SUMMARY
Several hypotheses have been proposed to explain how antiangiogenic drugs enhance the treatment efficacy of cytotoxic chemotherapy including impairing the ability of chemotherapy-responsive tumors to regrow after therapy. With respect to the latter, we show that certain chemotherapy drugs, e.g. paclitaxel, can rapidly induce pro-angiogenic bone marrow derived circulating endothelial cell (CEP) mobilization, and subsequent tumor homing, whereas others, e.g. gemcitabine, did not. Acute CEP mobilization was mediated, at least in part, by systemic induction of SDF-1α and could be prevented by various procedures such as treatment with anti-VEGFR2 blocking antibodies or by paclitaxel treatment in CEP-deficient Id-mutant mice, both of which resulted in enhanced anti-tumor effects mediated by paclitaxel, but not gemcitabine.
SIGNIFICANCE
Chemotherapy remains the most commonly employed form of systemic cancer treatment. Although partial or complete shrinkage of tumor mass is frequently induced in chemotherapy-responsive tumors, the survival benefits of such responses can be compromised by rapid regrowth of the drug-treated tumors. Our results illustrate how rapidly activated systemic host processes involving induction of certain cytokines and mobilization of CEPs from the bone marrow, can contribute to recovery of drug treated tumors, and moreover, how this can be blunted by combination treatment with a VEGF pathway targeted antiangiogenic drug. The results also implicate that CXCR4/SDF-1α in therapy-induced CEP responses mediated by certain chemotherapy drugs, and hence as a potential target for improving their anti-tumor effectiveness.
INTRODUCTION
A number of phase III clinical trials involving bevacizumab, the humanized antibody against VEGF, in combination with chemotherapy administered at the maximum tolerated dose (MTD) have shown median overall survival (OS) or progression free survival (PFS) benefits in metastatic breast, colorectal and small cell lung cancers (Hurwitz et al., 2004; Sandler et al., 2006; Miller et al., 2007). These trials include the use of 5-fluorouracil and irinotecan in first line colorectal cancer (Hurwitz et al., 2004), paclitaxel in first line metastatic breast cancer (Miller et al., 2007), and paclitaxel plus carboplatin in the first line treatment of non small cell lung cancer (Sandler et al., 2006). Despite these successes, some other phase III trials utilizing bevacizumab co-administered with conventional chemotherapy failed to show OS or PFS benefits, e.g. when administered with gemcitabine for the treatment of pancreatic cancer (Burris, III and Rocha-Lima, 2008). Factors such as type of tumor, stage, prior treatment, bevacizumab drug dose, pharmacogenomic status, or the nature of the chemotherapy drug combined with bevacizumab could all be factors in explaining whether or not, and to what extent clinical benefit is attained. This serves to emphasize how little is known about the mechanism(s) of action of bevacizumab, and possibly other antiangiogenic agents, especially when co-administered with chemotherapy.
Several hypotheses to explain how antiangiogenic drugs act as chemosensitizing agents have been proposed. One of them – the vessel normalization hypothesis - is based on the observation that enhanced tumor vessel leakiness produces elevated interstitial fluid pressures in tumors which can impede the delivery and diffusion of certain anti-cancer drugs. In addition the abnormal tumor vasculature is associated with reduced blood flow and perfusion, another function impending chemotherapy delivery, and also causing tumor hypoxia, which can cause resistance to chemotherapy and radiation. Treatment with certain antiangiogenic drugs can transiently reverse these abnormalities and enhanced chemotherapy (or radiation therapy) provided it is administered during the ‘normalization window’ (Jain, 2005; Winkler et al., 2004). An alternative or additional mechanism is related to the property of rapid tumor cell repopulation that can take place between successive MTD chemotherapy treatments. Addition of an antiangiogenic drug treatment during the chemotherapy drug-free break period should slow down tumor regrowth and thus increase the degree and durability of the tumor response (Kerbel, 2006; Hudis, 2005). A third hypothesis which essentially provides a mechanistic explanation to the second hypothesis, is based on our prior preclinical observations regarding the induction of CEP mobilization after treatment with a cytotoxic agent. We have demonstrated that lymphoma-bearing NOD/SCID mice treated with intensive 6-day cycles of MTD cyclophosphamide, separated by two week breaks, exhibited substantial increases in the viability and mobilization of CEPs post treatment after showing an initial decline during the cycles of therapy, a phenomenon which in some respect mimics the rebound of neutrophil counts after treatment with myelo-ablative chemotherapy (Bertolini et al., 2003). We suggested that such a mobilization effect in CEP levels may contribute to and facilitate tumor cell repopulation during the subsequent drug free break that is necessary to allow recovery from the toxic side effects of such therapy (Bertolini et al., 2003). This could occur by intrinsically promoting tumor vasculogenesis/angiogenesis, but also by suppressing the ability of chemotherapy to cause a local antiangiogenic effect in tumors by targeting the endothelial cells of the growing angiogenic neovasculature (Kerbel, 2006; Browder et al., 2000).
Chemotherapy-induced CEP mobilization is observed in patients treated with anthracycline and/or taxane-based neoadjuvant chemotherapy, i.e. increases in CEP levels observed at the end of the first and second cycles of chemotherapy treatment (Furstenberger et al., 2006). Furthermore, a surprisingly robust elevation in CEP levels has also been observed within hours of treatment with microtubule inhibiting cytotoxic-like vascular disrupting agents (VDAs) in mice (Shaked et al., 2006). We also found that CEPs and perhaps other bone marrow (pro-angiogenic) cells mobilized by VDA treatment, home to and colonize the remaining viable tumor rim commonly observed after treatment with a VDA. When an antiangiogenic drug, i.e. DC101, a VEGFR2 blocking antibody, was administered 24 hours prior to the VDA, the VDA-induced CEP surge was largely blocked, and the residual viable tumor rim was significantly suppressed, which was followed by increased anti-tumor efficacy (Shaked et al., 2006). In addition, preliminary evidence for the induction of CEPs after VDA treatment has been reported recently in phase I clinical trials using the vascular disrupting agents ZD6126 or AVE8062 (Beerepoot et al., 2006; Farace et al., 2007). Overall, these findings suggest that CEPs can contribute to some and perhaps even much of the rapid re-growth of tumors after treatment with a VDA.
VDAs have a unique mechanism of action as a result of targeting the abnormal vasculature of tumors, causing massive tumor hypoxia and inducing tumoral necrosis. Such affects could help trigger the acute CEP mobilization and tumor homing response. We therefore decided to analyze the impact of conventional chemotherapy drugs, which lack such acute and potent vascular disruptive effects, to determine if such drugs – still the main stay of systemic therapy for metastatic disease – nevertheless have similar inductive effects on CEP mobilization, tumor homing, and hence assisting the ability of tumors to recover from the exposure to such agents. We also decided to assess whether different chemotherapeutic drugs have variable abilities in inducing CEP mobilization and whether targeted antiangiogenic drugs or other agents can block chemotherapy induced CEP responses and hence amplify their effectiveness.
