Abstract
Tryptophan 2,3-dioxygenase (TDO) is the first enzyme in the tryptophan oxidation pathway. It is a hemoprotein and its heme prosthetic group is present as a heme-ferric (heme-Fe3+) form that is not active. To be able to oxidize tryptophan, the heme-Fe3+ form of the enzyme must be reduced to a heme-ferrous (heme-Fe2+) form and this study describes conditions that promote TDO activation. TDO is progressively activated upon mixing with tryptophan in a neutral buffer, which leads to an impression that tryptophan is responsible for TDO activation. Through extensive analysis of factors resulting in TDO activation during incubation with tryptophan, we conclude that tryptophan indirectly activates TDO through promoting the production of reactive oxygen species. This consideration is supported by the virtual elimination of the initial lag phase when either pre-incubated tryptophan solution was used as the substrate or a low concentration of superoxide or hydrogen peroxide was incorporated into the freshly tryptophan and TDO mixture. However, accumulation of these reactive oxygen species also leads to the inactivation of TDO, so that both TDO activation and inactivation proceed with the specific outcome depending greatly on the concentrations of superoxide and hydrogen peroxide. As a consequence, the rate of TDO catalysis varies depending upon the proportion of the active to inactive forms of the enzyme, which is in a dynamic relationship in the reaction mixture. These data provide some insight towards elucidating the molecular regulation of TDO in vivo.
Keywords: tryptophan 2, 3-dioxygenase, Aedes aegypti, superoxide, hydrogen peroxide, ascorbate
INTRODUCTION
Tryptophan 2,3-dioxygenase (TDO, EC 1.13.11.12) is a heme-containing dioxygenase involved in catalyzing the addition of molecular oxygen (O2) across the 2,3-double bond of the indole ring of tryptophan, leading to the cleavage of the indole ring to form N-formylkynurenine (NFK). TDO is the first and rate-limiting enzyme in the tryptophan oxidation pathway (or kynurenine pathway). Tryptophan is an essential amino acid for protein synthesis and also the precursor for production of a number of neurotransmitters (such as serotonin and melatonin). In mosquitoes, the kynurenine pathway is essential for the pigmentation of their compound (Li et al., 1999). Conceivably, the tryptophan metabolic pathways should be regulated in a well-balanced manner in mosquitoes as well as other species. The heme prosthetic group of TDO is present as a heme-ferric form (heme-Fe3+). To oxidize tryptophan, the heme-ferric form of the enzyme must be reduced to the heme-ferrous form (heme-Fe2+) (Tanaka and Knox, 1959; Hayaishi and Nozaki, 1969; Sono, 1989). Therefore, the tryptophan to kynurenine pathway might be regulated by a TDO activation/inactivation process.
It has been reported that reactive oxygen species (such as superoxide ) or hydrogen peroxide (H2O2) and reducing agents (such as ascorbate and sodium hydrosulfite) stimulate TDO activity through a reductive activation process (Tanaka and Knox, 1959; Hayaishi and Nozaki, 1969; Sono, 1989; Hitchcock and Katz, 1988). Earlier studies also indicated that TDO was subject to allosteric regulation and that the enzyme had at least two distinct sites for tryptophan binding, with one as a catalytic site and the other as an allosteric site (Schutz et al., 1972; Brady et al., 1971; Koike et al., 1969; Kobayashi et al., 1989). Hemoproteins have a major absorbance peak with a λmax around 400 nm in the visible region, and this is commonly termed the Soret band or Soret peak. The Soret peak of TDO may change as a consequence of exposure to , H2O2, or reducing agents in the presence or absence of tryptophan. For example, a spectral shift of the Soret peak towards longer wavelengths has been used as key evidence for suggesting the formation of an active form (heme-Fe2+) of TDO (Tanaka and Knox, 1959; Ishimura et al., 1970; Schutz and Feigelson, 1972; Littlejohn et al., 2000).
Using an expressed recombinant TDO from the yellow fever mosquito, Aedes (Ae.) aegypti, we further studied the roles that tryptophan, , H2O2, and reducing agents play in TDO-mediated reaction/inactivation. Our data indicate that reactive oxygen species and reducing agents modulate TDO function in a rather complicated manner. TDO activation in the presence of tryptophan is unlikely due to the allosteric induction of the enzyme by the substrate as previously suggested. Soret peak shift of TDO towards longer wavelengths, which has been considered the key indicator for the formation of active form of TDO in numerous previous studies, represents the accumulation of the inactivated form of TDO. These data raise some fundamental questions regarding some previous conclusions about TDO.
MATERIALS AND METHODS
Chemicals
Ascorbate, cysteine, hydrogen peroxide (H2O2), potassium superoxide (KO2), catalase, and superoxide dismutase were purchased from Sigma. NFK was synthesized by a described method (Auerbach and Knox, 1957).
TDO Cloning
A full-length TDO clone of Ae. aegypti was isolated by gene amplification of partial TDO cDNA using a forward (5′-CAGGCSTACGARCTKTGGTTCAA-3′) and a reverse degenerate oligonucleotide primer (5′-ACGTGRTTRTATCKCCACTTGG-3′) and subsequent screening of an Ae. aegypti larval cDNA library using the amplified partial TDO clone. The TDO sequence (AF325458) is available in the NCBI database.
Generation of Recombinant TDO Baculovirus
The coding region of the TDO sequence was amplified from its full-length cDNA clone using a specific forward primer containing a Pst I site (5′-CTGCAGATGAGTTGTCCCGTAGGA-3′) and a reverse primer containing an Sal I site (underlined nucleotide) and a 6-his tag at the 3′-end (5′-CGTCGACTAATGATGATGATGATGATGTTTCGCAG-CATAG-3′). The amplified products were cloned into a baculovirus transfer vector pBlueBac4.5 (Invitrogen, Carlsbad, CA) between the Pst I and the Sal I restriction sites. TDO recombinant baculoviruses were generated and purified in the same manner described in a previous study (Han and Li, 2002).
