Abstract
Pd and Ru catalyzed cycloisomerizations of 1,6-enynes are compared and contrasted. Such considerations led to the enantioselective synthesis of a cyathin terpenoid, (+)-allocyathin B2 (1). The synthesis features a Pd-catalyzed asymmetric allylic alkylation (AAA) to install the initial quaternary center, a Ru-catalyzed diastereoselective cycloisomerization to construct the six-membered ring, and a diastereoselective hydroxylative Knoevenagel reaction to introduce the final hydroxyl group. We demonstrate for the first time a mechanism-based stereochemical divergence in Pd- and Ru-catalyzed cycloisomerization reactions as well as in creation of alkene geometry with alkynes bearing a carboalkoxy group. Mechanistic rationalization is proposed for these observations.
Introduction
Cycloisomerizations represent atom economic approaches to ring construction. As a result, we have pioneered metal catalyzed cycloisomerizations of enynes to form cyclic 1,3- and 1,4-dienes.1–3 In the Pd catalyzed reaction (Fig. 1), a mechanistic rationale invokes HPd¬+ formed by protonation of Pd(0) as the catalytically active species.4 Preferential hydropalladation of the alkyne, and intramolecular carbapalladation of the alkene by the in situ generated vinylpalladium species followed by β-hydrogen elimination completes the cycle. β-Hydrogen elimination towards Ha creates a 1,3-diene; whereas, β-hydrogen elimination of Hb provides a 1,4-diene. Experimentally, both pathways have been observed and normally proceed with excellent regioselectivity depending upon the substrate. On the other hand, a Ru catalyzed process proceeds via a completely different pathway as shown in Fig. 2. This mechanism precludes the formation of 1,3-dienes since β-hydrogen elimination within the metallacycle is geometrically strongly disfavored. Further, the regioslectivity in the β-hydrogen elimination to form 1,4-dienes where such exists (i.e. with trisubstituted alkenes) also has been complementary. Despite these facts, divergences of the two quite distinct mechanisms remain to be elucidated.
Figure 1.
Pd-Catalyzed Cycloisomerization Mechanism.
Figure 2.
Ru-Catalyzed Cycloisomerizations Mechanism.
In the course of a total synthesis project, the issue of the differences between these two catalytic systems arose. In 1979, Ayer et al. reported the isolation and structure of (+)-allocyathin B2 (1, Scheme 1),5 a member of the cyathin terpenoids.5–13 Compound 1, a metabolite from the fruit bodies of Cyathus earlei Lloyd, is distinguished by an angularly fused 5-6-7 tricyclic skeleton with a highly unsaturated trienal motif embedded in the framework and 1,4-anti quaternary methyl groups located at the ring junctions. These terpenoids exhibit interesting biological activities against actinomycetes, Gram-postitive and Gram-negative bacteria as well as some fungi.6 Metabolites of other fungi14–17 and liverworts18 also share the cyathane backbone including a hydroxylated analogue, sarcodonin A (2, Scheme 1) from Sarcodon scabrosus which has anti-inflammatory activity,19 and a D-xylose conjugate of allocyathin B2, erinacine A (3, Scheme 1) from the mycelia of Hericum erinaceum which possesses potent stimulating activity for nerve growth factor (NGF) synthesis in vitro.15 The imposing structure and potential medicinal importance of these molecules have attracted a great deal of attention from many research teams since the disclosure of their structures,7,20–26 although only three groups have completed the racemic syntheses of allocyathin B2 27–29 and sarcodonin A30. In this paper, we report our studies contrasting these two metal catalyzed cycloisomerizations stimulated by this total synthesis. A preliminary report describing only the total synthesis has recently appeared.31
Scheme 1.
Retrosynthetic Analysis
Herein we report an enantioselective total synthesis of (+)-allocyathin B2. The goal of this synthesis was to develop a catalytic approach to enantioselectively install the first quaternary carbon and relay the stereochemical information to the remaining two stereogenic centers. Strategically, we planed to construct the cycloheptadiene unit of allocyathin B2 by a late stage intramolecular aldol condensation from aldehyde 4 (Scheme 1). A transition metal-catalyzed cycloisomerization was envisioned to build the six-membered ring of the 5-6-7 tricyclic cyathin core (compound 5).32 At the outset, it was recognized that this key disassembly allows several synthetic parameters, such as catalyst, substrate geometry, and appendage at the alkyne terminal, to be adjusted to achieve the requisite stereochemistry as well as to prepare for subsequent transformations. This disconnection also provides a rare opportunity to explore the Pd- and Ru-catalyzed cycloisomerizations related to the methods developed in this laboratory in a complex setting. The crucial intermediate, enyne 6 would be prepared through a Pd-catalyzed asymmetric allylic alkylation (AAA) reaction from ketone 7, another methodology recently developed in this group.33–35
Results and Discussion
Pd-catalyzed cycloisomerization of 1,6-enyne 14
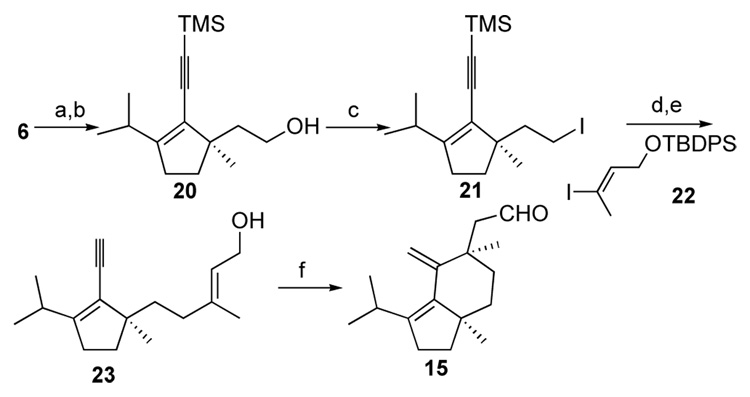
Since we had an efficient and reliable approach to enantioselectively install the C17 methyl group using a Pd-catalyzed enolate AAA reaction ( 8, Scheme 2),36 it was desirable to design a cyclization substrate from this compound in such a way that both sides of the resulting six-membered ring would be properly functionalized to allow the diastereoselective introduction of the C14 hydroxyl group as well as set the stage for the closure of the final seven-membered ring. With these considerations in mind, compound 14 was conceived as one of the viable substrates for the pivotal transition metal-catalyzed cycloisomerization. It should be noted that the Z geometry of the olefin in compound 14 was chosen at this stage because of its ease of assembly although we do anticipate that the olefin geometry will be a key factor to determine the stereochemical outcome of this reaction.
Scheme 2.
Synthesis of Substrate 14a
a (a) LDA, [(η3-C3H5)PdCl]2 (0.5 mol%), (S,S)-L* (1 mol%), allyl acetate, Me3SnCl, t-BuOH, 83%, 95% ee; (b) Me2CuLi, −20 °C to rt, 85%; (c) LDA, PhNTf2, 98%; (d) Pd2(dba) 3CHCl3 (2.5 mol%), PPh3 (20 mol%), CuI (5 mol%), TMS-acetylene, n-BuNH2, 50 °C, 85%; (e) 9-BBN; H2O2, NaOH, 87%; (f) (COCl)2, DMSO, Et3N, − 78 °C to rt, 84%; (g) CBr4, PPh3, 94%; (h) n-BuLi, ClCO2Me, −78 °C to rt, 99%; (i)Me2CuLi, −78 °C, 93%; (j) DIBAL-H, −78 °C to rt, 94%; (k) TBAF, 97%.
Alkyne 11 was synthesized in satisfactory yield through the addition of lithium dimethylcuprate to 8 followed by treatment of the resulting ketone 9 with LDA and PhNTf2 36 followed by a Sonogashira coupling reaction (Scheme 2).37 The elaboration of the Z-alkene 14 involved a straightforward chain elongation protocol38–40involving a stereospecific conjugate addition of lithium dimethylcuprate to ynoate 12.41
Many transition metals have been reported to catalyze the cycloisomerization of 1,6 and 1,7-enynes, notably Pd, Ru, Rh, and Ti.1,32 However, 1,7-enynes with tri-substituted olefins are challenges for most of the known methodologies, possibly due to the poor substrate coordination to the catalyst. Not surprisingly, [Rh(COD)Cl]2/BINAP/AgBF4 42,43 and Cp2Ti(CO)2 44 are ineffective catalysts in the cycloisomerization of 14. Prior efforts in our laboratory indicate that palladium systems are more tolerant of olefin substitution than ruthenium in these reactions.45 Therefore we decided to initially examine palladium catalysts in our cycloisomerization reaction.