RESULTS
Acute induction in CEP levels in peripheral blood of mice treated with certain chemotherapy drugs administered near or at the MTD
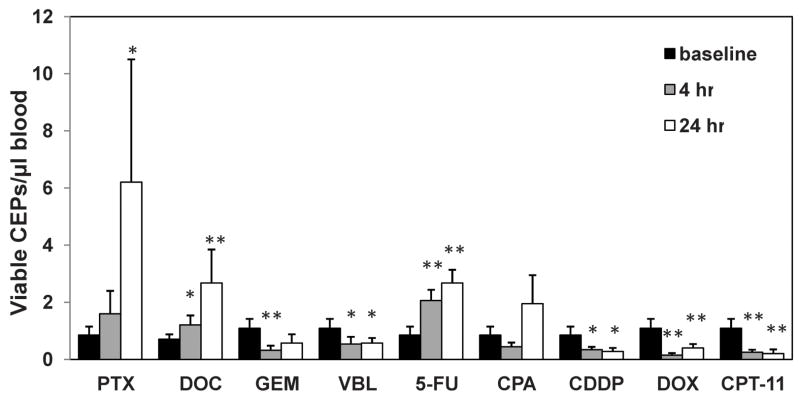
To study the impact of chemotherapy on tumor growth and angiogenesis mediated by bone marrow derived CEPs, we asked, similar to our previous observation with VDAs, whether chemotherapy administered at the MTD can induce a rapid induction in levels of viable CEPs. To this end, non-tumor bearing BALB/c mice were treated with a number of different chemotherapy drugs administered near or at the MTD (in doses indicated in supplemental Table S1), and blood was drawn from the retro-orbital sinus 4 and 24 hours later. CEP levels were evaluated using flow cytometry methodology, as previously described (Shaked et al., 2005a; Bertolini et al., 2003). The results in Figure 1 show that only certain drugs, e.g. most notably paclitaxel, 5-FU and docetaxel were found to cause acute elevations in viable CEP levels within 24 hours of a single bolus injection, whereas others failed to do this e.g., gemcitabine, cisplatinum, and doxorubicin.
Figure 1. Levels of viable CEPs in non-tumor bearing BALB/c mice treated with a variety of chemotherapy drugs near or at the MTD.
8–12 week old BALB/c mice (n=4–5 mice/group) were treated with 30mg/kg paclitaxel (PTX), 120mg/kg gemcitabine, (GEM), 40mg/kg docetaxel (DOC), 11mg/kg vinblastine (VBL), 100mg/kg 5 fluorouracil (5-FU), 250mg/kg cyclophosphamide (CPA), 6mg/kg cisplatinum (CDDP), 12mg/kg doxorubicin (DOX) or 100mg/kg irinotecan (CPT-11) as also indicated in Table S1. Four and 24 hours later mice were bled via retro-orbital sinus for the evaluation of viable CEPs by four color flow cytometry. *, 0.05>p>0.01; **, p<0.01.
The administration of an antiangiogenic drug prior to chemotherapy-induced CEP spike, blocks the rapid elevation in CEP levels
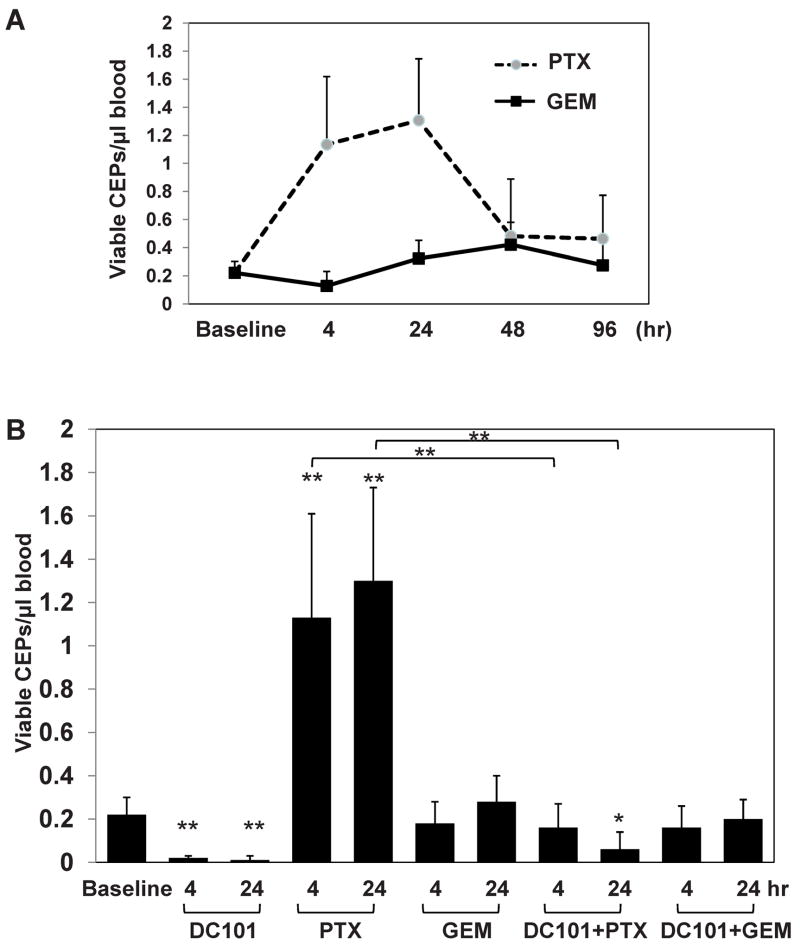
For subsequent studies we focused on experiments using two distinct chemotherapy drugs given at MTDs, i.e. 50mg/kg paclitaxel which induced rapid and marked elevations in CEP levels and 500mg/kg gemcitabine which did not when administered to C57Bl/6 mice. We first monitored levels of CEPs for up to 96 hours after chemotherapy drug injection in order to rule out delayed alterations in CEP levels. To do this, non tumor bearing C57Bl/6 mice were treated with either paclitaxel or gemcitabine at the indicated MTDs. Blood was drawn by retro-orbital sinus at several time points and processed for evaluation of viable CEPs. The results in Figure 2A demonstrate that levels of CEPs in paclitaxel group were rapidly increased within 24 hours and subsequently returned to the baseline levels by 48 hours. In contrast, levels of CEPs in gemcitabine treated group were maintained at baseline levels for the first 96 hours. Representative flow cytometry plots 4 hours after treatment are presented in Supplemental Figure S1A. Next, we asked whether similar to VDAs, the administration of DC101, an antiangiogenic anti-mouse VEGFR-2 monoclonal antibody (Prewett et al., 1999) 24 hours prior to the chemotherapy can block the rapid induction in CEP levels. The results in Figure 2B show that when DC101 was injected 24 hours before either chemotherapy, this resulted in a diminished CEP spike in the paclitaxel treated mice. No significant differences in CEP levels were observed in mice treated with the combination of DC101 and gemcitabine. Similar results were obtained when G6-31, a monoclonal neutralizing antibody to both mouse and human VEGF (Liang et al., 2006), were used in combination with paclitaxel or gemcitabine (Supplemental Figure S1B).
Figure 2. Evaluation of CEPs in mice treated with either paclitaxel or gemcitabine in combination with DC101.