Recombinant TDO (r-TDO) Expression and Purification
Sf9 cells were cultured at 27°C in an insect cell medium supplemented with 10 U of heparin (Sigma, St. Louis, MO) per ml in culture spinner flasks with constant stirring at 80 rpm. When the cell density reached 2 × 106 cells/ml, they were inoculated with the high-titer stock of the recombinant TDO baculovirus at 6 viral particles/cell. Cells were collected by centrifugation (1,000g for 20 min at 4°C) at 4 days after infection. Cell pellets were homogenized in binding buffer (20 mM Tris, 0.5 M NaCl, and 10 mM imidazole, pH 8.0), containing 0.1 mM hemin, 1.0 mM PMSF, and 0.5% triton X-100. The mixture was incubated for 1 h and centrifuged at 18,000g for 20 min. The supernatant was applied to a nickel-nitrilotriacetic acid column (Ni-NTA, Qiagen, Valencia, CA) pre-equilibrated with binding buffer. After extensive washing with the binding buffer, TDO was eluted with 10–300 mM gradient imidazole prepared with the binding buffer, and further purified by gel-filtration chromatography (Superdex 200, Pharmacia, Uppsala, Sweden) using 0.1 M Tris-HCl as eluting buffer.
TDO Activity Assay
NFK has an absorbance peak with a λ = 321 nm; therefore, TDO activity was based on the detection of the 321-nm peak in the reaction mixtures by spectral analysis or continuously monitored at 321 nm at 35°C using a Hitachi double-beam 2001 spectrophotometer equipped with water jacketed cuvette holders. A typical reaction mixture consisted of 10 mM tryptophan and 10 μg of TDO in 1.0 ml of 0.2 M phosphate buffer (pH 7.5). In all assays, tryptophan solutions were prepared within 2 min prior to the initiation of the enzyme activity assay. In some reactions, 500 U of superoxide dismutase and/or catalase were incorporated into the reaction mixtures to determine their effect on TDO activity, and in such cases the concentrations of substrate and enzyme and the total volume of the reaction mixture were kept constant by properly adjusting the volume and concentration of the tryptophan solution.
Effect of H2O2 and on TDO Activity
To determine the effect of H2O2 and on TDO activity, 0.02 to 10 mM of either H2O2 or KO2 was prepared fresh in a 0.2 M phosphate buffer (pH 7.5) just prior to the TDO assay, and 0.1 ml of KO2 or H2O2 solution was mixed with 0.895 ml of freshly prepared 11.17 mM tryptophan solution (which gave a 10 mM final concentration of tryptophan in a total volume of 1.0 ml reaction mixture). The reaction was initiated by addition of 10 μg of TDO in a 5-μl phosphate buffer (pH 7.5) into the reaction mixture. The final concentration of H2O2 or ranged from 0.002 to 1.0 mM. Absorbance increases of the reaction mixture at 321 nm were monitored continuously for 40 min at 35°C.
Effect of Ascorbate and Sodium Hydrosulfite on TDO Activity
Ascorbate (1–50 mM) and sodium hydrosulfite (0.5–10 mM) were prepared in a 0.2 M phosphate buffer (pH 7.5) just prior to the assays. After mixing 0.1 ml of the reducing agent in 0.895 ml of 11.17 mM tryptophan solution, the reaction was initiated by adding 10 μg of TDO into the tryptophan and reducing agent mixture. Accumulation of NFK in the reaction mixture was monitored continuously at 321 nm for 40 min at 35°C.
Spectral Changes of TDO in the Presence H2O2, , Ascorbate, and Sodium Hydrosulfite
Spectral changes of TDO (300–600 nm) in the presence of H2O2, , ascorbate, or sodium hydrosulfite were evaluated using the Hitachi U2001 double-beam spectrophotometer. Prior to spectral analysis, the baseline (300–600 nm) was zeroed with reference and sample cuvettes containing 0.2 M phosphate buffer, pH 7.5. After the baseline was adjusted, 120 μg TDO in a 60-μl phosphate buffer was mixed with 940 μl of phosphate buffer and its spectrum recorded as a control. Concentrated H2O2, KO2, ascorbate, or sodium hydrosulfite was prepared fresh in a 0.2 M phosphate buffer (pH 7.5) and 5 μl of the solution was added to the TDO solution. At 3 min after the addition of H2O2, KO2, ascorbate, or sodium hydrosulfite, spectral changes of TDO (300–600 nm) were recorded at 35°C.
RESULTS
Basic Characteristics of r-TDO
r-TDO expressed in insect cells was a major soluble protein in supernatant of cell lysate (Fig. 1A). After separation through affinity and gel filtration chromatographies, the protein sample was resolved as a single band on SDS-PAGE with a Mr = 42,000 (Fig. 1A). Spectral analysis of the purified protein revealed the typical Soret peak with a λmax at 405 nm (Fig. 1B). Addition of purified r-TDO into a tryptophan solution resulted in a progressive increase of the absorbance peak with a λmax = 321 nm (Fig. 1C). The species with a λmax at 321 nm was identified as NFK based on comparison with an authentic NFK standard. Production of NFK in the reaction mixture also was confirmed by reverse-phase HPLC with UV detection (not shown). These results indicate that the expressed r-TDO is biochemically active and that its activity can be accurately measured spectrophotometrically at 321 nm.
Fig. 1.