Although Pd2(dba)3 CHCl3/HOAc is known to be effective in catalyzing the formation of six-membered rings, only trace amounts of the cyclization product were obtained along with a significant amount of the starting material when 14 was exposed to the standard conditions (entry 1, Table 1). Despite the low conversion, a single diastereomer was obtained as demonstrated by NMR analysis. The obvious difficulty in the coordination of this 1,7-enyne to the catalyst prompted us to use the “ligandless” conditions developed in this group.46 Indeed, the yield significantly improved without the addition of BBEDA [bis(benzylidene)-ethylenediamine]. Since the use of HOAc as the co-catalyst led to the formation of an unidentified isomerization product, other acids were surveyed to avoid this problem.
Table 1.
Pd-Catalyzed Cycloisomerization of Compound 14a
 | |||||
|---|---|---|---|---|---|
| Entry | Ligand | Acid (mol%) | T (°C) | Time (h) | Yield of 15 (%) |
| 1 | BBEDAb | HOAc (25 mol%) | 60 | 24 | 4 (82% 14) |
| 2 | None | HOAc (25 mol%) | 60 | 20 | 18 (13% 14) |
| 3 | None | TFA (20 mol%) | 60 | 2 | 19 (8% 14) |
| 4 | None | HCO2H (200 mol%) | 70 | 0.8 | 23 (62% 16) |
| 5 | None | HCO2H (200 mol%) | 25 | 20 | 22 (64% 16) |
| 6 | None | o-CF3BzOH (20 mol%) | 70 | 12 | 60 |
All reactions were performed in toluene (0.1 M) with 2 mol% Pd2(dba)3CHCl3 at the indicated temperature.
No conversion was observed with other ligands such as (o-Tol)3P.
Among the acids assayed, o-trifluoromethylbenzoic acid was identified as the best choice (Table 1). Although formic acid was superior in terms of reactivity, the relatively sluggish cycloisomerization reaction allowed the formation of a significant amount of the reduced triene 16. Using the optimized conditions, compound 15 was obtained in 60% yield as a single diastereomer (entry 6, Table 1). The relative stereochemistry of the newly formed quaternary center was determined at a later stage (vide infra).
We then turned our attention to the construction of the final seven-membered ring. An interesting methodology developed by Nokami, the hydroxylative Knoevenagal reaction, seemed well suited to our goal of installing the C14 hydroxy group with concomitant extension of the carbon chain.47 Gratifyingly, after some experimentation, the desired transformation was accomplished to afford alcohol 17 as a single diastereomer (Scheme 3). Compound 17, which contains all three requisite stereogenic centers, was submitted for X-ray crystallographic structural determination. Unfortunately, the two quaternary methyls are 1,4-syn while the hydroxyl group possesses the correct configuration (Figure 1). Despite this setback we decided to pursue our synthesis using compound 17 as a model study and a possible intermediate to an epimer of (+)-allocyathin B2.
Scheme 3.
Synthesis of Epoxide 19a
a (a) PhS(O)CH2CN, piperidine, 69%; (b) 10% Pd/C, H2 (1 atm), 98%; (c) mCPBA, 76%; or NBS, water, 46%; or VO(acac)2, TBHP, 80%.
Surprisingly, the 1,4-reduction of the α,β-unsaturated nitrile (C12–C13) of compound 17 did not occur under a variety of conditions known for this purpose.48 On the other hand, by taking advantage of the unique conformation of nitrile 17, a chemoselective hydrogenation was achieved with heterogeneous catalysis. Thus, by subjecting compound 17 to 10% Pd/C and 1 atm of hydrogen, a near quantitative yield of the desired dihydro derivative 18 was obtained (Scheme 3).
Having established the requisite oxygen-bearing stereocenter, we found ourselves faced with the task of homologating the exocyclic olefin to an α,β-unsaturated aldehyde or equivalent. An osmium-catalyzed dihydroxylation of 18 failed to proceed with a number of co-oxidants (NMO, trimethylamine oxide, or K3Fe(CN)6) and additives (methane sulfonamide or pyridine).49 On the other hand, exposure of 18 to sterically less sensitive epoxidizing agents like mCPBA or NBS/water generated epoxide 19 due to a electron-rich nature of the tetrasubstituted double bond (Scheme 3).50 As predicted by analysis of a molecular model, a hydroxyl-directed epoxidation (VO(acac)2, TBHP)51 afforded the same product. These results, although discouraging, demonstrated the key role that the C6 stereochemistry plays in affecting the chemoselectivity. We therefore tried several approaches to reverse the diastereoselectivity of the cycloisomerization.
Pd-catalyzed cycloisomerization of compounds 23 and 28
As previously mentioned we anticipate the olefin geometry of the enyne to be a key factor in determining the stereochemical outcome of the cycloisomerization reaction. Thus, E-isomer 23 was synthesized from compound 6 as detailed in Scheme 4. The allyl side chain of compound 6 was oxidatively cleaved (OsO4, NMO; NaIO4), reduced (NaBH4), and iodinated (PPh3, I2, imidazole) to furnish compound 21. A Pd-catalyzed Negishi sp3-sp2 coupling reaction of the zinc derivative of alkyl iodide 21 and vinyl iodide 2252 followed by TBAF-mediated desilylation provided the desired E-allylic alcohol 23.53–55 When compound 23 was subjected to the established cycloisomerization conditions, surprisingly, the same aldehyde 15 was obtained in 54% yield.
Scheme 4.
Pd-Catalyzed Cycloisomerizaton of Compound 23a
a (a) OsO4 (1 mol%), NMO; NaIO4, 84%; (b) NaBH4, 94%; (c) PPh3, I2, ImH, 93%; (d) t-BuLi, ZnCl2, −78 °C to rt, then Pd(PPh3)4 (5 mol%), 22; (e) TBAF, 72% from 21; (f) Pd2(dba)3CHCl3, o-CF3BzOH, 70 °C, 54%.
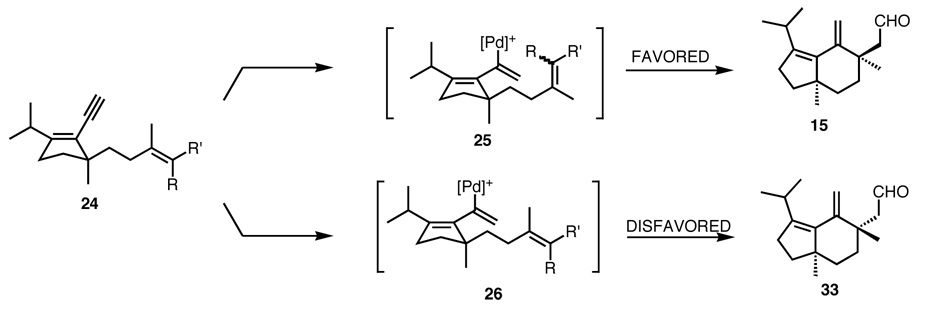
The outcome observed for both 14 and 23 in the Pd-catalyzed cycloisomerization can be rationalized by the proposed mechanism (Figure 4).56 For the substrate 24, after the initial hydropalladation, two intermediates 25 and 26 which places the bulky Pd catalyst approximately perpendicular to the five membered ring to minimize steric interactions with the isopropyl group are generated. In intermediate 25, the C-Pd bond and the π-bond of the acyclic alkene are syn-coplanar as required for the carbametalation. Thus, this orientation is energetically favorable and leads to the observed product. On the other hand, the alternative conformation 26 has the C-Pd and π-bond orthogonal. Such an unfavorable geometry for the carbametalation disfavors this cyclization and precludes formation of the desired stereochemistry. As this picture illustrates, the geometry of the trisubstituted double bond with respect to the product stereochemistry is irrelevant.
Figure 4.
Proposed Rationale for the Palladium-Catalyzed Cycloisomerization of Compound 24.