(A) 8–10 week old non-tumor bearing C57Bl/6 mice (n=4 mice/group) were treated with 50mg/kg paclitaxel (PTX), or 500mg/kg gemcitabine (GEM). Blood was drawn from the retro-orbital sinus at time points indicated in the figure, and processed for the evaluation of viable CEPs using flow cytometry. (B) In a separate experiment, mice were treated with paclitaxel (PTX) or gemcitabine (GEM) as described in (A), with or without DC101 given 24 hours prior to chemotherapy treatment. Blood was drawn via retro-orbital sinus and processed for the evaluation of viable CEPs using flow cytometry. *, 0.05>p>0.01; **, p<0.01.
The rapid elevation in CEPs after chemotherapy treatment resulted in bone marrow derived cell colonization of the treated tumors
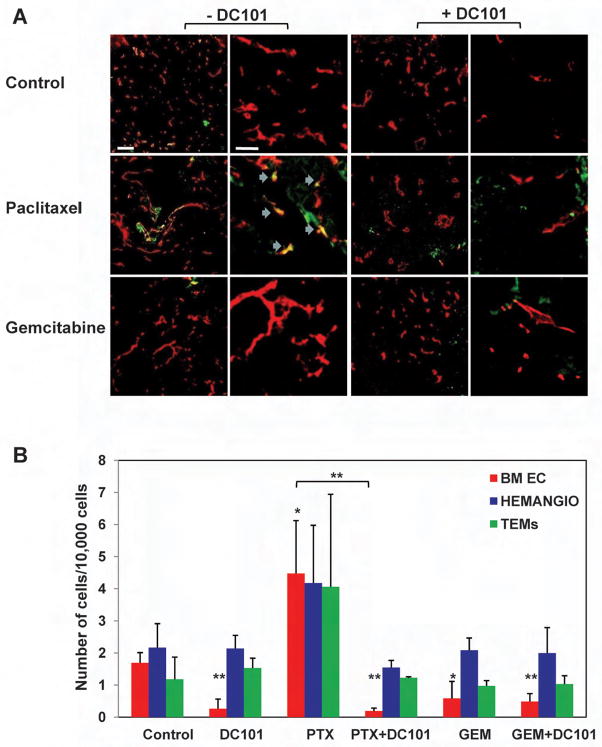
A growing body of evidence suggests that a number of different bone marrow derived cell types promote tumor angiogenesis and growth by various mechanisms. For example, hemangiocytes or recruited bone marrow circulating cells (RBCCs), and tie-2 expressing monocytes (TEMs) have recently been shown to reside at perivascular sites hence promote angiogenesis in a paracrine manner (Jin et al., 2006; De Palma et al., 2005; Grunewald et al., 2006; Udagawa et al., 2006; Kerbel, 2008). In order to track bone marrow cell homing and retention in treated tumors, experiments were undertaken using GFP+ bone marrow cells obtained from C57Bl/UBI/GFP mice which were transplanted into lethally irradiated C57Bl/6 mice (Shaked et al., 2006). Four weeks later, mice were used as recipients for an injection of Lewis-Lung carcinoma (LLC) cells. When tumors reached 500mm3, treatment with either bolus injected MTD paclitaxel or MTD gemcitabine was initiated. Three days later, tumors were removed for the evaluation of GFP+ bone marrow cell colonization and incorporation into the tumor vasculature using both confocal microscopy and flow cytometry techniques, as described in Experimental Procedures. We detected numerous bone marrow derived GFP+ cells in tumors that had been treated with paclitaxel in clear contrast to gemcitabine treated or untreated control tumors. When DC101 was administered 24 hours prior to chemotherapy, a substantial reduction in the number of GFP+ bone marrow cells was observed in paclitaxel treated and untreated tumors. No differences in GFP+ cell numbers were observed in gemcitabine treated tumors (Figure 3A and Supplemental Figure S2A). Of note, the antiangiogenic effect of DC101 on local angiogenesis is insignificant within the first 3 days as previously demonstrated (Franco et al., 2006).
Figure 3. Homing and colonization of GFP+ bone marrow cells in LLC tumors after treatment with paclitaxel or gemcitabine in combination with DC101.
C57Bl/6 mice (n=5 mice/group) that were previously lethally irradiated and subsequently transplanted with 107 GFP+ bone marrow cells obtained from UBI/GFP/C57Bl/6 mice, were used as recipients for a subcutaneous injection of LLC cells which were allowed to grow until they reached 500mm3, at which point treatment with paclitaxel (PTX), gemcitabine (GEM) with or without upfront treatment with DC101 was initiated. Three days later, tumors were removed and sections were prepared for the assessment of (A) GFP+ cells (in green) colonization of the tumors, CD31 staining (in red) as an endothelial cell marker, and blue arrows for colocalization of CD31 and GFP+ cells in the paclitaxel treated group (scale bars left 20μm, right 50μm), or (B) the number of bone marrow derived GFP+ endothelial cells (BM EC), hemangiocytes (Hemangio), and TEMs colonizing the tumor using tumors prepared as a single cell suspension and evaluated by flow cytometry.
Next, to further characterize some of the bone marrow cell types colonizing the tumors, tumors from all groups (n=5/group) were prepared as single cell suspension and subsequently stained for the evaluation of bone marrow derived endothelial cells, TEMs, and hemangiocytes using flow cytometry, as described in Experimental Procedures. The results in Figure 3B show significant increases in bone marrow derived endothelial cells as well as increases in hemangiocytes and TEMs (although the latter two did not reach significance) in the paclitaxel treated group. The administration of DC101 prior to chemotherapy treatment inhibited bone marrow cell colonization of tumors. Overall, these results suggest that paclitaxel treatment induces bone marrow derived cell mobilization and colonization of tumors and hence may promote tumor cell repopulation and angiogenesis by various mechanisms.
Enhanced anti-tumor and antiangiogenic activities in mice treated with paclitaxel plus DC101
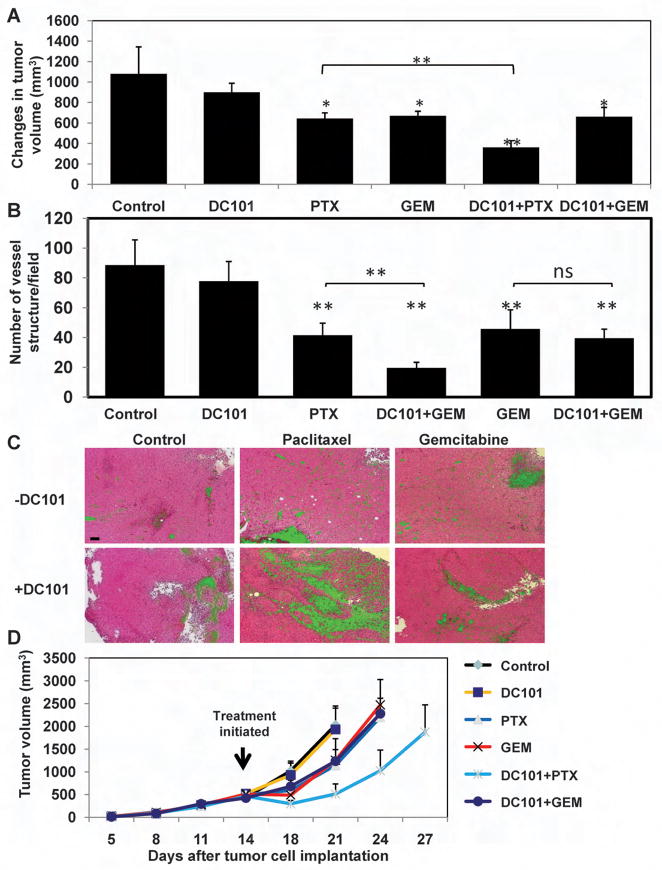
To further characterize the anti-tumor and anti-angiogenic effects of the combination of paclitaxel plus DC101 in comparison to gemcitabine plus DC101, LLC tumors (n≥5 tumors/group) were evaluated for volume, necrosis, and microvessel density, 3 days after either paclitaxel or gemcitabine treatment with or without prior administration of DC101. The results in Figure 4A-C and Supplemental Figure S2B demonstrate significant reductions in tumor volume and microvessel density, and increases in overall tumor necrosis in tumors treated with the combination of DC101 and paclitaxel in comparison to treatment with pacliatxel alone. In contrast, no significant differences were observed between gemcitabine treated and DC101/gemcitabine treated tumors. Also noteworthy is the observation that a single dose of paclitaxel reduced microvessel density - indicating damage to the tumor vasculature, where a single injection of DC101 did not cause a drop in microvessel density (Figure 4B).