Basic characteristics of r-TDO. A: SDS PAGE showing the relative amount of r-TDO in soluble protein of TDO recombinant baculovirus-infected insect cells (lane 1), and purity of r-TDO after affinity and gel filtration chromatography (lane 2). Ladder = migration profile of protein standards. B: Spectral characteristics of purified r-TDO (about 1 mg in 1.0 ml of phosphate buffer, pH 7.5). C: Production of NFK in TDO and tryptophan reaction mixtures. The baseline from 280–400 nm in C was set to zero immediately after mixing 30 μg of r-TDO with the tryptophan solution to subtract the absorbance of tryptophan and TDO. The total volume of the reaction mixture was 1 ml and spectral changes were recorded at 5-min intervals at 35°C.
Presence of an Initial Lag Period During TDO-Mediated Tryptophan Oxidation
During TDO-mediated tryptophan oxidation, there was an apparent initial lag period that lasted about 20–25 min at the applied conditions (see Fig. 1C). Increases in tryptophan concentration up to 10 mM augmented the oxidation rate, but showed no apparent impact on the duration of the lag phase (Fig. 2A). During the lag period, the rate of tryptophan oxidation increased gradually and after the lag period, a seemingly linear increase in NFK concentrations was observed in the reaction mixtures (Fig. 2A). Analysis of TDO activity in the linear range at different pH values and temperatures showed that TDO had its maximum activity at 40°C and at pH = 8.0 (data not shown). TDO activity was approximately proportional to the amounts of enzyme added to the reaction mixtures at the applied assay conditions, but the progressive increase in TDO activity and the subsequent linear KFN accumulation were diminished when catalase and superoxide dismutase were incorporated into the reaction mixture (Fig. 2B).
Fig. 2.
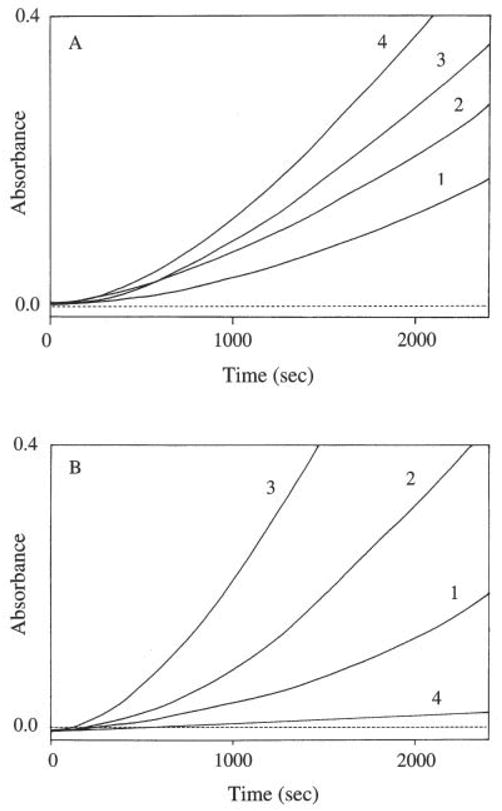
The presence of an initial lag period during r-TDO-catalyzed tryptophan oxidation. A: Traces 1, 2, 3, and 4 illustrate absorbance changes in TDO and tryptophan reaction mixtures with tryptophan concentrations at 1, 3, 6, and 10 mM, respectively. B: Traces 1, 2, and 3 show absorbance changes in the TDO and tryptophan reaction mixtures with TDO contents at 10, 20, and 30 μg, respectively, and trace 4 represents absorbance changes in a reaction mixture identical to that used to produce trace 3, but containing both catalase (500 U) and superoxide dismutase (500 U). The total volume of the reaction mixtures was 1 ml. The amount of enzyme (20 μg) used in A and the concentration of tryptophan (10 mM) in B remained constant, and the temperature was 35°C.
Effect of Pre-Incubated Tryptophan Solution on TDO Activity
When a tryptophan solution prepared in a 0.2 M phosphate buffer (pH 7.5) was incubated at 37°C for 40–160 min prior to mixing with TDO, the initial lag period was reduced greatly as compared to that of the reaction mixture containing freshly prepared tryptophan solution (Fig. 3). Addition of superoxide dismutase and catalase to the pre-incubated tryptophan solution for 5 min prior to mixing with TDO diminished its effect on TDO activation (Fig. 3).
Fig. 3.

Effect of pre-incubated tryptophan on TDO activity. Tryptophan solution was prepared in 0.2 M phosphate buffer (pH 7.5) and incubated for 40, 120, and 160 min at 37°C prior to mixing with TDO. Traces illustrate absorbance changes at 321 nm in the reaction mixtures with freshly prepared tryptophan (trace 1) and with tryptophan pre-incubated at 37°C for 40 min (trace 2), 120 min (trace 3), and 160 min (trace 4). Trace 5 was generated with tryptophan solution pre-incubated at 37°C for 160 min, but the solution was treated with 500 U of catalase and superoxide dismutase for 5 min prior to the addition of TDO.
Effect of Exogenous H2O2 and on TDO Activity
The presence of a lag period during TDO-mediated tryptophan oxidation is consistent with the interpretation that the enzyme must be activated. Furthermore, the reduction of the lag period in the reaction mixture containing pre-incubated tryptophan and the inhibition of TDO activity by catalase or superoxide dismutase indicate that both and H2O2 are likely involved in TDO activation. Incorporation of low concentrations (2–10 μM) of H2O2 into the TDO and tryptophan reaction mixture substantially reduced the lag period, with the most effective H2O2 concentration at 5 μM (Fig. 4A).
Fig. 4.