To further test this mechanistic proposal and provide an alternative strategy for our synthesis, we decided to prepare the de-methylated substrate 28 and subject this compound to the Pd-catalyzed cycloisomerization reaction (Scheme 5). Toward this end, Moffatt-Swern oxidation of compound 11 followed by a Wittig reaction with PPh3CHCO2Et furnished E-olefin 27 as the only product.39 Reduction of the ester and desilylation produced alcohol 28. The Pd-catalyzed cycloisomerization of enyne 28 proceeded smoothly under the previously established conditions to afford aldehyde 29 in 54% yield as a single diastereomer. As expected, the same stereoselectivity was achieved as in the case of E-olefin 23.57 This result provides further evidence to support our rationale that the interaction between the fused five-membered ring and the vinyl substituent dominates the stereo-determinating step. It is also noteworthy that, in this transformation, no 1,3-diene 30 was detected (Scheme 5). Previous studies show that Pd-catalyzed enyne cycloisomerizations of disubstituted olefins often generate mixtures of 1,4- and 1,3-dienes.58 Further, the presence of an allylic hydroxyl substituent tends to favor the 1,3-diene (i.e. 30).59 Presumably, the overwhelming bias toward the formation of a non-conjugated 1,4-diene in this reaction derives from the conformational restriction of the bicyclic system and the strain associated with placing a double bond exocyclic to a six membered ring.58–60 We observed a similar effect in our application of this cycloisomerization in our synthesis of saponaceolide.60
Scheme 5.
Pd-Catalyzed Cycloisomerizaton of Compound 28a
a (a) (COCl)2, DMSO, NEt3, 88%; (b) Ph3PCHCO2Et, 98%; (c) DIBAL-H, −78 °C to rt, 96%; (d) TBAF, 92%; (e) Pd2(dba)3CHCl3, o-CF3BzOH, 70 °C, 54%.
Ru-catalyzed cycloisomerization
Because the Pd-catalyzed cycloisomerization only generated the cyclized product with a 1,4-syn relationship, we considered employing CpRu(CH3CN)3PF6 as the catalyst.61,62 This choice was based on the notion that Pd- and Ru-catalyzed cycloisomerizations undergo different reaction mechanisms.32 Unfortunately, precedent indicated that this cycloisomerization is sensitive to olefin substitution and that 1,7-enynes with trisubstituted olefins are not particularly good substrates. Consequently, we first carried out a model study with compound 31 to establish the diastereoselectivity of this process and as proof of concept. Previous efforts in our group suggested the use of an allylic silyl ether as the substrate.36 Indeed, no reaction occurred with compound 28 under standard conditions with CpRu(CH3CN)3PF6 (10 mol%) while the reaction of silyl ether 31 cleanly generated an enol ether63 which was hydrolyzed (TFA, H2O) to deliver aldehyde 32 as a single diastereomer (Scheme 6). To our delight, comparison of the spectral data of compound 29 with those of 32 clearly indicated that the opposite diastereoselectivity was achieved with Ru catalysis. This result provided support to our hypothesis that different reaction mechanisms might induce complementary diastereoselectivity in transition metal-catalyzed cycloisomerization reactions.
Scheme 6.
Ru-Catalyzed Cycloisomerizaton of Compound 31a
a (a) TBSCl, ImH, 99%; (b) CpRu(CH3CN)3PF6 (10 mol%), 53%; (c) TFA, H2O, 0 °C to rt, 83%.
Encouraged by this result, compounds 14 and 23 as well as their silyl ethers were subjected to the Ru-catalyzed reaction. Unlike the disubstituted model 31, the silyl protected derivatives of 14 and 23 didn’t give any desired product.64 With some optimization, we found that exposure of compound 23 to CpRu(CH3CN)3PF6 (20 mol%) in 2-butanone generated a cyclization product in 15% yield. Gratifyingly, comparison of NMR data of this product with those of compound 15 indicates that only the desired diastereomer 33 was obtained (Scheme 7). This result agreed with our observations in the reactions of disubstituted substrates. The excellent diastereoselectivity was certainly exciting. However, improvement on the conversion was desirable to render the reaction synthetically practical.65 A yield of 29% was achieved by increasing the catalyst loading to 35 mol%. Further increase of the catalyst was detrimental due to significant decomposition of the starting material. On the other hand, the best yield (41%) was obtained upon addition of 100 mol% DMF with 20 mol% catalyst (vide infra).
Scheme 7.
Synthesis of Epoxide 40.a
a (a) CpRu(CH3CN)3PF6 (35 mol%), 29%; (b) PhS(O)CH2CN, piperidine, 44%, dr > 20:1; (c)10% Pd/C, H2 (1 atm), 87%; (d) VO(acac)2, TBHP, 52%.
Although there was no difference in reactivity or diastereoselectivity in the Pd-catalyzed cycloisomerization of Z and E isomers 14 and 23, this was the not case in the Ru-catalyzed reaction. In fact only a small amount of cyclization product (5%) was obtained when compound 14 was subjected to the optimized cycloisomerization conditions (20 mol% catalyst).66 More surprisingly, examination of the NMR spectra revealed that a 1:1 mixture of both diastereomers, compounds 33 and 15, was obtained.
To explain our observations in the Ru-catalyzed cycloisomerization of compounds 14 and 23, especially the difference in diastereoselectivity, the following mechanistic rationale was proposed (Figure 5). Presumably, after the initial chelation of the coordinatively unsaturated Ru catalyst to the enyne, the ratelimiting formation of a ruthenacyclopentene intermediate occurs.3,61 Between the two conformers 34 and 35, the syn-coplanar orientation of the alkene and alkyne in the former compared to an orthogonal orientation in the latter should favor formation of ruthenacycle 36 over 37. In the case of Z-substrate 14, these intermediates (34, 35: R = H, R’ = CH2OH) lead to bad steric interactions in both 36 and 37 leading to both being disfavored. Thus, the reaction is poorer and both products are observed. With the E substrate 23, the upper pathway via 36 is now favored since it is devoid of most bad intereactions.
Figure 5.
Mechanistic Rationale for the Ru-Catalyzed Cycloisomerization of Compounds 14 and 23.
With these results we demonstrate for the first time a mechanism-based stereochemical divergence in Pdand Ru-catalyzed cycloisomerizations. By varying substrate geometry and more importantly, catalyst choice, we can enable access to all four diastereomers with excellent stereocontrol. The complementarity of Pd and Ru catalysts revealed by our study further broadens the scope of transition metal-catalyzed cycloisomerization reactions and provides valuable insight into the mechanistic aspects of these interesting transformations.
With aldehyde 33 available, it was subjected to the hydroxylative Knoevenagel reaction to furnish nitrile 38 with excellent diastereoselectivity (dr > 20:1, Scheme 7).67 Based on previous results, the stereochemistry of the hydroxyl group was assigned as R and would have to be inverted at a later stage. After the selective hydrogenation of the C13–C14 double bond to produce diene 39, we were again in a position to address the homologation of the exocylic olefin. As observed with compound 18 (vide supra), no reaction occurred under osmium-catalyzed dihydroxylation conditions. We anticipated that, by changing the stereochemistry at C6, the chemoselectivity of the hydroxyl-directed epoxidation would be different since the tetrasubstituted olefin is no longer accessible from the bottom face. Indeed, treatment of compound 39 with VO(acac)2/TBHP furnished the desired epoxide 40 as the only product. However, our attempts to open the epoxide with a cyanide source (KCN; TMSCN)68 were thwarted by the poor stability of the substrate. In appraisal of the difficulty of this route, we decided to pursue a more feasible alternative.
Before discussing the revised approach, it is necessary to examine the unusual diastereoselectivity in the formation of compound 38. The accepted mechanism of the transformation from 42 to 43 involved the initial Knoevenagel reaction followed by double bond migration, sulfoxide-sulfenate 2,3-sigmatropic rearrangement and desulfurization.69 We believe that, in this case, the double bond migration generates an olefin that is oriented perpendicular to the plane of the bicyclic system to avoid the steric interaction with the exocyclic olefin (Scheme 8). By analogy to previous studies in this group, a quickly equilibrating mixture of roughly equal amounts of two diastereomers 42 and 43 is expected.70 Since the nitrile group has to be situated to lead to an E double bond, each diastereomer can only rearrange as depicted in Scheme 8.71 Intermediate 42 rearranges much faster to 44 from the front than from the back due to the steric interaction with the exocyclic olefin. Since only the final S–O bond cleavage by piperidine is irreversible it doesn’t matter which intermediate, 44 or 45, is favored in the equilibrium. Only the relative rate of the sigmatropic rearrangement controls the diastereoselectivity, which represents a Curtin-Hammett situation (Scheme 8). It is worth noting that in similar instances, only 50–75% de was obtained through the β–chiral carbon induction.70
Scheme 8.