Figure 4. Assessment of LLC tumor volume, microvessel density, necrosis, and long-term tumor growth in mice treated with paclitaxel, or gemctibine, in combination with DC101.
500mm3 LLC bearing C57Bl/6 mice (n=4–5 mice/group) were treated with paclitaxel (PTX), gemcitabine (GEM) in combination with DC101 administered 24 hours prior to the chemotherapy drug. (A) tumor volumes were assessed before and three days after treatment. The changes in tumor volume are shown. Three days after treatment tumors were removed and evaluated for (B) microvessel density after CD31 staining for vessel structure. Data presented as the number of vessel structures per field (n>10 fields/tumor), or (C) necrosis (in green) on H&E staining (scale bar, 100μm)(see Supplemental Figure S2B for summary of quantitative data). (D) In a separate experiment, LLC tumors implanted in C57Bl/6 mice were allowed to reach 500mm3, at which point treatment with paclitaxel, gemcitabine (administered at the MTDs) and DC101 was initiated. Tumors were measured regularly using a caliper, and tumor growth was plotted as per number of days from tumor cell implantation. *, 0.05>p>0.01; **, p<0.01.
Next, to explore a long-term anti-tumor effect of DC101 when administered in combination with paclitaxel or gemcitabine, 5×105 LLC cells were subcutaneously implanted in the flanks of C57Bl/6 mice. When tumors reached 500 mm3, a single dose of DC101 was administered followed by paclitaxel or gemcitabine injection 24 hours later. The results in Figure 4D show that the combination of DC101 and paclitaxel resulted in a substantial anti-tumor effect manifested by a delayed tumor growth endpoint in comparison to tumors treated with gemcitabine alone, paclitaxel alone, or the combination of DC101 and gemcitabine. Comparable results for long term enhanced treatment efficacy were obtained for the combination of paclitaxel and DC101 in C57Bl/6 mice bearing B16F1 melanomas (Supplemental Figure S3A). Moreover, we did not observe enhanced treatment benefit when DC101 was administered prior to doxorubicin (which does not induce a CEP spike – see Figure 1) in C57Bl/6 mice bearing LLC (Supplemental Figure S3B). Overall, these results reinforce our hypothesis that the administration of an antiangiogenic drug just prior to a chemotherapy drug which is competent to induce a rapid CEP spike results in enhanced treatment efficacy whereas little or no enhanced anti-tumor activity is obtained when it is combined with a chemotherapy drug that does not induce such a CEP spike.
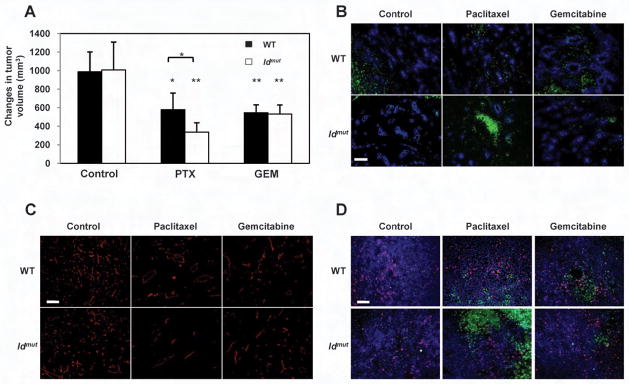
Superior anti-tumor and anti-angiogenic activities in Id mutant mice treated with paclitaxel compared to mice treated with gemcitabine
To further evaluate the treatment efficacy of paclitaxel can be enhanced in the absence of a CEP spike, we tested the anti-tumor effects of paclitaxel or gemcitabine in Id1+/−Id3−/− mutant mice and compared the treatment effects to that observed in wt controls. Id mutant mice cannot mobilize CEPs (Lyden et al., 1999), but are not deficient for other bone marrow derived pro-angiogenic cells such as TEMs, tumor associated macrophages, or tumor associated neutrophils (Ciarrocchi et al., 2007). Thus enhanced efficacy of the chemotherapeutic drug can be ascribed directly to the lack of CEP mobilization, as opposed to inhibition of other VEGF responsive bone marrow derived cells. For this approach, LLC tumors implanted in mice were allowed to reach 500mm3, at which point treatment with either drug was initiated. Of note, a 3 day tumor growth delay at the 500mm3 point (20.3 days in Id mutant mice versus 17.2 days in wt mice, post tumor implantation) was observed in tumors grown in Id mutant mice in comparison to the respective tumors grown in wt mice, in line with previous publications (Lyden et al., 1999; Shaked et al., 2006) (data not shown). Three days after treatment, tumors (n≥5 tumors/group) were measured, and then removed for the evaluation of tumor hypoxia, vessel perfusion, micovessel density, cell proliferation and apoptosis. Consistent with our hypothesis, Id loss in the host animal had no influence on gemcitabine effectiveness as no significant differences in tumor growth, perfusion, hypoxia, or microvessel density were observed. In contrast, a significant decrease in tumor volume, accompanied by increases in tumor hypoxia, and reduction in blood perfusion and microvessel density, were observed in tumors grown in the Id mutant mice treated with paclitaxel compared to the wt mice (Figure 5A-C and Supplemental Figure S4A-C). In addition, we found significant increases in tumor cell apoptosis in the paclitaxel treated tumors grown in Id mutant mice, in comparison to tumors treated in the wt mice. No significant differences in tumor cell apoptosis or proliferation were observed in tumors treated with gemcitabine grown in Id mutant versus wt mice (Figure 5D and Supplemental Figure S4D-E). Overall, these results provide further evidence for the tumor growth enhancing role that acutely mobilized bone marrow-derived cells may play with respect to those chemotherapy drugs which induce their mobilization, followed by subsequent homing to tumors. Blocking this chemotherapy-induced host reactive process resulted in increased treatment efficacy. In previous studies using VDA treatment combined with DC101, these marked short term tumor-associated differences were found to be predictive of long term anti-tumor effects including prolonged survival (Shaked et al., 2006).