Effect of H2O2 and on TDO activity. Freshly prepared tryptophan and H2O2 or KO2 solutions were mixed and the reaction initiated by the addition of TDO. Spectral changes at 321 nm were monitored continuously for 40 min at 35°C. A: Traces illustrate absorbance increases at 321 nm in TDO and tryptophan reaction mixtures in the absence of H2O2 (trace 1) and in the presence of 2 (trace 2), 5 (trace 3), 10 (trace 4), and 50 μM of H2O2 (trace 5), respectively. B: Traces show absorbance increases at 321 nm in TDO and tryptophan reaction mixtures in the absence of KO2 (trace 1) and in the presence of 1 (trace 2), 2 (trace 3), 5 (trace 4), 10 (trace 5), and 50 μM of KO2 (trace 6), respectively. The total volume of the reaction mixture was 1 ml, the amount of TDO was 10 μg, and the concentration of tryptophan was 10 mM.
H2O2 was less effective at stimulating TDO activity at concentrations above 10 μM and became an effective inhibitor at concentrations ≥0.1 mM (Fig. 4A). Addition of low concentrations of KO2 to the TDO and tryptophan reaction mixtures also reduced the initial lag period (Fig. 4B). At the applied assay conditions, KO2 showed the optimum effect on stimulating TDO activity at concentrations from 2–10 μM, was less effective at concentrations from 20–50 μM, and became an effective inhibitor of the enzyme at ≥0.1 mM, a result similar to that observed for H2O2.
Effect of Reducing Agents on TDO Activity
Addition of ascorbate affected the lag phase and the overall amounts of NFK produced during a 40-min incubation period. In the presence of relatively low concentrations (0.1–3 mM) of ascorbate in TDO and tryptophan reaction mixtures, the lag period was reduced and the overall amounts of NFK produced were much greater than that of the reaction mixture without ascorbate (Fig. 5A). In the presence of a relatively high concentration of ascorbate (>5 mM), the lag period was reduced, but after a short linear period (lasting about 5–10 min) of enzymatic reaction, most of the enzyme seemed to be inactivated because the curve of NFK in the reaction mixtures became flat during the rest of the incubation period (Fig. 5A). When superoxide dismutase and catalase also were incorporated into the reaction mixture containing 5 mM of ascorbate, the linear period of NFK accumulation was extended to about 25 min (Fig. 5A). In the presence of high concentrations of ascorbate (>10 mM), TDO was inactivated more rapidly because the enzyme lost its activity just a few minutes after incubation; consequently, much smaller amounts of NFK were produced in the reaction mixture during the 40-min period (data not shown) than that of control without ascorbate. Addition of relatively low concentrations (0.05–0.2 mM) of sodium hydrosulfite into the TDO and tryptophan mixture also stimulated the TDO activity with an optimum effect around 0.1 mM (Fig. 5B). Sodium hydrosulfite was less effective in stimulating TDO activity at 0.5 mM and became an effective inhibitor of the enzyme at concentrations ≥1 mM (Fig. 5B). The complete inhibition of TDO in the presence of 1 mM sodium hydrosulfite (Fig. 5B, trace 6) is both surprising and intriguing and requires further investigation.
Fig. 5.
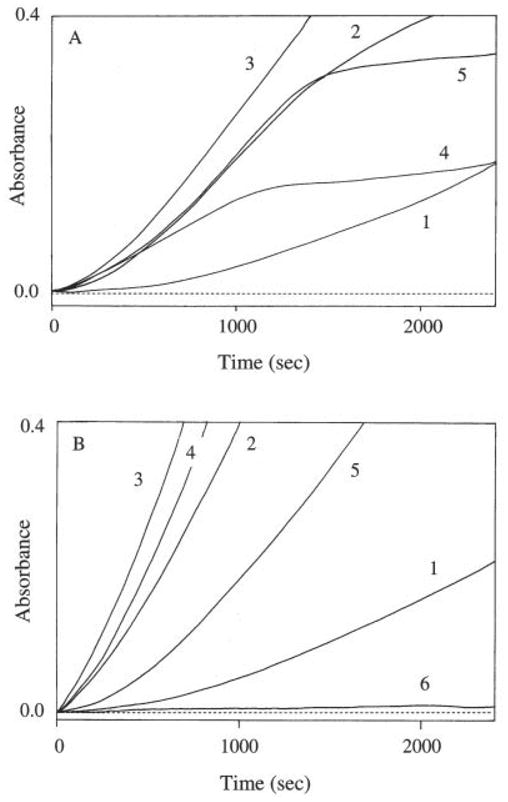
Effect of ascorbate and sodium hydrosulfite on TDO activity. Freshly prepared tryptophan and ascorbate or sodium hydrosulfite solutions were first mixed and the reaction was initiated by the addition of TDO. Spectral change at 321 nm was monitored continuously for 40 min at 35°C. A: Traces illustrate absorbance increases at 321 nm in TDO and tryptophan reaction mixtures in the absence of ascorbate (trace 1), in the presence of 0.1 (trace 2), 1.0 (trace 3), and 5 mM (trace 4) of ascorbate, and in the presence of 5 mM ascorbate plus 500 U of both superoxide dismutase and catalase (trace 5). B: Traces show absorbance increases at 321 nm in TDO and tryptophan reaction mixtures in the absence of sodium hydrosulfite (trace 1) and in the presence of 0.05 (trace 2), 0.1 (trace 3), 0.2 (trace 4), 0.4 (trace 5), and 1.0 mM sodium hydrosulfite (trace 6). The total volume of the reaction mixture was 1 ml, the amount of TDO was 10 μg, and the concentration of tryptophan was 10 mM.