Mechanistic Rationale for the Hydroxylative Knoevenagel Reaction to Form 38.
Revised Strategy
To avoid the difficulty in the homologation of the exocyclic double bond, we decided to introduce the requisite carbon as an alkynyl substituent before the cyclization. However, this strategy has several potential drawbacks. First, after the initial ruthenacyclopentene formation, significant steric interaction between the extra carbon at the terminus of the alkyne and the isopropyl group might slow down the already sluggish reaction. Furthermore, the mechanism of both the Pd and Ru catalyzed cycloisomerizations indicate the E geometry of the exocyclic olefin should form exclusively which will require a subsequent isomerization in some way to allow the intramolecular aldol reaction to form a seven-membered ring. On the other hand, the introduction of an alkynyl substituent might benefit the cycloisomerization. The presence of a terminal alkyne in the substrate slows the Ru catalyzed cyclization since the Ru catalyst gets tied up in formation of a vinylidene complex. While this is reversible, the rates of these steps are not so fast compared to the cycloisomerization. Preventing such a pathway should therefore speed up the cycloisomerization as well as shut down decomposition pathways via the reactive vinylidene intermediates. While the cycloisomerization might suffer from the strain between the substituent at the alkyne and the isopropyl group, the same strain might promote the isomerization of the resulting exocyclic olefin to give the desired Z-product.54 The fact that the initial C-bound Ru enolate can slip to form an Obound enolate may provide a kinetic pathway for equilibration (see Scheme 9).3, 61
Scheme 9.
Preparation of Substrates 47 and 48a–c and their Cycloisomerization Reactionsa
a (a) t-BuLi, ZnCl2, −78 °C to rt, then Pd(PPh3)4 (5 mol%), 22; (b) K2CO3, MeOH, 68% from 21; (c) n-BuLi, ClCO2NEt2 (47); ClCO2CH3 (48a); ClCO2i-Pr (48b); ClCO2t-Bu (48c), 99%; (d) TBAF, 52–55%; (e) CpRu(CH3CN)3PF6 (20 mol%).
Four derivatives of compound 23 were prepared in a straightforward fashion by mono-desilylation of the Negishi coupling product followed by acylation and deprotection to remove the TBDPS group (47–48a–c, Schemes 9). When amide 47 was subjected to the standard Ru-catalyzed cycloisomerization conditions no reaction occurred presumably due to the conformation rigidity associated with amides and the coordination of the amide moiety to the ruthenium catalyst.72 Reaction of ester 48a produced three compounds along with some recovered starting material. We identified two separable cyclization products 49a and 50a in a ratio of 1.8 to 1 as well as a hydroxyl group transposition product 51a (Scheme 9). Satisfactorily, both 49a and 50a constitute only the desired diastereomer. It is worth noting that this is the first reported double bond isomerization in enyne cycloisomerization reactions to form six-membered rings.73
While we were certainly delighted to observe the excellent diastereoselectivity and the isomerization of the exocyclic double bond, the poor yield and the low geometric selectivity represent two major hurdles to overcome. The issue of conversion was addressed first using compound 48a as a model. Previous experiences in this laboratory with CpRu(CH3CN)3PF6 indicate that the reaction can be sensitive to the water content of the solvent. However, no significant change was observed when freshly distilled 2-butanone was used. Interestingly, the conversion deteriorated with the addition of 4 Å molecular sieves and the ratio of compound 48a to 49a was completely reversed (entry 2, Table 2). On the other hand, adding water (100 mol%) to the reaction actually doubled the yield although the selectivity of the two geometric isomers dropped slightly (entry 3, Table 2). We ascribe the beneficial effect of water to its function as a weak ligand. Following this lead, we assayed a variety of ligands74 and improved the yield to 62% with DMF as an additive (100 mol%) although the product ratio is still poor (entry 5, Table 2).75 It is noteworthy that the conversion-promoting effect of DMF was also observed in the reaction of compound 23 as the yield increased from 29 % with 35 mol% catalyst to 41 % with 20 mol% catalyst with 100 mol% of DMF as the additive.
Table 2.
Ru-Catalyzed Cycloisomerization of Compouds 48a–ca
| Entry | Substrate | Additive | Yield (49+50)%b | Ratio (49/50)c |
|---|---|---|---|---|
| 1 | 48a | None | 23 (30% 51a) | 1.8 : 1 |
| 2 | 48a | 4Å mol. sieves | 14 | 1 : 8.2 |
| 3 | 48a | Water (100 mol%) | 54 | 1.2 : 1 |
| 4 | 48a | DMF (50 mol%) | 29 | 1.2 : 1 |
| 5 | 48a | DMF (100 mol%) | 62 | 1.2 : 1 |
| 6 | 48b | DMF (100 mol%) | 60 | 1.5 : 1 |
| 7 | 48c | DMF (100 mol%) | 55 | 6.7 : 1 |
All reactions were performed with 20 mol% CpRu(CH3CN)3PF6 in 2-butanone (0.1 M) at rt for 2 h.
Yields of 51a–c were not determined except entry 1.
Ratio was determined upon isolation. Both geometric isomers were obtained as single diastereomers.
As E-isomer 50 is not a viable candidate for the intramolecular aldol cyclization to form a seven-membered ring we attempted to further increase the ratio of compound 49.76 Using the proposed rationale as a guide, we reasoned that by increasing the size of the ester we might be able to accentuate the strain and thus promote the double bond isomerization. Indeed, we observed a drastic improvement in the ratio of compound 49 by changing methyl ester (48a) to tert-butyl ester (48c) as the most relief was achieved in the later case (Table 2). In the event, a 55% combined yield of the 6.7:1 ratio of products 49c and 50c was obtained and the pure diasatereomer 49c was isolated in 48% yield.
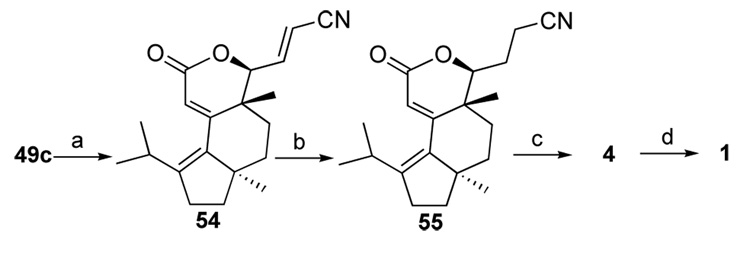
In the next hydroxylative Knoevenagel reaction, we obtained lactone 54 in good yield as one diastereomer instead of the expected alcohol (Scheme 10).67 Although tert-butyl esters have been used to block the possible lactonization, the rigid conformation of our substrates greatly accelerated the cyclization process. The C12–C13 double bond was then selectively hydrogenated to generate compound 55 in satisfactory yield.
Scheme 10.
Synthesis of (+)-allocyathin B2a
a (a) PhS(O)CH2CN, piperidine, 75%; (b) 10% Pd/C, H2, 83%; (c) DIBAL-H; (d) KOH, MeOH, 60 °C, 51% from 55.
The absolute configuration of the oxygen-bearing stereocenter was later shown to be S by comparison to the final product. This stereochemical outcome is the opposite of what was expected based on the results of compound 38. The rationale for this unusual observation is outlined in Scheme 11. We propose that the E-vinyl ester forces the migrated olefin to be parallel to the plane of the bicycle instead of perpendicular as in the previous case shown in Scheme 8. The preferred route of sigmatropic rearrangement would be from the bottom (intermediate 56) instead of from the top (intermediate 57) where a quaternary methyl group interferes (Scheme 11). The altered rearrangement trajectory leads to a reversal from the stereoselectivity predicted by previous experiments.
Scheme 11.
Mechanistic Rationale for the Hydroxylative Knoevenagel Reaction to Form 54.