Figure 5. Assessment of LLC tumor volume, hypoxia, perfusion, microvessel density, and tumor cell proliferation and apoptosis of tumors grown in Id mutant mice or their wt controls after treatment with paclitaxel, or gemctibine.
500mm3 LLC bearing Id mutant mice were treated with paclitaxel (PTX) or gemcitabine (GEM). (A) tumor volumes were assessed before and three days after treatment. The changes in tumor volume are shown. Three days after treatment tumors were removed and evaluated for (B) vessel perfusion (in blue) and hypoxia (in green) (scale bar, 50μm), (C) microvessel density (CD31 staining in red)(scale bar, 50μm), and (D) proliferation (in red) and apoptosis (in green)(scale bar, 50μm). ns, not significant; *, 0.05>p>0.01; **, p<0.01. See Supplemental Figure S4 for summary of quantitative data, respectively.
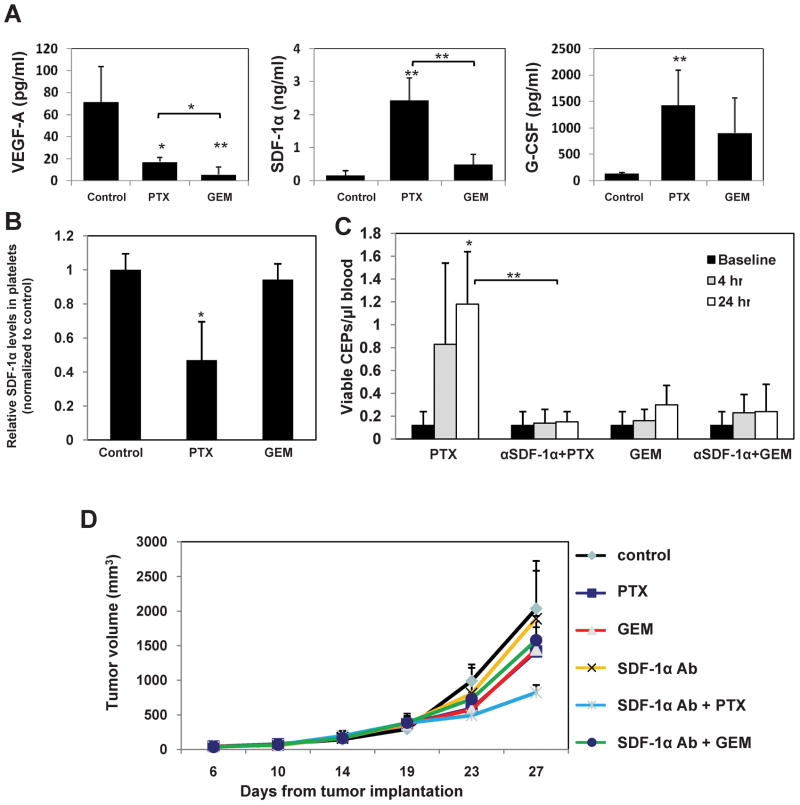
Rapid induction in SDF-1α levels may account for the acute CEP mobilization after treatment with paclitaxel
To further assess the molecular mechanisms responsible for the acute paclitaxel-induced CEP mobilization, plasma samples from non-tumor bearing C57Bl/6 mice (n=4 mice/group) were obtained 4 hours after treatment with either paclitaxel or gemcitabine administered at the MTDs, and circulating VEGF-A, SDF-1α, and G-CSF levels were evaluated, as they are all known to mobilize bone marrow derived cells including CEPs (Asahara et al., 1999; Jin et al., 2006; Powell et al., 2005). As shown in Figure 6A, both gemcitabine and paclitaxel treated mice exhibited significant increases in circulating G-CSF plasma levels and decreases in VEGF-A plasma levels, although not to the same extent. However, levels of SDF-1α were significantly induced only in the paclitaxel treated mice in comparison to untreated control or gemcitabine treated mice. No increases in SDF-1α levels were also observed in C57Bl/6 mice (n=4 mice/group) 4 hours after they were treated with MTD doxorubicin which does not induce a CEP spike (Supplemental Figure S5)
Figure 6. Circulating levels of VEGF-A, G-CSF and SDF-1α four hours after treatment with paclitaxel or gemcitabine and the impact of SDF-1α neutralizing antibody treatment on viable CEPs and tumor growth.
Non tumor bearing C57Bl/6 mice (n=4 mice/group) were treated with paclitaxel (PTX) or gemcitabine (GEM). Four hours later, mice were bled by cardiac puncture and plasma was collected. (A) Levels of murine VEGF-A, G-CSF and SDF-1α were analyzed by ELISA. (B) Analysis of SDF-1α content stored in isolated circulating platelets from C57Bl/6 mice, 4 hours after they were treated with paclitaxel or gemcitabine at MTDs. (C) Non-tumor bearing C57Bl/6 (n=4–5 mice/group) mice were treated with SDF-1α neutralizing antibodies. Twenty-four hours later, mice were treated with paclitaxel (PTX) or gemcitabine (GEM). After 4 hours, mice were bled from the retro-orbital sinus for the evaluation of viable CEPs by flow cytometry. (D) In C57Bl/6 mice, LLC tumors were allowed to growth until they reached 500mm3, at which point the mice were treated with polyclonal SDF-1α neutralizing antibodies in combination with either paclitaxel or gemcitabine. Control mice received non-specific antisera treatment. Tumors were measured regularly using a caliper, and tumor growth was plotted as per number of days from tumor cell implantation. *, 0.05>p>0.01; **, p<0.01.
Jin et.al., have recently reported that SDF-1α is stored in platelets, and thus hemangiocytes as well as other bone marrow cells expressing CXCR4 may rapidly mobilize from the bone marrow and promote angiogenesis in response to acutely induced SDF-1α secretion from circulating activated platelets (Jin et al., 2006; Avecilla et al., 2004). To test this possibility, platelets isolated from non-tumor bearing C57Bl/6 mice were incubated in-vitro for 4 hours with either 5μM paclitaxel or 50μM gemcitabine, as previously reported (Kroep et al., 1999). Subsequently, platelet cell lysates were generated, and the concentration of SDF-1α content was evaluated by ELISA. No significant differences were observed between any of the groups (data not shown). Next, since Jin et.al. (Jin et al., 2006) have reported that various cytokines may induce release of SDF-1α from platelets, we asked whether paclitaxel may indirectly promote the release of SDF-1α from platelets. To this end, non-tumor bearing C57Bl/6 mice (n=4 mice/group) were treated with either paclitaxel or gemcitabine administered at the MTDs. After 4 hours, mice were bled by cardiac puncture, and platelets were isolated as described in Experimental Procedures. Levels of SDF-1α were evaluated on platelet lysates following normalization of protein content. The results in Figure 6B show that only in mice treated with paclitaxel was there a significant reduction in SDF-1α content in platelets observed, as opposed to similar SDF-1α levels in platelets obtained from untreated or gemcitabine treated mice. Taken together, these results suggest that paclitaxel may induce CEP spike, at least in part, by the acute release of stored SDF-1α from platelets.