Effect of and H2O2 on the TDO Spectrum
After low concentrations of H2O2 were mixed with the TDO solution, small decreases were observed in the TDO Soret peak (5–10%) and the edge of the Soret band in the short wavelength range shifted slightly toward longer wavelengths, but there was no noticeable shift of its λmax, and the edge of the Soret band in the long wavelength coincided with that of TDO before H2O2 addition (Fig. 6). Decrease of the Soret peak was the consequence of an interaction between TDO and H2O2 rather than by dilution because only 5 μl of concentrated H2O2 solution was incorporated into 1.0 ml of TDO solution (0.5% increase of its original volume). In addition, at low concentrations of H2O2 (≤50 μM), there was no noticeable change of the minor absorbance peak at 500 nm. Increase of H2O2 concentrations in the TDO solution further decreased the Soret peak and these were accompanied by a shift of the λmax towards longer wavelengths (Fig. 6). At relatively high concentrations of H2O2, there were some increases in baseline absorbance within 500–600 nm, and an isosbestic point around 480 nm was observed (Fig. 6). Spectral changes of TDO after incorporation of relatively low and high concentrations of KO2 (data not shown) were similar to those observed for TDO in the presence of H2O2. TDO showed maximum activity at relatively low concentrations of or H2O2 and its activity was diminished when the concentrations of these reactive oxygen species were ≥0.1 M. Accordingly, a considerable portion of TDO likely is present as the active form in the presence of low concentrations (5–50 μM) of H2O2 or and the majority of the enzyme is likely present in an inactive or an inactivated form in the presence of relatively high concentrations of H2O2 or (>0.1 M).
Fig. 6.

Spectral changes of TDO in the presence of H2O2. The baseline from 250 to 600 nm was zeroed in the presence of a 0.2 M phosphate buffer (pH 7.5) in both reference and sample cuvettes. After the baseline was adjusted, 120 μg of r-TDO in 60 μl of phosphate buffer was mixed into 940 μl of 0.2 M phosphate buffer and the spectrum of TDO from 300.600 nm was recorded before and after the addition of H2O2. Traces illustrate the spectrum of TDO before the addition of H2O2 (trace 1) and the spectral changes of TDO at 3 min after the addition of 0.005 (trace 2), 0.02 (trace 3), 0.1 (trace 4), 0.5 (trace 5), and 1.0 mM of H2O2 (trace 6).
Comparison of the spectral change of TDO with that of horseradish peroxidase in the presence of H2O2 suggests that TDO might be oxidized further at relatively high concentrations of or H2O2. Figure 7A and B illustrate the spectral shift of the horseradish peroxidase Soret peak in the presence of a low (10 μM) and high concentration (8 mM) of H2O2. Heme-containing peroxidase was oxidized to compound I (or heme-Fe+5) in the presence of relatively low concentrations of H2O2. In the presence of relatively high concentrations of the H2O2, peroxidase tends to be gradually oxidized further to compound III, an inactivated form (Dunford, 1991). The similarity in spectral shift of the TDO Soret peak towards longer wavelengths as that observed for HRP in the presence H2O2 suggests that TDO-heme-Fe3+ is oxidized further in the presence of a relatively high concentration of and H2O2, leading to the formation of an inactivated form of TDO.
Fig. 7.

Spectral changes of HRP in the presence of H2O2. A: Traces show the spectrum of HRP (0.5 mg in 1 ml of 0.2 M phosphate buffer, pH 7.5) in the absence of H2O2 (trace 1) and the spectral change of HRP at 3 min after the addition of H2O2 at 10 μM final concentration (trace 2). B: Traces illustrate the spectrum of HRP in the absence of H2O2 (trace 1) and in the presence of H2O2 at 8 mM final concentration (traces 2–8). In the presence of a low concentration of H2O2, there was no further significant decrease of the Soret band after 3 min of incubation, so only the trace at 3 min after incubation was presented in A. In the presence of 8 mM H2O2 in B, the Soret peak decreased continuously and traces 2–8 represented the spectral changes of HRP at each 3-min interval after H2O2 addition.
Effect of Reducing Agents on the TDO Spectrum
Treatment of TDO with ascorbate (1–10 mM) resulted in a 7–10% decrease of its Soret peak, but no noticeable shift of the TDO spectrum was observed (Fig. 8A). The Soret peak decreased about 20 and 50% in the presence of 0.2 and 0.5 mM sodium hydrosulfite, respectively (Fig. 8B). In the presence of 0.2 mM sodium hydrosulfite, there was no noticeable change of the λmax of the Soret band and its limit at the longer wavelength side coincided with that of untreated enzymes. Furthermore, the edge of its Soret band on the short wavelength side shifted slightly towards longer wavelengths (Fig. 8B). These changes were similar to those observed for TDO in the presence of low concentrations of either KO2 or H2O2. With sodium hydrosulfite at 0.5 mM, there was no apparent shift of the λmax (405 nm) of the Soret band, but the base of its Soret band on the longer wavelength side extended beyond the boundary of the untreated enzyme (Fig. 8B, curve 3). A further increase in sodium hydrosulfite to 1.0 mM shifted the λmax of the Soret band from 405 to 432 nm (Fig. 8B, curve 4). Because both ascorbate and sodium hydrosulfite at their optimum TDO stimulating concentrations (around 1 mM for ascorbate and 0.1 mM for sodium hydrosulfite, see Fig. 5) decreased the 405-nm Soret band without changing its spectrum (Fig. 8), the spectra of the active and inactive forms of TDO appeared essentially the same in the visible region. In the presence of 1 mM sodium hydrosulfite, TDO showed no activity to tryptophan, suggesting that the form with a λmax at 432 nm is not the active form of the enzyme.
Fig. 8.

Spectral changes of TDO in the presence of ascorbate (A) and sodium hydrosulfite (B). The baseline was adjusted as described in Figure 6. Traces in A illustrate the spectrum of TDO in the absence of ascorbate (trace 1) and the spectral changes of TDO in the presence of 1 (trace 2), 5 (trace 3), and 10 mM of ascorbate (trace 4). Traces in B show the spectrum of TDO in the absence of sodium hydrosulfite (trace 1) and the spectral changes of TDO at 3.0 min after addition of 0.2 (trace 2), 0.5 (trace 3), and 1.0 mM of sodium hydrosulfite (trace 4).
