 4C). In females, gabapentin administered at higher doses (150 or 200mg/kg, n=11) did not cause a significant decrease in seizure activity (median seizure score = 10, range 0 – 24), relative to lower doses of gabapentin (0, 50, or 100 mg/kg; n=15, median seizure score = 8, range = 0 – 97, NS; Figure 4D). The seizure activity in male and female control mice at low doses (0, 50 or 100 mg/kg gabapentin) was not significantly different.
Figure 4. Sex differences in acute seizures and brain injury.
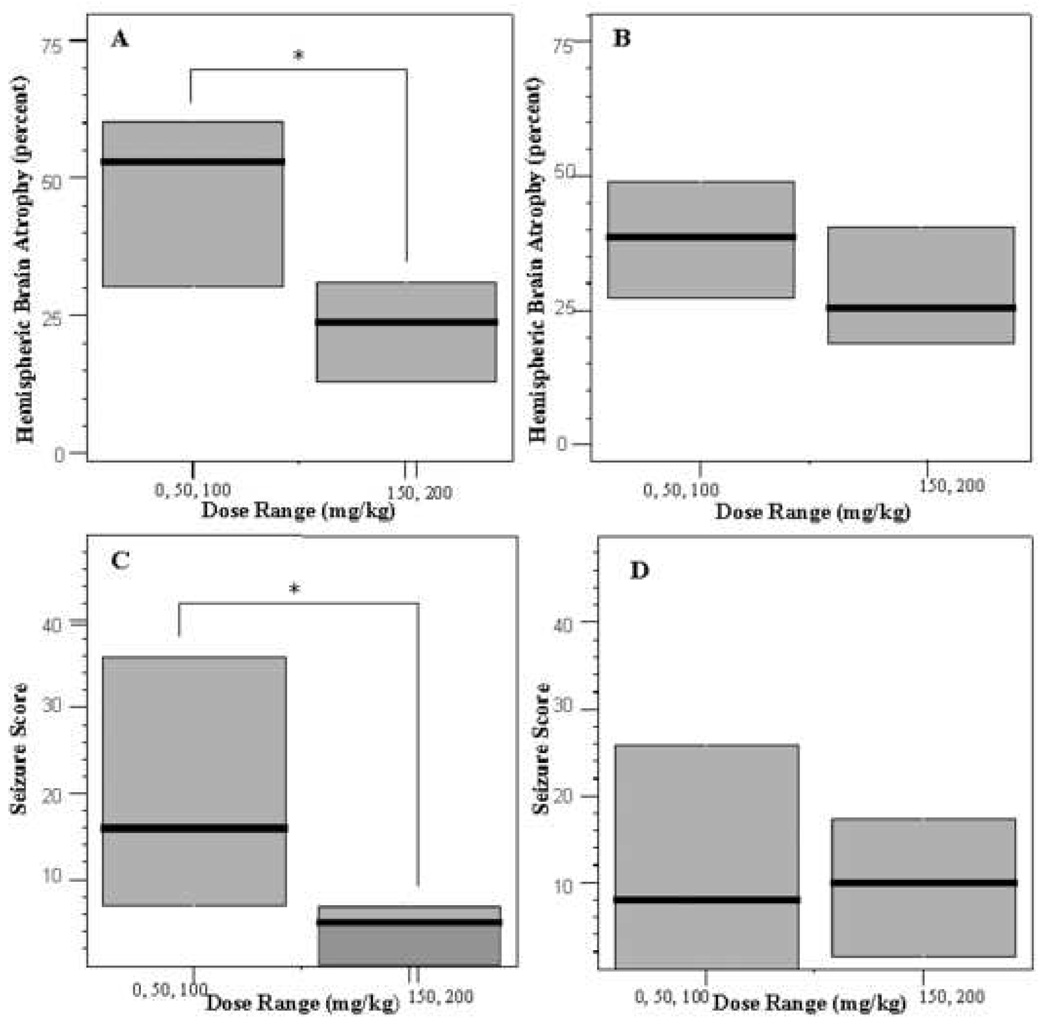
Panel A. Boxplots of hemispheric brain atrophy in male pups. Hemispheric brain atrophy was reduced in the mice treated with high dose gabapentin (150 or 200 mg/kg) compared with vehicle/low dose treated (0, 50 or 100 mg/kg, p<0.05). Panel B. No significant difference in hemispheric brain atrophy was seen in female mice. Panels C and D. Seizure score was significantly reduced in the male, but not female mice, treated with high dose gabapentin. Difference in atrophy or seizures between males and females receiving vehicle or low dose gabapentin was not significantly different.