Neutralizing SDF-1α levels enhances the anti-tumor activity of chemotherapy-induced CEP spikes
With the aim of assessing whether SDF-1α can account for the rapid CEP mobilization observed after treatment with paclitaxel, non tumor-bearing C57Bl/6 mice were treated with neutralizing anti-SDF-1α antibodies (n=5 mice/group). Twenty-four hours later, mice were treated with either gemcitabine or paclitaxel, and 4 and 24 hours later evaluation of CEP levels was undertaken. The results in Figure 6C revealed that the SDF-1α neutralizing antibodies substantially blocked induction in CEP levels within 24 hours in the paclitaxel treated mice. No significant differences were observed in mice treated with gemcitabine. To further assess whether blocking SDF-1α may enhance paclitaxel treatment efficacy, mice bearing LLC tumors were treated with a polyclonal anti-SDF-1α neutralizing antibodies 24 hours prior to either paclitaxel or gemcitabine treatment. Control mice were treated with non-specific antisera, as previously described (Addison et al., 2000; Phillips et al., 2003). The results in Figure 6D demonstrate that only in mice treated with the combination of SDF-1α neutralizing antibodies and paclitaxel was there evidence of enhanced anti-tumor efficacy. This enhancement was not observed when the SDF-1α neutralizing antibodies were combined with gemcitabine. Overall, these results suggest that the rapid increase in SDF-1α levels accounts for the acute CEP mobilization after paclitaxel treatment, and as such, SDF-1α neutralizing antibodies can be used as a de facto antiangiogenic/anti-vasculogenic-like treatment strategy.
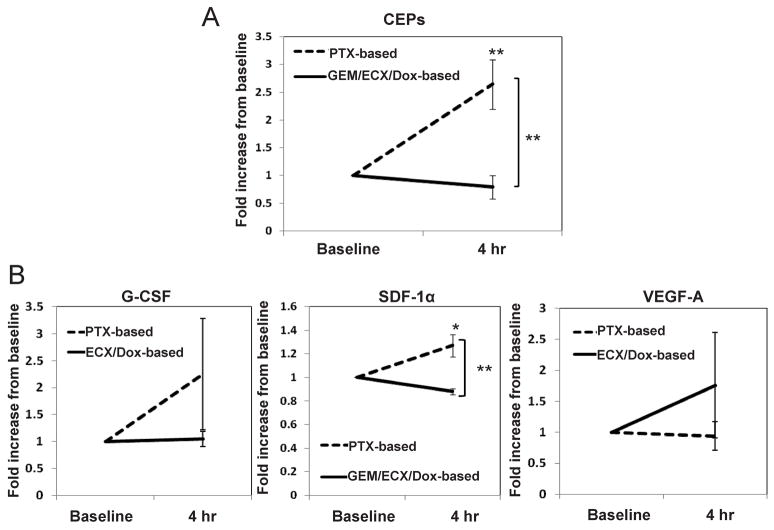
Increases in CEP and SDF-1α plasma levels induced in patients treated with paclitaxel
The preclinical results we previously obtained using VDAs, i.e., rapid elevations in CEP levels within 4 hours after drug administration, have been reproduced by a number of clinical studies (Beerepoot et al., 2006; Farace et al., 2007)(Personal Communication, Paul Nathan). The next aim, therefore, was to evaluate whether there is indication that our aforementioned preclinical results using chemotherapy are also observed clinically. To this end, a number of cellular and molecular assays were undertaken using clinical samples from cancer patients treated with chemotherapy in two different centers (the European Institute of Oncology, Milan, Italy, and University Medical Center Utrecht, Utrecht, The Netherlands). Cancer patients were treated with paclitaxel or paclitaxel-based therapy and the results were compared to patients who were treated with gemcitabine, doxorubicin-, or cisplatin-based therapies as indicated in detail in Experimental Procedures. The results in Figure 7A revealed that similar to our preclinical observations, levels of CEPs significantly and acutely increased (from baseline) in patients receiving paclitaxel-based therapy. Furthermore, significance was also reached when paclitaxel-based therapy was compared to the other treatment groups. In addition, plasma concentrations of SDF-1α, G-CSF and VEGF were evaluated 4 hours after paclitaxel-based therapy, compared to the plasma concentrations of patients treated with gemcitabine, doxorubicin-, or cisplatinum-based therapies. The results in Figure 7B revealed that only SDF-1α plasma concentrations were rapidly and significantly increased in patients treated with paclitaxel-based therapy in contrast to patients treated with other chemotherapies. A non- significant trend was observed in both G-CSF and VEGF plasma concentrations of either group. Overall, based on the preclinical data and the preliminary clinical data obtained, these clinical results indicate that our preclinical mechanistic explanation for the enhanced anti-tumor activity of bevacizumab when administered in combination with certain chemotherapy drugs may also hold clinically. Future prospective randomized clinical trials will be necessary to confirm this.
Figure 7. Levels of CEPs as well as G-CSF, SDF-1α and VEGF plasma concentrations in cancer patients 4 hours after they were treated with various chemotherapy drugs administered at the MTDs.
Cancer patients (n=30) were treated with paclitaxel (n=8), paclitaxel plus carboplatin (n=4)(both of which designated as PTX-based therapy), gemcitabine (n=8)(GEM), epirubicin, cisplatin plus capecitebin (n=5)(ECX), or doxorubicin +/− cyclophosphamide (n=5)(Dox-based therapy). Four hours later, patients were bled intravenously for the evaluation of (A) CEPs (n=12 for PTX-based, and n=18 for GEM/ECX/Dox-based therapies) as well as (B) G-CSF (n=3 for PTX-based, and n=10 for ECX/Dox-based therapies), SDF-1α (n=12 for PTX-based, and n=15 for GEM/ECX/Dox-based therapies) and VEGF (n=3 for PTX-based, and n=10 for ECX/Dox-based therapies) plasma concentrations. Results were normalized to the baseline level of each patient to reduce variability that may occur due to tumor type, stage, and values obtained from two different centers. *, 0.05>p>0.01; **, p<0.01.
DISCUSSION
Our results provide a new perspective regarding the impact that conventional chemotherapy can have on tumor angiogenesis and hence how a combination with antiangiogenic drugs may amplify the anti-tumor effects of chemotherapy. Previously, if anything, chemotherapy has been reported to have the potential to cause local tumor antiangiogenic effects by virtue of targeting cycling endothelial cells in sprouting angiogenic blood vessel capillaries within tumors (Browder et al., 2000; Klement et al., 2000; Miller et al., 2001). But at approximately the same time, some chemotherapy drugs administered at MTDs can cause a systemic host mediated counter-regulating response from the bone marrow, comprised, at least in part, by acute mobilization of CEPs which subsequently has the potential to stimulate tumor angiogenesis and vasculogenesis. This host response may not only help abrogate the potential local antiangiogenic effect, but intrinsically stimulate tumor vasculogenesis/angiogenesis as well, and thus act to limit the duration of tumor responses induced by the cytotoxic chemotherapy drug treatment.