With the stage set for the endgame, we attended to the closure of the final seven-membered ring through an intramolecular aldol condensation of lactol-aldehyde 4 as summarized in Scheme 10. 77,78 The spectroscopic properties of the synthetic sample were in full agreement with those reported in literature.27–29 Since the preparation of erinacine A (3) through the glycosidation of 1 has been described by Snider et al.,28,29 this asymmetric route also constitutes a formal synthesis of this natural product. An alternative approach for the asymmetric synthesis of allocyathin B2 also appeared recently.79
Summary
We demonstrate for the first time a mechanism-based stereochemical divergence in Pd- and Ru-catalyzed cylcoisomerizations. Building upon these enyne cycloisomerizations, we have achieved an efficient enantioselective synthesis of a cyathin diterpene, (+)-allocyathin B2 (1). Additional, highlights of this route include a Pd-catalyzed enolate AAA reaction to build the first quaternary carbon and a unique hydroxylative Knoevenagel reaction to stereoselectively introduce the oxygen-bearing center. The unusual olefin isomerization in the Ru-catalyzed cycloisomerization was also investigated and exploited The synthesis consists of a longest linear sequence of 17 steps and a total of 19 steps from 2-methylcyclopentanone.
Experimental Procedures
[(5S,7aS)-3-Isopropyl-5,7a-dimethyl-4-methylene-2,4,5,6,7,7a-hexahydro-1H-inden-5-yl]-acetaldehyde (15)
To a solution of compound 14 (55.0 mg, 0.224 mmol) in toluene (2.2 mL) was added Pd2(dba)3·CHCl3 (3.5 mg, 0.00335 mmol) and o-trifluoromethylbenzoic acid (6.3 mg, 0.0335 mmol) at room temperature. The mixture was stirred at 70 °C for 15 h and cooled to room temperature. The mixture was directly purified by column chromatography (5% ether in petroleum ether) to afford compound 15 as a clear oil (33.1 mg, 60%). [α]D = +206.3° (c 3.1, 24.4 °C, CH2Cl2); IR (neat) 2959, 1723, 1459, 1370, 900 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.60 (dd, J = 4.4, 4.0 Hz, 1 H), 5.02 (d, J = 1.6 Hz, 1 H), 4.79 (d, J = 1.6 Hz, 1 H), 2.81 (sept, J = 7.2 Hz, 1 H), 2.56 (dd, J = 14.8, 1.2 Hz, 1 H), 2.04 (dd, J = 14.4, 4.8 Hz, 1 H), 1.79 (m, 1 H), 1.64-1.57 (m, 6 H), 1.49 (m, 1 H), 1.22 (s, 3 H), 1.00 (dd, J = 6.8, 1.6 Hz, 3 H), 0.93 (m, 6 H); 13C NMR (CDCl3, 125 MHz) δ 204.2, 148.4, 142.3, 139.1, 110.2, 50.0, 48.7, 39.6, 39.5, 37.8, 36.8, 28.2, 26.4, 25.1, 23.1, 21.6, 21.4; HRMS Calc’d for C17H26O [M+]: 246.1984. Found: 246.1990.
(E)-(S)-4-Hydroxy-4-[(5S,7aR)-3-isopropyl-5,7a-dimethyl-4-methylene-2,4,5,6,7,7ahexahydro-1H-inden-5-yl]-but-2-ene nitrile (17)
To a solution of compound 15 (30.0 mg, 0.122 mmol) in benzene (1 mL) was added phenylsulphinyl acetonitrile (24.1 mg, 0.146 mmol) followed by piperidine (12.5 mg, 0.146 mmol) at room temperature. The mixture was stirred at room temperature for 15 h and was directly purified by column chromatography (30% ether in petroleum ether) to afford compound 17 as white crystals (23.9 mg, 69%), mp. 181–182 °C. [α]D = +102.3° (c 0.72, 23.5 °C, CH2Cl2); IR (neat) 3455, 2232, 1630, 1458 cm−1; 1H NMR (CDCl3, 500 MHz) δ 6.70 (dd, J = 16.0, 3.0 Hz, 1 H), 5.67 (dd, J = 16.0, 2.5 Hz, 1 H), 5.02 (d, J = 1.5 Hz, 1 H), 4.85 (d, J = 1.5 Hz, 1 H), 4.46 (m, 1 H), 2.82 (sept, J = 7.0 Hz, 1H), 2.33 (m, 2 H), 1.85 (m, 1 H), 1.78 (m, 1 H), 1.65 (m, 1 H), 1.56-1.53 (m, 3 H), 1.01 (d, J = 7.0 Hz, 3 H), 0.93 (m, 9 H); 13C NMR (CDCl3, 125 MHz) δ 155.5, 147.0, 142.5, 139.5, 118.0, 112.4, 99.4, 71.7, 49.0, 45.5, 39.9, 36.7, 33.6, 28.4, 26.7, 23.3, 21.8, 21.6, 18.7; HRMS Calc’d for C19H27NO [M+]: 285.2093. Found: 285.2083.
(E)-(R)-5-(2-Ethynyl-3-isopropyl-1-methyl-cyclopent-2-enyl)-3-methyl-pent-2-en-1-ol (23)
To a solution of the compound 21 (110 mg, 0.293 mmol) and anhydrous ZnCl2 (39.8 mg, 0.293 mmol) in THF (3 mL) was added t-BuLi (1.4 M in pentane, 0.627 mL, 0.818 mmol) at −78 °C in 10 min. The mixture was stirred for 5 min at this temperature and warmed to room temperature for 1 h. The resulting yellow solution was transferred through cannular to a mixture of vinyl iodide 22 (100.4 mg, 0.322 mmol) and Pd(PPh3)4 (16.9 mg, 0.146 mmol) at room temperature. The reaction mixture was stirred overnight before the addition of ether (30 mL) and water (15 mL). After separation of the layers the aqueous layer was extracted with ether (2 × 10 mL). The combined organic layers were washed with brine (10 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. The residue was immediately purified with column chromatography (3% ether in petroleum ether) to furnish the coupled product as a light yellow liquid which was contaminated with traces of unreacted vinyl iodide. 1H NMR (CDCl3, 300 MHz) δ 7.62 (m, 4 H), 7.36 (m, 6 H), 5.27 (t, J = 4.2 Hz, 1 H), 4.15 (d, J = 6.0 Hz, 2 H), 2.92 (sept, J = 7.0 Hz, 1H), 2.27 (m, 2 H), 1.90 (m, 1 H), 1.77 (m, 1 H), 1.44 (m, 1 H), 1.38 (s, 3 H), 1.21-1.06 (m, 3 H), 1.00 (m, 3 H), 0.99 (s, 9 H), 0.98 (m, 6 H), 0.15 (s, 6 H).
To a solution of the above product in THF (2 mL) was added TBAF (1 M in THF, 0.585 mL, 0.585 mmol) at room temperature. The mixture was stirred for 1 h and treated with brine (5 mL) and ether (15 mL). After separation of the layers the aqueous phase was extracted with ether (20 mL). The combined organic layers were dried (MgSO4), filtered, and concentrated under reduced pressure. The residue was purified with column chromatography (25% ether in petroleum ether) to provide compound 23 (51.8 mg, 72%) as a light yellow liquid, [α]D = + 2.26 (c 0.53, 23.3°C, dichloromethane). IR (neat) 2960, 2137, 1458, 1249 cm−1; 1H NMR (CDCl3, 300 MHz) δ 5.40 (t, J = 4.2 Hz, 1 H), 4.11 (d, J = 6.9 Hz, 2 H), 3.08 (s, 1H), 2.94 (sept, J = 6.6 Hz, 1H), 2.39 (t, J = 7.8 Hz, 2 H), 1.94 (m, 2 H), 1.82 (m, 1 H), 1.67 (s, 3 H), 1.62-1.41 (m, 4 H), 1.07 (s, 3 H), 1.01 (d, J = 1.5 Hz, 3 H), 0.98 (d, J = 1.5 Hz, 3 H); 13C NMR (CDCl3, 75 MHz) δ 157.9, 140.7, 123.6, 122.9, 81.8, 79.6, 59.4, 49.9, 38.3, 34.8, 34.7, 29.2, 28.8, 26.0, 21.0, 20.8, 16.4; HRMS Calc’d for C17H26O [M+]: 246.1984; Found: 246.1975.