Animal Sex and Hemispheric Brain Atrophy
Median percent hemisphere brain atrophy for male mice administered a 150 or 200 mg/kg dose of gabapentin (n = 11, median = 24%, range = 2 – 48%) was reduced compared to median percent hemispheric brain atrophy in male mice administered a 0, 50, or 100 mg/kg dose gabapentin (n = 15, median 53%, range 8 – 77%, p < 0.05, see Figure 4A). In females, the high dose treated mice had less injury but this reduction was not statistically significant (median hemispheric atrophy in low-dose treated animals = 39%, range = 0 – 73% versus 26%, range = 2 – 77% in high dose treated group, NS; Figure 4B). The median percent hemispheric brain atrophy between male and female control mice at low dose (0, 50, or 100 mg/kg) gabapentin was not significantly different.
Animal Sex and Correlation between Seizures and Atrophy
A correlation was found between median seizure score and median percent hemispheric brain atrophy in male and female mice administered low (0, 50, or 100 mg/kg) dose gabapentin (Spearman’s rho = 0.856, p < 0.001, n = 15 males; Spearman’s rho = 0.857, p < 0.001, n=15 females). At high doses of gabapentin (150 or 200 mg/kg), all correlation was lost in both males and females (n = 11 males, Spearman’s rho = 0.140, NS and n = 11 females, Spearman’s rho = 0.133, NS).
DISCUSSION
Because neonatal strokes present acutely with seizures, it is important to identify anticonvulsants that effectively reduces acute seizure and ameliorate ischemic brain damage. We found that gabapentin, given immediately after carotid ligation in P12 CD1 mice, effectively reduced acute seizures and brain atrophy in injured animals. The seizure suppression and neuroprotective effects were only seen at the higher doses. These higher doses of gabapentin did not result in any notable acute behavioral side effects. We further determined that the higher doses decreased the number of epochs with seizure scores over the range of seizure severity and eliminated the most severe seizures (i.e. seizure score of 6).
Similar to prior studies, the severity of acute seizures correlated with the severity of hemispheric brain atrophy in the vehicle-treated mice. The correlation persisted after administration of low doses gabapentin, but was lost after administration of high doses. Loss of correlation at higher doses of gabapentin is likely because the most injured animals drive the correlation that is lost when higher doses suppress the seizures and injury.
The ischemic pathway overlaps greatly with seizure processes and therefore anticonvulsants have been proposed as possible neuroprotective agents (17). Felbamate was protective in a rat pup model of neonatal hypoxia-ischemia when administered before or up to four hours after injury (18, 19). Phenytoin pretreatment attenuated hypoxic-ischemic brain injury in neonatal rats (20) and in utero hypoxic brain injury in fetal guinea pigs (21). Zonisamide pretreatment reduced hypoxic-ischemic brain injury in rat pups, but did not decrease acute electrographic seizures (22). A single dose of lamotrigine reduced hippocampal neuronal damage in the rat neonatal hypoxia-ischemia model (23). With the unilateral carotid ligation mouse model, it is now possible to determine the impact of anticonvulsants on both the acute seizures and the brain injury.