Our results also provide a potential explanation why not all chemotherapy drugs will necessarily have their efficacy enhanced by the addition of an antiangiogenic agent when the mechanism involves blunting CEP mobilization acutely induced by the chemotherapy drug. It should be noted that our experiments were conducted using only a single dose of DC101 prior to chemotherapy, for the purpose of inhibiting CEP mobilization, as we previously demonstrated with VDAs (Shaked et al., 2006). We have not tested the efficacy of repetitive combination treatments since it has already been demonstrated that DC101 has an anti-tumor effect due to antiangiogenic mechanisms, when administered in such a fashion as a single agent (Prewett et al., 1999). The results may also be pertinent to explaining some of the benefit of other therapeutic approaches which target CEPs. For example, the administration of chemotherapy at close regular intervals using low, non-toxic doses, with no prolonged breaks (“metronomic” chemotherapy)(Kerbel and Kamen, 2004) not only avoids acute CEP mobilization but can even target CEPs (Bertolini et al., 2003; Shaked et al., 2005b). It will also be of interest to determine whether and to what degree, other types of bone-marrow derived proangiogenic cells(Grunewald et al., 2006; De Palma et al., 2005; Udagawa et al., 2006) may be induced (or suppressed) by MTD chemotherapy and thus potentially contribute to tumor recovery after treatment (or response). Notably, some of these populations, e.g. Gr1+/CD11b+ myeloid cells may not be suppressed by drugs which target the VEGF-A pathway of angiogenesis (Shojaei et al., 2007). However, our experiments performed in Id mutant mice indicate that CEPs play the major role in the systemic response, as these animals are not deficient for other pro-angiogenic cells (Ciarrocchi et al., 2007).
Our results raise a number of important questions relevant to antiangiogenic drugs and the impact of CEPs in tumor angiogenesis. For example, as antiangiogenic small molecule oral receptor tyrosine kinase inhibitors (RTKIs) which target multiple RTKs including VEGF receptors have not yet shown an ability to enhance the efficacy of conventional chemotherapy in phase III trials in contrast to bevacizumab (Kerbel, 2008), could this be due to an inability of such drugs to block CEP mobilization? In this regard, we have recently reported that one such drug, sunitinib, can cause marked elevations in multiple circulating growth factors, cytokines and chemokines in a dose-dependent and tumor independent fashion (Ebos et al., 2007). These factors include VEGF, PlGF, SCF, G-CSF, SDF-1α and SCF. Since the receptors for G-CSF and SDF-1α are not affected by sunitinib and both G-CSF and SDF-1α are known to mobilize CEPs (Asahara et al., 1999; Jin et al., 2006; Powell et al., 2005), targeting VEGF receptors and c-kit using a drug such as sunitinib may not be sufficient to blunt chemotherapy-induced CEP spikes when they occur. Second, might our results help resolve some of the ongoing controversy regarding the importance of CEPs to tumor angiogenesis? Most studies have shown low (Peters et al., 2005) or even non-existent (Purhonen et al., 2008) incorporation of CEPs in tumor blood vessels in mouse tumor models (Bertolini et al., 2006); however, as we previously reported for VDAs - which are not yet clinically approved drugs and are being tested only in small numbers of patients – some commonly used chemotherapy drugs such as paclitaxel can also cause a robust mobilization of CEPs which subsequently can home to the drug-treated tumors and incorporate into newly forming vessels. Importantly, such incorporation may be influenced by damage to the tumor (neo)vasculature thus creating the physiologic need (‘signal’) for rapid replacement of damaged or destroyed endothelium in the tumor vasculature. Rapid mobilization of CEPs and homing to vessels damaged by adverse cardiovascular events (Urbich and Dimmeler, 2004) could be taken as a model for this host process in the context of cytotoxic drug-induced damage to the tumor vasculature. In this regard, while VDAs are well known to cause damage to the tumor vasculature, such a property is less appreciated with respect to chemotherapy. However, there is an expanding literature of chemotherapy-induced damage to endothelial cells in the tumor vasculature (Browder et al., 2000; Klement et al., 2000; Miller et al., 2001) which in some cases can be very rapid (Farace et al., 2007)(Personal Communication, Paul Nathan). Indeed our own results, represented here, indicate that MTD chemotherapy can cause rapid drops in tumor microvessel density, e.g., even after a single MTD dose as shown in Figure 4B.
Our preclinical results are supported by limited clinical observations testing levels of CEPs and SDF-1α, GCSF and VEGF plasma concentrations in cancer patients treated with chemotherapy using paclitaxel. Jin et.al. have recently suggested that elevated levels of SDF-1α induce mobilization of CXCR4+ cells from the bone marrow (Jin et al., 2006), among them perhaps CEPs as the majority of them express CXCR4 (Yamaguchi et al., 2003; Athanassakis et al., 2001). Based on our preliminary results, platelets could be one source of the released SDF-1α. The rapid induction of various cytokines may promote platelet activation and hence cause the release SDF-1α stored in platelets (Jin et al., 2006; Rafii et al., 2008). It has also been suggested that mobilization of activated megakaryocytes from the bone marrow niche can upregulate levels of SDF-1α(Avecilla et al., 2004)
Finally, it will be of interest to evaluate the contribution of the mechanism we have proposed here to account for antiangiogenic drug mediated enhancement of standard chemotherapy using drugs such as bevacizumab, relative to other proposed mechanisms such as transiently induced vessel normalization (Jain, 2005; Winkler et al., 2004), or enhancement of the extent of local damage to the tumor vasculature mediated by chemotherapy, in the clinical setting.
EXPERIMENTAL PROCEDURES
Blood samples obtained from cancer patients
Blood samples were collected from cancer patients receiving chemotherapy. Sixteen patients with stage IV metastatic breast cancer were treated with either paclitaxel (n=8) or gemcitabine (n=8) at the European Institute of Oncology, Milan, Italy. The study followed the rules of the European Institute of Oncology Ethics Committee and written informed consent was obtained from all patients. In addition, four patients with ovarian cancer were treated with carboplatin and paclitaxel (paclitaxel-based therapy); five patients were treated with either doxorubicin monotherapy or in combination with cyclophosphamide (doxorubicin-based therapy) for respectively malignant sarcoma and breast cancer; and five patients with esophageal cancer were treated with the combination epirubicin, cisplatin and capecitebin (cisplatin-based therapy) at the clinic of the Department of Medical Oncology, University Medical Center, Utrecht, the Netherlands. The study was approved by the Institutional Ethical Review Board at The University Medical Center Utrecht and written informed consent was obtained from all patients.
Tumors and Animal Models
Eight to twelve week old C57Bl/6 or BALB/c mice (obtained from the Jackson Laboratory West, Sacramento, CA) were treated with chemotherapy drugs. Lewis Lung carcinoma (LLC) cells (0.5×106) (ATCC, Manassas, VA)were subcutaneously implanted into immunocompetent C57Bl/6 mice (The Jackson Laboratory), or C57Bl/6 mice previously irradiated and then transplanted with green fluorescent protein+ (GFP+) -bone marrow cells or were injected into ld1+/−ld3−/−(ldmut) and wildtype C57Bl/6 mice. B16F1 melanoma cells (0.5×106) (ATCC, Manassas, VA) were implanted into the flanks of immunocompetent C57Bl/6 mice. Tumor size was assessed regularly with Vernier calipers by using the formula width2 × length × 0.5. When tumors reached 500 mm3, treatment was initiated. All in vivo studies mice were randomly grouped (n=4–6/group). All animal studies were performed according to Sunnybrook Health Sciences Centre Animal Care Committee and Canadian Council on Animal Care (Toronto, Ontario, Canada) or Institutional Animal Care and Use Committee at Sloan-Kettering Cancer Center (New York, NY, USA).