Spectral Changes of TDO in the Presence of Both Tryptophan and Ascorbate
After ascorbate was mixed with the TDO and tryptophan mixture, a small decrease of the 405-nm Soret band and a slight shift of the edge of the short wavelength side of the Soret band to longer wavelengths were observed, but there was no noticeable change in its λmax (Fig. 9A). In addition, a rapid accumulation of NFK, indicated by the detection of the 321-nm peak, also was observed after ascorbate addition (Fig. 8). As incubation proceeded, the base of the Soret peak on the longer wavelength side was extended beyond the borderline around 418 nm, leading to the formation of a shoulder also at the right side of the Soret peak (Fig. 9A). When catalase and superoxide dismutase were incorporated into the TDO, tryptophan, and ascorbate reaction mixtures, the appearance of the right side shoulder was delayed substantially and the dimension of the right side shoulder also was smaller than that observed in the reaction mixture without catalase and superoxide dismutase (see Fig. 9A, B). After a number of repeats of the same experiment, it was found that the time when the right side shoulder of the Soret peak was observed closely corresponded to the time when TDO was no longer active in the reaction mixtures (see Fig. 4A as reference). Therefore, the formation of the right side shoulder of the Soret peak signals the loss of enzyme activity for TDO.
Fig. 9.

Spectral changes of TDO in the presence of both tryptophan and ascorbate in the absence or presence of catalase and superoxide dismutase. A: The baseline was zeroed using 5 mM tryptophan in 0.2 M phosphate. B: The baseline was zeroed using 5 mM tryptophan in the presence of 500 U of both superoxide dismutase and catalase in a 0.2 M phosphate buffer. Traces in A illustrate the spectrum of TDO right after its mixing into tryptophan solution (trace 1) and the spectral changes of TDO at 3 min (trace 2) and 6 min (trace 3) after addition of 5 mM ascorbate into the same reaction mixture. Traces in B show the spectrum of TDO right after its mixing into tryptophan, superoxide dismutase, and catalase mixture (trace 1) and the spectral changes of TDO at 3 (trace 2), 6 (trace 3), 9 (trace 4), and 12 (trace 5) min after the addition of 5 mM ascorbate into the same reaction mixture.
DISCUSSION
The results of a number of experiments have been interpreted to indicate that TDO is present as an inactive form with its prosthetic group in the heme-ferric form, and that for it to oxidize tryptophan, its heme-Fe3+ form must be reduced to the heme-Fe2+; tryptophan functions as an allosteric inducer for TDO; H2O2 and reducing agents can activate the enzyme; and formation of active TDO is accompanied by a visible spectral shift of its Soret peak towards longer wavelengths (Taniguchi et al., 1979; Sono, 1989; Hitchcock and Katz, 1988). Data obtained in this study support some of these conclusions, but also raise some fundamental questions about the interpretations of the phenomena observed during TDO-mediated tryptophan oxidation. Our study dealing with the effects of tryptophan, , H2O2, and reducing agents on TDO activity and the influences of these species on TDO spectral characteristics provides some details towards a more comprehensive understanding of the biochemical processes/mechanisms leading to the activation/inactivation of the enzyme. We show that and H2O2 are involved in TDO activation as well as its inactivation, and that the rate of tryptophan oxidation at a particular time during a reaction in vitro is dependent on the proportion of the active form to the inactive form(s) of the enzyme. This latter seems to be a dynamic process closely related to the applied reaction conditions; the active form of the enzyme has essentially the same spectral characteristics in the visible region as the inactive form; and the form with its λmax at the Soret peak shifted to longer wavelengths under some specific conditions represents an inactive or inactivated form of TDO.
Production of NFK was minimal during the first few minutes after TDO was mixed with a tryptophan solution, but as incubation proceeded, the rate of tryptophan oxidation gradually increased, and after the lag period the rate was linear. Because TDO gradually became active following the addition of tryptophan, this substrate has been considered to be the allosteric inducer of the enzyme (Schutz et al., 1972; Koike el al., 1969; Kobayashi et al., 1989). Binding of substrate may lead to some structural change of the enzyme, especially at its catalytic center, but our data do not support a direct role of tryptophan in TDO activation through inducing allosteric induction. We interpret our results to indicate that the progressive activation of TDO following tryptophan addition is due to the production of and H2O2 from tryptophan. This interpretation is supported by the following factors: the decrease in the lag period with pre-incubated tryptophan solution as the substrate preparation or with freshly-prepared tryptophan in the presence of low concentrations of either or H2O2; the diminishing of TDO activation in pre-incubated tryptophan solution after being treated with catalase and peroxidase; and the prevention of TDO activation in freshly prepared tryptophan solution with both superoxide dismutase and catalase added at the beginning of the reaction. In the TDO and tryptophan reaction mixture, the following reactions likely proceed. Because the oxidation/reduction process is driven not only by the redox potentials of the oxidant and reductant, but also by their concentrations, the following reactions (except 2 and 5) should be reversible. Apparently, there is a complicated equilibrium process depending upon the redox potentials of O2, , H2O2, TDO-heme-Fe2+, and TDO-heme-Fe3+ and their concentrations in the reaction mixture.