Gabapentin could reduce the activity of the Na+ or K+ channels, implicated in excitotoxicity (24, 25). However, the most important mechanism of gabapentin anticonvulsant effects is probably in blocking the influx of calcium into neurons via the α2δ-1 and α2δ-2 subunits of voltage-dependent calcium channels. The cortex and hippocampus have been shown to have high densities of the α2δ-2 subunits. Via this mechanism, gabapentin may reduce synaptic release of excitatory neurotransmitters and post-synaptic neuronal excitation. However the molecular processes involved in the effect of gabapentin upon these calcium channel subunits that results in its anticonvulsant effect are currently unknown (26). Reduced pre- and post-synaptic excitation would be expected to blunt both acute ischemic seizures and brain injury. The use of gabapentin during pregnancy to reduce brain damage in new-born infants at risk for perinatal asphyxia has been proposed (27).
Our data also suggests that female and male mouse pups in this model may respond differently to gabapentin. Compared with mice receiving vehicle or low dose gabapentin, male mice receiving high dose gabapentin after carotid ligation had significantly less severe seizures and hemispheric brain injury. No significant difference in seizures or injury was found in the female mice between those administered vehicle or low dose gabapentin and those administered high dose gabapentin. A caveat in this finding is that the acute seizures and chronic injury were lower in the females compared with males administered the vehicle/low dose of gabapentin, although this difference was not significant. These data suggest that a sex-related difference in response to gabapentin may exist in this model and gabapentin may be acting in a different fashion in females versus males. This could suggest differences in the impact of gabapentin upon the calcium channels in males versus females or a difference in the relative contribution of calcium channel function to the ischemic cascade in the immature male and female mice. Gender differences have been reported in the incidence of cerebral palsy, which can result from perinatal brain injuries, as well as in the cell signaling cascades that mediate cell death in the immature brain (28). In female neonatal rodents, ischemic injury is mediated predominantly by activation of caspases, while apoptosis inducing factor (AIF) plays a greater role in males (29). Du et al also reported that in vitro neurons from male rodents are more sensitive to toxicity from glutamate and nitric oxide than female neurons (30). Renolleau recently reported that the third generation caspase inhibitor Q-VD-OPh preferentially protects female 7 day old rat pups from stroke compared to males (31). However, the relative contribution of the voltage-dependent calcium channels to these pathways in male and female pups is unclear. Gender specific differences in the voltage gated coronary calcium channel expression and currents (increased in males) (32, 33), and in mesenteric artery responses to voltage gated coronary calcium channel agonists (also increased in males) (34) have been reported in adult tissue and linked to sex hormone levels. The relevance of these findings to possible sex-related differences in the immature animal is unknown. Alternatively, there could be a sex-dependent difference in the renal clearance of gabapentin, although this is not the case in adult rodents and humans (35, 36). This possible sex-dependant difference in the effect of gabapentin upon ischemic seizures and injury should be confirmed in other immature ischemia models.
In conclusion, in this immature model of ischemic seizures and brain injury, gabapentin suppresses seizures and reduces chronic injury at higher doses. These effects may differ according to animal sex. Gabapentin should be further tested in other relevant immature animal models. If further research supports these findings, clinicians could consider giving gabapentin when a neonate presents with a seizure due to a stroke or suspicion of brain asphyxia, particularly in boys. An intravenous preparation of gabapentin would be helpful in this regard. Gabapentin could also be considered as an anticonvulsant for infants with vascular malformations at risk for seizures and stroke. It will be important to determine which anticonvulsants provide the most neuroprotection, the impact of anticonvulsant combinations, and their use optimal with moderate hypothermia. Anticonvulsants could be very promising tools to minimize damage after ischemia in the neonatal or infant brain.
Acknowledgements
The authors thank Andrew Hooper for his technical assistance and Lisa Ferenc for editing this manuscript.
Financial Support: This study was supported by grants from the Howard Hughes Summer Undergraduate Research Fellowship program, National Institutes of Health National Institute of Neurological Disorders and Stroke K02 NS052166 and Hunter’s Dream for a Cure Foundation.