(5S,7aR)-(3-Isopropyl-5,7a-dimethyl-4-methylene-2,4,5,6,7,7a-hexahydro-1H-inden-5-yl)-acetaldehyde (33)
To a solution of compound 23 (165 mg, 0.67 mmol) and DMF (49.0 mg, 0.67 mmol) in 2-butanone (7 mL) was added CpRu(CH3CN)3PF6 (58 mg, 0.134 mmol) in one portion at room temperature. The mixture was stirred for 2 h and concentrated under reduced pressure. The crude material was purified by column chromatography (8% ether in petroleum ether) to provide compound 33 (67.6 mg, 41%) as a clear liquid, [α]D = + 120.0 (c 1.2, 26.3 °C, dichloromethane). IR (neat) 2927, 1727, 1451 cm−1; 1H NMR (CDCl3, 500 MHz) δ 9.88 (m, 1 H), 4.78 (d, J = 1.0 Hz, 1 H), 4.65 (d, J = 1.0 Hz, 1 H), 2.77 (sept, J = 7.0 Hz, 1 H), 2.50 (dd, J = 15.5, 3.0 Hz, 1 H), 2.42 (dd, J = 15.5, 3.0 Hz, 1 H), 2.27 (m, 2 H), 1.80 (m, 2 H), 1.69 (m, 1 H), 1.54 (m, 3 H), 1.42 (m, 1 H), 1.03 (s, 3 H), 0.92 (d, J = 7.0 Hz, 3 H), 0.88 (d, J = 7.0 Hz, 3 H), 0.86 (s, 3 H); 13C NMR (CDCl3, 125 MHz) δ 204.2, 149.9, 142.5, 139.3, 108.9, 53.5, 39.9, 39.4, 37.0, 35.8, 29.9, 28.5, 26.7, 25.3, 23.4, 21.8, 21.6; HRMS Calc’d for C17H26O [M+]: 246.1984; Found: 246.1990.
(E)-(R)-4-Hydroxy-4-[(5S,7aR)-(3-isopropyl-5,7a-dimethyl-4-methylene-2,4,5,6,7,7ahexahydro-1H-inden-5-yl)]-but-2-enenitrile (38)
To a solution of compound 33 (66.0 mg, 0.268 mmol) and phenylsulphinyl acetonitrile (53.1 mg, 0.322 mmol) in benzene (2.7 mL) was added piperidine (27.4 mg, 0.322 mmol) dropwise at room temperature. The mixture was stirred for 24 h and purified directly by column chromatography (2:1, petroleum ether/ether) to give compound 38 as a pale yellow solid (33.6 mg, 44%). [α]D = + 150.6 (c 1.16, 22.7 °C, dichloromethane); IR (neat) 3476, 2225, 1678, 1450, 1257 cm−1; 1H NMR (CDCl3, 500 MHz) δ7.09 (dd, J = 16.0, 4.0 Hz, 1 H), 5.79 (dd, J = 16.0, 2.5 Hz, 1 H), 4.98 (s, 1 H), 4.79 (s, 1 H), 4.54 (m, 1 H), 2.83 (sept, J = 7.0 Hz, 1H), 2.34 (m, 2 H), 1.96 (d, J = 5.0 Hz, 1 H), 1.74 (m, 2 H), 1.59 (m, 3 H), 1.03 (s, 3 H), 0.99 (d, J = 7.0 Hz, 3 H), 0.95 (d, J = 7.0 Hz, 3 H), 0.91 (s, 3 H); 13C NMR (CDCl3, 125 MHz) δ 154.4, 148.0, 142.6, 139.3, 117.6, 109.1, 100.6, 76.1, 48.6, 48.2, 45.4, 43.1, 39.0, 36.2, 31.3, 28.4, 26.5, 26.3, 25.0, 24.1, 23.1, 21.6, 21.4, 20.4; Anal. Calc’d for C19H27NO: C, 79.95; H, 9.53; N, 4.91; Found: C, 80.12; H, 9.54; N, 4.78.
(E)-[(5S,7aR)-3-Iso-propyl-5,7a-dimethyl-5-(2-oxo-ethyl)-1,2,5,6,7,7a-hexahydro-inden-4-ylidene]-acetic acid tert-butyl ester (49c)
To a solution of compound 48c (184.5 mg, 0.533 mmol) in 2-butanone (5.3mL) and DMF (38.9 mg, 0.533 mmol) was added CpRu(CH3CN)3PF6 (46.3 mg, 0.107 mmol). The yellow solution was stirred at room temperature for 2 h and concentrated under reduced pressure. The crude material was purified by column chromatography (4% ether in petroleum ether) to give compounds 49c (less polar) and 50c (more polar). Compound 49c (88.6 mg, 48%, light yellow oil): [α]D = + 266.0 (c 1.80, 23.7 °C, dichloromethane). IR (neat) 2959, 1750, 1708, 1456, 1368, 1143 cm−1; 1H NMR (CDCl3, 400 MHz) δ9.83 (t, J = 3.6 Hz, 1 H), 5.51 (s, 1 H), 3.06 (dd, J = 18.0, 4.0 Hz, 1 H), 2.91 (dd, J = 18.0, 4.0 Hz, 1 H), 2.86 (sept, J = 6.8 Hz, 1H), 2.34 (m, 2 H), 1.71 (m, 2 H), 1.62 (m, 2 H), 1.51 (m, 1 H), 1.48 (s, 9 H), 1.42 (m, 1 H), 1.32 (s, 3 H), 1.01 (m, J = 6.4 Hz, 3 H), 0.99 (s, 3 H), 0.98 (d, J = 6.4 Hz, 3 H); 13C NMR (CDCl3, 100 MHz) δ 203.3, 166.4, 154.2, 145.8, 140.9, 118.7, 80.6, 51.1, 48.2, 40.3, 38.9. 36.2, 35.3, 28.5, 28.1, 26.5, 25.5, 24.5, 21.6, 21.4; HRMS Calc’d for C22H34O3 [M+]: 346.2508; Found: 346.2508.
(E)-(3S,3aR,5aR)-3-(1-Isopropyl-3a,5a-dimethyl-8-oxo-2,3,3a,4,5,5a,6,8-octahydro-7-oxacyclopenta[a]naphthalen-6-yl)-acrylonitrile (54)
To a solution of compound 49c (20.7 mg, 0.060 mmol) and phenylsulphinyl acetonitrile (13.8 mg, 0.084 mmol) in benzene (0.6 mL) was added piperidine (7.1 mg, 0.084 mmol) at room temperature. The mixture was stirred for 36 h and directly purified by column chromatography (petroleum ether/ether, 2:1) to give compound 54 as a white solid (13.9 mg, 75%), mp. 130–131 °C, [α]D = + 667.9 (c 0.57, 24.6 °C, dichloromethane). IR (neat) 2957, 2227, 1724, 1678, 1451, 1247 cm−1; 1H NMR (CDCl3, 500 MHz) δ 6.71 (dd, J = 16.0, 3.5 Hz, 1 H), 5.95 (dd, J = 16.0, 2.0 Hz, 1 H), 5.77 (s, 1 H), 4.75 (dd, J = 4.0, 2.0 Hz, 1 H), 2.89 (sept, J = 6.5 Hz, 1 H), 2.48 (m, 2 H), 1.84 (m, 2 H), 1.76 (m, 2 H), 1.65 (m, 2 H), 1.57 (s, 3 H), 1.05 (d, J = 7.0 Hz, 3 H), 0.99 (s, 3 H), 0.98 (s, 3 H); 13C NMR (CDCl3, 100 MHz) δ 163.8, 160.0, 153.1, 146.3, 133.8, 130.3, 116.7, 113.7, 103.1, 82.8, 48.4, 40.1, 39.0, 35.4, 32.3, 29.3, 27.1, 23.4, 21.6, 21.4, 17.8; HRMS Calc’d for C20H25NO2 [M+]: 311.1885; Found: 311.1865.
(+)-Allocyathin B2 (1)
To a solution of compound 55 (10.0 mg, 0.032 mmol) in CH2Cl2 (0.5 mL) was added DIBAL-H (1 M in hexanes, 0.128 mL, 0.128 mmol) at −78 °C. The mixture was stirred at this temperature for 2 h and quenched with addition of 1 M NaHSO4 (0.4 mL). The suspension was warmed up to room temperature for 15 min and extracted with ether (2 × 5 mL). The organic layer was washed with brine (2 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. The crude aldehyde 4 was used directly for the next step. Aldehyde 4: 1H NMR (CDCl3, 500 MHz, mixture of two diastereomers) major: δ 9.88 (s, 1 H), 5.88 (m, 1 H), 5.22 (m, 1 H), 3.85 (d, J = 6.5 Hz, 1 H), 3.74 (m, 1 H), 3.66 (m, 1 H), 2.86 (m, 1 H), 2.57-2.39 (m, 4 H), 2.33 (m, 2 H), 1.98 (m, 1 H), 1.72 (m, 1 H), 1.67 (m, 2 H), 1.52 (m, 3 H), 1.35 (m, 2 H), 1.03 (m, 3 H), 0.99-0.96 (m, 6 H), 0.94 (s, 3 H); minor: δ 9.88 (s, 1 H), 5.37 (m, 1 H), 5.09 (m, 1 H), 3.87 (d, J = 6.5 Hz, 1 H), 3.75 (m, 1 H), 3.58 (m, 1 H), 2.86 (m, 1 H), 2.33 (m, 2 H), 1.98 (m, 1 H), 1.72 (m, 1 H), 1.67 (m, 2 H), 1.52 (m, 3 H), 1.35 (m, 2 H), 1.03 (m, 3 H), 0.99-0.96 (m, 6 H), 0.92 (s, 3 H).