It also is apparent that relatively high concentrations of and H2O2 inhibit or inactivate TDO. Conceivably, oxidation of heme-Fe2+ to heme-Fe3+ by or H2O2 may be more feasible than the reduction of heme-Fe3+ to heme-Fe2+ by these reactive oxygen species even at relatively low concentrations. However, because incorporation of low concentrations of or H2O2 results in a rapid accumulation of NFK in the TDO and tryptophan reaction mixture (i.e., activation of TDO by or H2O2 becomes detectable), the role of or H2O2 in TDO activation has been taken for granted. TDO showed essentially no activity with exogenous or H2O2 at concentrations ≥0.2 mM. Inability to oxidize tryptophan by TDO in the presence of relatively high concentrations of or H2O2 could not be explained simply by an or H2O2-mediated equilibrium process of heme-Fe2+ and heme-Fe3+, because if or H2O2 affected only the equilibrium between the active form (TDO-heme-Fe2+) and the inactive form (TDO-heme-Fe3+) of the enzyme, a considerable portion of TDO-heme-Fe3+ should have been reduced to its active form (TDO-heme-Fe2+) in the presence of relatively high concentrations of or H2O2. As a result, a substantial amount of NFK would have been produced in the reaction mixture. It is clear that high concentrations (>0.2 mM) of or H2O2, instead of activating the enzyme, effectively inhibit or inactivate TDO. Spectral analysis of TDO indicates that the heme-Fe3+ group may be oxidized further in the presence of relatively high concentrations of and H2O2. Although the mechanism of TDO activation involves reduction of its heme-Fe3+ group to its heme-Fe2+ group and low concentrations of H2O2 (2–10 μM) favor the TDO activation pathway, it seems apparent that relatively high concentrations of H2O2 drive the heme-Fe3+ towards further oxidation because the spectral change of TDO resembles that of HRP in the presence of relatively high concentrations of H2O2. However, compared to HRP, TDO is highly sensitive to H2O2 and appears to be rapidly inactivated by H2O2 at a concentration >0.2 mM, regardless the presence or absence of tryptophan. The effects of and H2O2 in TDO activation and inhibition/inactivation suggest that during TDO-mediated tryptophan oxidation, the relative amount of inactive form, active form, and inactivated form of TDO must be in a dynamic process, depending upon the reaction conditions.
Ascorbate has been used extensively in studies dealing with the activation of TDO. However, the overall effects of ascorbate on TDO-catalyzed tryptophan oxidation deserve some careful reexamination. It has generally been considered that ascorbate could not activate TDO in the absence of tryptophan (Koike el al., 1969; Kobayashi et al., 1989). This consideration was based primarily on the spectral changes of ascorbate-treated TDO in the absence or presence of tryptophan. Addition of ascorbate to the TDO solution did not lead to any apparent spectral change even at fairly high concentrations, but in the presence of both ascorbate and tryptophan, a shoulder at the right side of the Soret peak was observed a few minutes after incubation (compare Figs. 7A and 8A). These results, in conjunction with a significant reduction of the lag period and rapid accumulation of NFK seen in the TDO and tryptophan reaction mixtures in the presence of ascorbate (Fig. 4A), could easily lead to a conclusion that the spectral change or the formation of the right side shoulder of the TDO Soret peak was due to the formation of the active form of TDO. This spectral change has since been considered a key indicator for the formation of the active form (heme-Fe2+) of TDO and IDO (Tanaka and Knox, 1959; Schutz et al., 1972; Ishimura et al., 1970; Schutz and Feigelson, 1972; Matsumura et al., 1984).
Through careful comparisons of the results obtained in this study, however, we come up with some different conclusions about the roles of ascorbate in TDO activation/inactivation and some different interpretations about the spectral change of the TDO Soret peak. Our data indicate that ascorbate in the TDO and tryptophan reaction mixtures also promotes the production of and H2O2, which contribute in part to the overall effect of TDO activation by ascorbate. However, an accelerated accumulation of and H2O2 due to presence of ascorbate in turn leads to more TDO molecules being inactivated, thereby decreasing the linear period of the enzymatic reaction. These conclusions were based on the apparent effects of ascorbate in reducing the initial lag period, but decreasing its overall linear period during TDO-catalyzed tryptophan oxidation, especially at relatively high concentrations of ascorbate (Fig. 4, trace 4), and the effect of superoxide dismutase and catalase in increasing the linear period of the TDO-mediated tryptophan oxidation in the presence of ascorbate (see Fig. 4, trace 5). Other than the reactions proposed in the TDO and tryptophan reaction mixtures in the absence of reducing agents, the following additional reactions might proceed in the presence of ascorbate.
Formation of a small shoulder at the right site of the TDO Soret peak was not observed after TDO was treated with ascorbate alone (Fig. 8A), but easily observed several minutes after both tryptophan and ascorbate were mixed with TDO (Fig. 9A). Such a spectral change was attributed initially by many previous reports to result from the accumulation of the active form (heme-Fe2+) of TDO (Koike el al., 1969; Kobayashi et al., 1989). However, it was subsequently noticed that after ascorbate was mixed with TDO and tryptophan reaction mixtures, there was always a rapid accumulation of NFK during the first few minutes, but other than a slight decrease of the TDO Soret peak, no apparent spectral shift of the Soret peak was observed. In contrast, when NFK was no longer produced in the mixture, the right side shoulder of the Soret peak was invariably observed. Ascorbate promoted the production of and/or H2O2 in TDO and tryptophan reaction mixtures and over-accumulation of these reactive oxygen species might inactivate TDO. Coincidentally, addition of superoxide dismutase and catalase extended the linear period of NFK production in the reaction mixture (Fig. 4A) and delayed the formation of the longer-wavelength shoulder of the Soret peak (compare Fig. 9A and B). Because the occurrence of this shift is accompanied by the loss of TDO activity, the observed spectral change of TDO likely represents the formation of inactivated TDO, due presumably to the accumulation of and/or H2O2. The virtually complete inhibition of TDO activity accompanied with the spectral shift of TDO Soret peak towards a longer wavelength in the presence of relatively high concentrations of H2O2 (>0.2 mM) support this conclusion.