Abbreviations
- P12
postnatal day 12
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lynch JK, Nelson KB. Epidemiology of perinatal stroke. Curr Opin Pediatr. 2001;13:499–505. doi: 10.1097/00008480-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Giroud M, Lemesle M, Gouyon JB, Nivelon JL, Milan C, Dumas R. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: a study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995;48:1343–1348. doi: 10.1016/0895-4356(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 3.Schoenberg BS. Risk factors for stroke in infants and children. Adv Neurol. 1979;25:313–324. [PubMed] [Google Scholar]
- 4.Comi AM, Weisz CJ, Highet BH, Johnston MV, Wilson MA. A new model of stroke and ischemic seizures in the immature mouse. Pediatr Neurol. 2004;31:254–257. doi: 10.1016/j.pediatrneurol.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Comi AM, Johnston MV, Wilson MA. Strain variability, injury distribution and seizure onset in a mouse model of stroke in the immature brain. Dev Neurosci. 2005;27:127–133. doi: 10.1159/000085984. [DOI] [PubMed] [Google Scholar]
- 6.Comi AM, Highet BH, Mehta P, Hana CT, Johnston MV, Wilson MA. Dextromethorphan protects male but not female mice with brain ischemia. Neuroreport. 2006;17:1319–1322. doi: 10.1097/01.wnr.0000220136.98918.41. [DOI] [PubMed] [Google Scholar]
- 7.Koelfen W, Freund M, Varnholt V. Neonatal stroke involving the middle cerebral artery in term infants: clinical presentation, EEG and imaging studies, and outcome. Dev Med Child Neurol. 1995;37:204–212. doi: 10.1111/j.1469-8749.1995.tb11993.x. [DOI] [PubMed] [Google Scholar]
- 8.Delsing BJ, Catsman-Berrevoets CE, Appel IM. Early prognostic indicators of outcome in ischemic childhood stroke. Pediatr Neurol. 2001;24:283–289. doi: 10.1016/s0887-8994(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 9.Johnston MV, Hoon AH., Jr Possible mechanisms in infants for selective basal ganglia damage from asphyxia, kernicterus, or mitochondrial encephalopathies. J Child Neurol. 2000;15:588–591. doi: 10.1177/088307380001500904. [DOI] [PubMed] [Google Scholar]
- 10.Downen M, Belkowski S, Knowles H, Cardillo M, Prystowsky MB. Developmental expression of voltage-gated potassium channel beta subunits. Brain Res Dev Brain Res. 1999;117:71–80. doi: 10.1016/s0165-3806(99)00100-5. [DOI] [PubMed] [Google Scholar]
- 11.Jensen FE. The role of glutamate receptor maturation in perinatal seizures and brain injury. Int J Dev Neurosci. 2002;20:339–347. doi: 10.1016/s0736-5748(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 12.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 13.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 14.Comi AM. Neuroprotection for Ischemic Injury in the immature brain. Curr Pediatr Rev. 2005;1:135–145. [Google Scholar]
- 15.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 16.Morrison RS, Wenzel HJ, Kinoshita Y, Robbins CA, Donehower LA, Schwartzkroin PA. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J Neurosci. 1996;16:1337–1345. doi: 10.1523/JNEUROSCI.16-04-01337.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calabresi P, Cupini LM, Centonze D, Pisani F, Bernardi G. Antiepileptic drugs as a possible neuroprotective strategy in brain ischemia. Ann Neurol. 2003;53:693–702. doi: 10.1002/ana.10603. [DOI] [PubMed] [Google Scholar]
- 18.Wasterlain CG, Adams LM, Hattori H, Schwartz PH. Felbamate reduces hypoxic-ischemic brain damage in vivo. Eur J Pharmacol. 1992;212:275–278. doi: 10.1016/0014-2999(92)90343-3. [DOI] [PubMed] [Google Scholar]