To a solution of the crude dialdehyde 4 in MeOH (1.0 mL) was added 5% KOH in MeOH (1.0 mL). The mixture was stirred at 60 °C for 1 h. The reaction was quenched with addition of 10% citric acid solution (3 mL) and was concentrated under reduced pressure. The aqueous residue was extracted with ether (2 × 5 mL). The combined organic layers were washed with brine (2 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (5:1 EtOAc/petroleum ether) to give (+)-allocyathin B2 (1, 4.9 mg, 51%) as a pale yellow oil, [α]D = + 482.3 (c 0.18, 23.6 °C, methanol); lit. [α]D = + 144 (c 0.18, methanol).15,79 IR (neat) 3442, 1668, 1571, 2959 cm−1; 1H NMR (CDCl3, 500 MHz) δ 9.45 (s, 1 H), 6.82 (dd, J = 8.0, 6.5 Hz, 1 H), 5.94 (d, J = 8.0 Hz, 1 H), 3.73 (m, 1 H), 3.16 (dd, J = 18.5, 6.0 Hz, 1 H), 2.83 (sept, J = 6.5 Hz, 1 H), 2.55 (br, d, J = 18.5 Hz, 2 H), 2.53 (m, 1 H), 2.42 (dd, J = 9.5, 2.5 Hz, 1 H), 2.41 (d, J = 13.5 Hz, 1 H), 1.74 (ddd, J = 12.5, 7.5, 5.0 Hz, 1 H), 1.69-1.65 (m, 3 H), 1.61 (br, m, 1 H), 1.34 (dt, J = 14.0, 3.5 Hz), 1.05 (d, J = 7.0 Hz, 3 H), 1.00 (s, 3 H), 0.97 (d, J = 7.0 Hz, 3 H), 0.96 (s, 3 H); 13C NMR (CDCl3, 125 MHz) δ194.2, 155.1, 146.5, 144.4, 151.8, 137.7, 119.3, 74.0, 49.1, 48.2, 38.2, 36.5, 33.9, 29.2, 29.0, 27.0, 26.5, 23.9, 21.5, 21.5; HRMS Calc’d for C20H28O2 [M+]: 300.2089; Found: 300.2077.
Supplementary Material
Full experimental procedures and characterization data as well as copies of the NMR spectra (PDF). This material is free of charge via the Internet at http://pubs.acs.org.
Figure 3.
X-ray Crystallography of Compound 17.
Acknowledgement
We thank the National Institutes of Health and the National Science Foundation for their generous support of our program. Mass spectra were provided by the Mass spectrometry Facility, University of California, San Francisco, supported the NIH Division of Research Resources. We are grateful to Prof. H. Kawagishi for sharing the optical rotation and NMR spectra of (+)-allocyathin B2.
Reference
- 1.Trost BM, Krische MJ. Synlett. 1998:1–16. [Google Scholar]
- 2.Trost BM, Surivet J-P, Toste FD. J. Am. Chem. Soc. 2004;126:15592–15602. doi: 10.1021/ja046824o. [DOI] [PubMed] [Google Scholar]
- 3.Trost BM, Toste FD. J. Am. Chem. Soc. 2002;124:5025–5036. doi: 10.1021/ja012450c. [DOI] [PubMed] [Google Scholar]
- 4.Trost BM. Chem. Eur. J. 1998;4:2405–2412. [Google Scholar]
- 5.Ayer WA, Lee SP. Can. J. Chem. 1979;57:3332–3337. [Google Scholar]
- 6.Allbutt AD, Ayer WA, Brodie HJ, Johri BN, Taube H. Can. J. Microbiol. 1971;17:1401–1402. doi: 10.1139/m71-223. [DOI] [PubMed] [Google Scholar]
- 7.Ayer WA, Browne LM. Tetrahedron. 1981;37:2199–2248. [Google Scholar]
- 8.Ayer WA, Browne LM, Mercer JR, Taylor DR, Ward DE. Can. J. Chem. 1978;56:717–721. [Google Scholar]
- 9.Ayer WA, Lee SP, Nakashima TT. Can. J. Chem. 1979;57:3338–3343. [Google Scholar]
- 10.Ayer WA, Taube H. Tetrahedron Lett. 1972;13:1917–1920. [Google Scholar]
- 11.Ayer WA, Taube H. Can. J. Chem. 1973;51:3842–3843. [Google Scholar]
- 12.Ayer WA, Ward DE, Browne LM, Delbaere LTJ, Hoyano Y. Can. J. Chem. 1981;59:2665–2672. [Google Scholar]
- 13.Ayer WA, Yoshida T, Van Schie DMJ. Can. J. Chem. 1978;56:2113–2120. [Google Scholar]
- 14.Kawagishi H, Shimada A, Hosokawa S, Mori H, Sakamoto H, Ishiguro Y, Sakemi S, Bordner J, Kijima N, Furukawa S. Tetrahedron Lett. 1996;37:7399–7402. [Google Scholar]
- 15.Kawagishi H, Shimada A, Shirai R, Okamoto K, Ojima F, Sakamoto H, Ishiguro Y, Furukawa S. Tetrahedron Lett. 1994;35:1569–1572. [Google Scholar]
- 16.Kawagishi H, Simada A, Shizuki K, Mori H, OKamoto K, Sakamoto H, Furukawa S. Heterocyclic Commu. 1996;2:51–54. [Google Scholar]
- 17.Lee EW, Shizuki K, Hosokawa S, Suzuki M, Suganuma H, Inakuma T, Li J, Ohnishi-Kameyama M, Nagata T, Furukawa S, Kawagishi H. Biosci. Biotechnol. Biochem. 2000;64:2402–2405. doi: 10.1271/bbb.64.2402. [DOI] [PubMed] [Google Scholar]
- 18.Toyota M, Nakaisi E, Asakawa Y. Phytochemistry. 1996;43:1057–1064. [Google Scholar]
- 19.Shibata H, Tokunaga T, Karasawa D, Hirota A, Nakayama M, Nozaki H, Tada T. Agric. Biol. Chem. 1989;53:3373–3375. [Google Scholar]
- 20.Wright DL, Whitehead CR. Org. Prep. and Proc. 2000;32:307–320. [Google Scholar]
- 21.Thominiaux C, Chiaroni A, Desmaele D. Tetrahedron Lett. 2002;43:4107–4110. [Google Scholar]
- 22.Tekeda K, Nakane D, Tekeda M. Org. Lett. 2000;2:1903–1905. doi: 10.1021/ol0059753. [DOI] [PubMed] [Google Scholar]
- 23.Wender PA, Bi FC, Brodney MA, Gosselin F. Org. Lett. 2001;3:2105–2108. doi: 10.1021/ol0160699. [DOI] [PubMed] [Google Scholar]
- 24.Wright DL, Whitehead CR, Sessions EH, Ghiviriga I, Frey DA. Org. Lett. 1999;1:1535–1538. doi: 10.1021/ol991032y. [DOI] [PubMed] [Google Scholar]
- 25.Magnus P, Shen L. Tetrahedron. 1999;55:3553–3560. [Google Scholar]
- 26.Dahnke KR, Paquette LA. J. Org. Chem. 1994;59:885–899. [Google Scholar]
- 27.Tori M, Toyoda N, Sono M. J. Org. Chem. 1998;63:306–313. [Google Scholar]
- 28.Snider BB, Vo NH, O'Neil SV. J. Org. Chem. 1998;63:4732–4740. [Google Scholar]
- 29.Snider BB, Vo NH, O'Neil SV, Foxman BM. J. Am. Chem. Soc. 1996;118:7644–7645. [Google Scholar]
- 30.Piers E, Cook KL. Chem. Commun. 1996:1879–1880. [Google Scholar]; Piers E, Gilbert M, Cook KL. Org. Lett. 2000;2:1407–1410. doi: 10.1021/ol0057333. [DOI] [PubMed] [Google Scholar]
- 31.Trost BM, Dong Li, Schroeder GM. J. Am. Chem. Soc. 2005;127:2844–2845. doi: 10.1021/ja0435586. [DOI] [PubMed] [Google Scholar]
- 32.Aubert C, Buisine O, Malacria B. Chem. Rev. 2002;102:813–834. doi: 10.1021/cr980054f. [DOI] [PubMed] [Google Scholar]
- 33.Trost BM, Schroeder GM. J. Am. Chem. Soc. 1999;121:6759–6760. [Google Scholar]
- 34.Trost BM, Schroeder GM, Kristensen J. Angew. Chem. Int. Ed. 2002;41:3492–3495. doi: 10.1002/1521-3773(20020916)41:18<3492::AID-ANIE3492>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.Trost BM. Chem. Pharm. Bull. 2002;50:1–14. doi: 10.1248/cpb.50.1. [DOI] [PubMed] [Google Scholar]