Our results provide a reasonable basis to suggest that the spectral shift of TDO Soret peak towards a longer wavelength is due to formation of the inactivated TDO, which leads to an important question as to what is the spectral characteristic of active TDO. TDO displays maximum activity in the presence of relatively low concentrations of , H2O2, and sodium hydrosulfite; therefore, a portion of TDO should be present in its active form under these conditions. Otherwise, the rapid accumulation of NFK in the reaction mixture is difficult to explain. The similarity of the spectrum of TDO, treated with optimum stimulative concentrations of , H2O2, and sodium hydrosulfite, to that of untreated native enzyme provides the basis to suggest that the spectrum of the active form is similar to the inactive form. It is also possible that although TDO shows high activity under the optimum stimulative conditions, there is only a small portion of TDO present in its active form, which does not lead to apparent spectral change.
Acknowledgments
The authors thank Lynn Olson for help in preparing the manuscript. This work was supported by a grant from NIH, number AI 44399.
Abbreviations used
- NFK
N-formylkynurenine
- r-TDO
recombinant TDO
- TDO
tryptophan 2,3-dioxygenase
Footnotes
Contract grant sponsor: National Institutes of Health; Contract grant number: AI 44399.
LITERATURE CITED
- Auerbach VH, Knox WE. L-Kynurenine and N1-formyl-L-kynurenine. Methods Enzymol. 1957;3:620–623. [Google Scholar]
- Brady FO, Forman HJ, Feigelson P. The role of superoxide and hydroperoxide in the reductive activation of tryptophan-2,3-dioxygenase. J Biol Chem. 1971;246:7119–7124. [PubMed] [Google Scholar]
- Dunford HB. In: Peroxidases in chemistry and biology. Everse J, Everse KE, Grisham MB, editors. Vol. 2. Boca Raton: CRC Press; 1991. pp. 1–24. [Google Scholar]
- Han Q, Li J. Comparative characterization of Aedes 3-hydroxykynurenine transaminase/alanine glyoxylate transaminase and Drosophila serine pyruvate aminotransferase. FEBS Lett. 2002;527:199–204. doi: 10.1016/s0014-5793(02)03229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaishi O, Nozaki M. Nature and mechanisms of oxygenases. Science. 1969;164:389–396. doi: 10.1126/science.164.3878.389. [DOI] [PubMed] [Google Scholar]
- Hitchcock MJ, Katz E. Purification and characterization of tryptophan dioxygenase from Streptomyces parvulus. Arch Biochem Biophys. 1988;261:148–160. doi: 10.1016/0003-9861(88)90113-0. [DOI] [PubMed] [Google Scholar]
- Ishimura Y, Nozaki M, Hayaishi O. The oxygenated form of L-tryptophan 2,3-dioxygenase as reaction intermediate. J Biol Chem. 1970;245:3593–3602. [PubMed] [Google Scholar]
- Li J, Beerntsen BT, James AA. Oxidation of 3-hydroxykynurenine to produce xanthommatin for eye pigmentation: a major branch pathway of tryptophan catabolism during pupal development in the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1999;29:329–338. doi: 10.1016/s0965-1748(99)00007-7. [DOI] [PubMed] [Google Scholar]
- Littlejohn TK, Takikawa O, Skylas D, Jamie JF, Walker MJ, Truscott RJ. Expression and purification of recombinant human indoleamine 2, 3-dioxygenase. Protein Expr Purif. 2000;19:22–29. doi: 10.1006/prep.2000.1214. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hayashi K, Sono M. Effects of tryptophan and pH on the kinetics of superoxide radical binding to indoleamine 2,3-dioxygenase studied by pulse radiolysis. J Biol Chem. 1989;264:15280–15283. [PubMed] [Google Scholar]
- Koike K, Poillon WN, Feigelson P. Influence of allosteric effector substances on the structure and catalytic activity of tryptophan oxygenase. J Biol Chem. 1969;244:3457–3462. [PubMed] [Google Scholar]
- Matsumura M, Osada K, Aiba S. L-tryptophan 2,3-dioxygenase of a moderate thermophile, Bacillus brevis. Purification, properties and a substrate-mediated stabilization of the quaternary structure. Biochim Biophys Acta. 1984;786:9–17. doi: 10.1016/0167-4838(84)90147-x. [DOI] [PubMed] [Google Scholar]
- Schutz G, Feigelson P. Purification and properties of rat liver tryptophan oxygenase. J Biol Chem. 1972;247:5327–5332. [PubMed] [Google Scholar]
- Schutz G, Chow E, Feigelson P. Regulatory properties of hepatic tryptophan oxygenase. J Biol Chem. 1972;247:5333–5337. [PubMed] [Google Scholar]
- Sono M. The roles of superoxide anion and methylene blue in the reductive activation of indoleamine 2,3-dioxygenase by ascorbic acid or by xanthine oxidase-hypoxanthine. J Biol Chem. 1989;264:1616–1622. [PubMed] [Google Scholar]
- Tanaka T, Knox WE. The nature and mechanism of the tryptophan pyrrolase (peroxidase-oxidase) reaction of Pseudomonas and of rat liver. J Biol Chem. 1959;234:1162–1170. [PubMed] [Google Scholar]
- Taniguchi T, Sono M, Hirata F, Hayaishi O, Tamura M, Hayashi K, Iizuka T, Ishimura Y. Indoleamine 2,3-dioxygenase. Kinetic studies on the binding of superoxide anion and molecular oxygen to enzyme. J Biol Chem. 1979;254:3288–3294. [PubMed] [Google Scholar]