- 19.Wasterlain CG, Adams LM, Schwartz PH, Hattori H, Sofia RD, Wichmann JK. Posthypoxic treatment with felbamate is neuroprotective in a rat model of hypoxia-ischemia. Neurology. 1993;43:2303–2310. doi: 10.1212/wnl.43.11.2303. [DOI] [PubMed] [Google Scholar]
- 20.Hayakawa T, Hamada Y, Maihara T, Hattori H, Mikawa H. Phenytoin reduces neonatal hypoxic-ischemic brain damage in rats. Life Sci. 1994;54:387–392. doi: 10.1016/0024-3205(94)00696-2. [DOI] [PubMed] [Google Scholar]
- 21.Lampley EC, Jr, Mishra OP, Graham E, Delivoria-Papadopoulos M. Neuroprotective effect of phenytoin against in utero hypoxic brain injury in fetal guinea pigs. Neurosci Lett. 1995;186:192–196. doi: 10.1016/0304-3940(95)11308-j. [DOI] [PubMed] [Google Scholar]
- 22.Hayakawa T, Higuchi Y, Nigami H, Hattori H. Zonisamide reduces hypoxic-ischemic brain damage in neonatal rats irrespective of its anticonvulsive effect. Eur J Pharmacol. 1994;257:131–136. doi: 10.1016/0014-2999(94)90704-8. [DOI] [PubMed] [Google Scholar]
- 23.Papazisis G, Kallaras K, Kaiki-Astara A, Pourzitaki C, Tzachanis D, Dagklis T, Kouvelas D. Neuroprotection by lamotrigine in a rat model of neonatal hypoxic-ischaemic encephalopathy. Int J Neuropsychopharmacol. 2007:1–9. doi: 10.1017/S1461145707008012. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Qin N, Reitz T, Wang Y, Flores CM. Inhibition of the rat brain sodium channel Nav1.2 after prolonged exposure to gabapentin. Epilepsy Res. 2006;70:263–268. doi: 10.1016/j.eplepsyres.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Bertrand S, Nouel D, Morin F, Nagy F, Lacaille JC. Gabapentin actions on Kir3 currents and N-type Ca2+ channels via GABAB receptors in hippocampal pyramidal cells. Synapse. 2003;50:95–109. doi: 10.1002/syn.10247. [DOI] [PubMed] [Google Scholar]
- 26.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Bucay AH. Use of gabapentin during pregnancy to reduce brain damage in new-born infants that are premature or at risk of perinatal asphyxia. Med Hypotheses. 2007;68:1422. doi: 10.1016/j.mehy.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007;49:74–78. doi: 10.1017/s0012162207000199.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
- 30.Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 31.Renolleau S, Fau S, Goyenvalle C, Joly LM, Chauvier D, Jacotot E, Mariani J, Charriaut-Marlangue C. Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: a role for gender. J Neurochem. 2007;100:1062–1071. doi: 10.1111/j.1471-4159.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- 32.Bowles DK. Gender influences coronary L-type Ca(2+) current and adaptation to exercise training in miniature swine. J Appl Physiol. 2001;91:2503–2510. doi: 10.1152/jappl.2001.91.6.2503. [DOI] [PubMed] [Google Scholar]
- 33.Bowles DK, Maddali KK, Ganjam VK, Rubin LJ, Tharp DL, Turk JR, Heaps CL. Endogenous testosterone increases L-type Ca2+ channel expression in porcine coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2091–H2098. doi: 10.1152/ajpheart.00258.2004. [DOI] [PubMed] [Google Scholar]
- 34.Hall J, Jones TH, Channer KS, Jones RD. Mechanisms of agonist-induced constriction in isolated human mesenteric arteries. Vascul Pharmacol. 2006;44:427–433. doi: 10.1016/j.vph.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Boyd RA, Turck D, Abel RB, Sedman AJ, Bockbrader HN. Effects of age and gender on single-dose pharmacokinetics of gabapentin. Epilepsia. 1999;40:474–479. doi: 10.1111/j.1528-1157.1999.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 36.Radulovic LL, Turck D, von Hodenberg A, Vollmer KO, McNally WP, DeHart PD, Hanson BJ, Bockbrader HN, Chang T. Disposition of gabapentin (neurontin) in mice, rats, dogs, and monkeys. Drug Metab Dispos. 1995;23:441–448. [PubMed] [Google Scholar]