- 36.Trost BM, Pissot-Soldermann C, Chen I, Schroeder GM. J. Am. Chem. Soc. 2004;126:4480–4481. doi: 10.1021/ja0497025. [DOI] [PubMed] [Google Scholar]
- 37.Corey EJ, Kang M, Desai MC, Ghosh AK, Houpis IN. J. Am. Chem. Soc. 1988;110:649–651. doi: 10.1021/ja00210a083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown CA, Coleman RA. J. Org. Chem. 1979;44:2328–2329. [Google Scholar]
- 39.Mancuso AJ, Swern D. Synthesis. 1981:165–168. [Google Scholar]
- 40.Corey EJ, Fuchs PL. Tetrahedron Lett. 1972;36:3769–3772. [Google Scholar]
- 41.Corey EJ, Katzenellenbogen JA. J. Am. Chem. Soc. 1969;91:1851–1852. [Google Scholar]
- 42.Lei A, He M, Wu S, Zhang X. Angew. Chem. Int. Ed. 2002;41:3457–3460. doi: 10.1002/1521-3773(20020916)41:18<3457::AID-ANIE3457>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Cao P, Zhang X. Angew. Chem. Int. Ed. 2000;39:4104–4106. doi: 10.1002/1521-3773(20001117)39:22<4104::aid-anie4104>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 44.Sturla SJ, Kablaoui NM, Bulchwald SL. J. Am. Chem. Soc. 1999;121:1976–1977. [Google Scholar]
- 45.Trost BM. Acc. Chem. Res. 1990;23:34–42. [Google Scholar]
- 46.Trost BM, Li Y. J. Am. Chem. Soc. 1996;118:6625–6633. [Google Scholar]
- 47.Nokami J, Mandai T, Imakura Y, Nishiuchi K, Kawada M, Wakabayashi S. Tetrahedron Lett. 1981;22:4489–4490. [Google Scholar]
- 48.Reduction with in situ-generated copper hydride, Mo-catalyzed hydrosilane reduction or Lindlar's catalyst reduction gave no reaction.
- 49.Minato M, Yamamoto K, Tsuji J. J. Org. Chem. 1990;55:766–768. [Google Scholar]
- 50.Sato T, Nagata T, Maeda K, Ohtsuka S. Tetrahedron Lett. 1994;35:5027–5030. [Google Scholar]
- 51.Mihelich ED, Daniels K, Eickhoff DJ. J. Am. Chem. Soc. 1981;103:7690–7692. [Google Scholar]
- 52.Nesnas N, Rando RR, Nakanishi K. Tetrahedron. 2002;58:6577–6584. [Google Scholar]
- 53.Gagneur S, Montchamp JL, Negishi E. Organometallics. 2000;19:2417–2419. [Google Scholar]
- 54.Negishi E-I, Valente LF, Kobayashi M. J. Am. Chem. Soc. 1980;102:3298–3299. [Google Scholar]
- 55.Negishi E, Swanson DR, Rousset CJ. J. Org. Chem. 1990;55:5406–5409. [Google Scholar]
- 56.Trost BM, Romero DL, Rise F. J. Am. Chem. Soc. 1994;116:4268–4278. [Google Scholar]
- 57.The relative stereochemitry was assigned by comparison of the NMR spectra of compound 29 with those of compound 15.
- 58.Trost BM, Lee DC, Rise F. Tetrahedron Lett. 1989;30:651–654. [Google Scholar]
- 59.Trost BM, Chung JYL. J. Am. Chem. Soc. 1985;107:4586–4588. [Google Scholar]
- 60.Trost BM, Corte JR, Gudiksen MS. Angew. Chem. Int. Ed. 1999;38 [PubMed] [Google Scholar]
- 61.Trost BM, Toste FD, Pinkerton AB. Chem. Rev. 2001;101:2067–2096. doi: 10.1021/cr000666b. [DOI] [PubMed] [Google Scholar]
- 62.Trost BM, Toste FD. J. Am. Chem. Soc. 2000;122:714–715. [Google Scholar]
- 63.The enol ether was obtained as a single E isomer based on the NMR analysis
- 64.A rapid decomposition was observed with TBS protected compounds in the presence of CpRu(CH3CN)3PF6 in acetone while a clean desilylation occurred in DMF after 4 h. On the other hand, TBDPS derivatives are very stable and were quantitatively recovered in both solvents. Protection of the free hydroxyl group as an acetate led to no reaction.
- 65.We attempted to use CpRu(COD)Cl which is an effective catalyst in the intermolecular Alder-ene reaction. There was no reaction with this catalyst alone and a complex mixture was obtained with the addition of AgOTf as the co-catalyst. Since the free allylic alcohol was perceived as a potential problem due to its possible coordination with Ru, some additives were added to tentatively mask the hydroxyl group. However, no reaction occurred when the substrate was premixed with AlMe3 before the addition of the catalyst. On the other hand, TMS protected starting material was isolated if BSA was added.
- 66.The rest of the mass is recovered starting material along with some of the corresponding allylic aldehyde. Rigorous exclusion of oxygen from the solvent minimized the formation of the oxidation by-product. This is also true for the reaction of compound 23.
- 67.Ono T, Tamaoka T, Yuasa Y, Matsuda T, Nokami J, Wakabayashi S. J. Am. Chem. Soc. 1984;106:7890–7893. [Google Scholar]
- 68.Chini M, Crotti P, Favero L, Macchia F. Tetrahedron Lett. 1991;36:4775–4778. [Google Scholar]
- 69.Trost BM, Flemming I, Paquette LA. In: Comprehensive Organic Synthesis. Trost BM, editor. Vol. VI. Oxford: Pergamon Elsevier; 1991. pp. 899–904. [Google Scholar]
- 70.Trost BM, Mallert S. Tetrahedron Lett. 1993;34:8025–8028. [Google Scholar]
- 71.Hoffmann RW. Angew. Chem. Int. Ed. 1979;18:563–572. [Google Scholar]
- 72.We also synthesized the corresponding unsaturated nitrile and aldehyde. The reaction of the nitrile derivative gave recovered starting material because of its strong coordination with Ru and subsequent deactivation of the catalyst. The aldehyde derivative generated a cyclization product which is too labile to be isolated.
- 73.For complete double bond isomerization in seven-membered ring formation see ref. 61.
- 74.Other weak phosphorus-containing ligands such as triphenylphosphine oxide and phosphonate suppressed the conversion to less than 3%.
- 75.No reaction was observed with DMF as solvent.
- 76.Attempts to isomerize the double bond by treatment with diphenyl disulfide in the presence of light led to decomposition of the starting material.
- 77.Various conditions were attempted including CSA/benzene reflux, L-proline, pyrrolidine/acetic acid as well as alumina.
- 78.Ho P-T. Tetrahedron Lett. 1978;19:1623–1626. [Google Scholar]
- 79.Takano M, Umino A, Nakada M. Org. Lett. 2004;6:4897–4990. doi: 10.1021/ol048010i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full experimental procedures and characterization data as well as copies of the NMR spectra (PDF). This material is free of charge via the Internet at http://pubs.acs.org.