Drugs and MTD drug concentrations
The following antibodies were used in-vivo for therapy: 800μg/mouse, DC101 (ImClone Systems Inc, New York, NY), which is a rat monoclonal blocking antibody specific for mouse VEGFR2/flk-1; and 50μg/mouse, monoclonal SDF-1α neutralizing antibodies (R&D systems, Minneapolis, MN); goat (polyclonal) anti-SDF-1α neutralizing antibodies or goat non-specific anti-sera control after Fc fragment digestion; and 5mg/kg G6-31, a monoclonal anti mouse/human VEGF neutralizing antibody (Genentech Inc., South San Francisco, CA), the doses of which were previously determined for optimal activity (Prewett et al., 1999; Schober et al., 2003; Phillips et al., 2003; Liang et al., 2006). Chemotherapy drugs were administered near or at the maximum tolerated dose as indicated in (Supplemental Table S1). All drugs were administered intraperitoneally (i.p.) as a bolus injection. Control mice received the relevant vehicles. DC101, G6-31, and SDF-1α neutralizing antibodies when used with chemotherapy were given 24 hours prior to the chemotherapy drug injection.
Flow Cytometry
For preclinical evaluation of viable CEPs, blood was obtained from anaesthetized mice via retro-orbital sinus bleeding, and prepared for CEP labeling using, as previously described (Bertolini et al., 2003; Shaked et al., 2005a). For clinical samples, CEPs were evaluated in patients as described (Bertolini et al., 2006). For detailed information see Supplemental Experimental Procedures.
For the evaluation of GFP+ bone marrow derived cells, hemangiocytes, and TEMs resident in tumors, 100–300 μm of tumor tissue (n=5 samples/group) were prepared as single cell suspension as previously described (Baeten et al., 2002). Hemangiocytes were defined as (GFP+) CD45+/CXCR4+/VEGF-1+ (Jin et al., 2006). TEMs were defined as (GFP+) CD45+/Tie-2+/CD11b+ (De Palma et al., 2005). Acquisition of at least 50, 000 cells per sample was undertaken. All bone marrow cell types were plotted as the absolute cell number in 10, 000 cells.
For all flow cytometry experiments, CD133 was purchased from Miltenyi Biotec Inc., Tie-2 is produced in-house (Sunnybrook Health Sciences Centre), and VEGFR-1 was purchased from R&D systems. All other antibodies were purchased from BD Biosciences.
Quantitation and visualization of tissue necrosis, hypoxia and vessel perfusion, tumor cell proliferation and apoptosis
Tissue processing and immunohistochemistry were performed as described previously (Shaked et al., 2006). For detailed information see Supplemental Experimental Procedures.
Microscopic image acquisition and analysis
Tumor sections were visualized under a Carl Zeiss Axioplan 2 microscope (Carl Zeiss Canada Inc. Toronto, ON, Canada). Images were captured with a Zeiss Axiocam digital camera connected to the microscope using AxioVision 3.0 software. The number of fields per tumor sample varied from 4 to 10, depending on the tumor size. Analysis of tumor hypoxia, perfusion and necrosis as well as tumor cell proliferation and apoptosis was carried out by calculating the fraction of the tumor area positively stained for the indicated parameter, using Adobe Photoshop 6.0 software (Adobe systems incorporated, San Jose, CA). For the analysis of microvessel density, the total number of vascular structures (CD31-positive) per field were counted per each tumor sample. At least 10 fields per tumor representing all tumor area were taken (n≥5 tumors/group).
Analysis of VEGF-A, SDF-1α and G-CSF plasma concentration
Blood samples obtained by cardiac puncture of mice under anesthesia, or intravenously from cancer patients were collected either in Microtainer (Becton Dickinson, Franklin Lakes, NJ) plasma separating tubes (for mice) or EDTA tubes (for human) and centrifuged at 4°C, and subsequently stored at −70°C until assayed. Levels of mouse or human VEGF-A, SDF-1α and G-CSF were assessed by using commercially available sandwich ELISAs (R&D Systems).
Isolation of platelets and the analysis of SDF-1α
Experiments were performed as previously described (Jin et al., 2006). For detailed information see Supplemental Experimental Procedures.
Bone Marrow Transplantation
Experimental procedures were carried out as previously described (Shaked et al., 2006). For detailed information see Supplemental Experimental Procedures.
Statistical Analysis
Data are expressed as mean ± S.D. and the statistical significance of differences in mean values was assessed by the two-tailed Student’s t-test. Differences between designated groups compared to control-untreated group (unless indicated otherwise) were considered significant at values of 0.05 > P > 0.01 (*) or P < 0.01 (**). For human samples, data are expressed as mean ± SEM. Comparisons between baseline and 4 hours after treatment were made using paired t-test. Comparisons between groups of patients treated with paclitaxel-based versus other chemotherapy-based therapies were made using unpaired t-test. Significance was set on 0.05 > P > 0.01 (*) or P < 0.01 (**).
Supplementary Material
Acknowledgments
We thank Cassandra Cheng for her excellent secretarial assistance, for Petia Stefanova for her excellent technical help with tissue processing and immunohistochemistry, and for Genentech Inc. for kindly providing G6-31 antibodies for this study. This work was supported by grants from the National Institutes of Health (NIH), CA-41233 to RSK; NIH and Breast Cancer Research Foundation to RB; the National Cancer Institute of Canada and Canadian Institutes of Health Research to RSK; the Associazione Italiana per la Ricerca sul Cancro (AIRC), Istituto Superiore di Sanità (ISS), and the sixth EU Framework Programme (Integrated Project “Angiotargeting” contract no. 504743) in the area of “Life sciences, genomics and biotechnology for health.” to FB; and a sponsored research agreement with ImClone Systems New-York (RSK), and Dr. Saal van Zwanenberg Stichting fellowship to LD. LW and ZZ are employees of Imclone Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addison CL, Arenberg DA, Morris SB, Xue YY, Burdick MD, Mulligan MS, Iannettoni MD, Strieter RM. The CXC chemokine, monokine induced by interferon-gamma, inhibits non-small cell lung carcinoma tumor growth and metastasis. Hum Gene Ther. 2000;11:247–261. doi: 10.1089/10430340050015996. [DOI] [PubMed] [Google Scholar]
- Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanassakis I, Papadimitriou L, Vassiliadis S. Murine ectoplacental cone-derived trophoblast cells express chemokine receptors. J Reprod Immunol. 2001;50:105–119. doi: 10.1016/s0165-0378(01)00062-6. [DOI] [PubMed] [Google Scholar]
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, Hackett NR, Crystal RG, Witte L, Hicklin DJ, Bohlen P, Eaton D, Lyden D, de Sauvage F, Rafii S. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- Baeten CI, Wagstaff J, Verhoeven IC, Hillen HF, Griffioen AW. Flow cytometric quantification of tumour endothelial cells; an objective alternative for microvessel density assessment. Br J Cancer. 2002;87:344–347. doi: 10.1038/sj.bjc.6600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerepoot LV, Radema SA, Witteveen EO, Thomas T, Wheeler C, Kempin S, Voest EE. Phase I clinical evaluation of weekly administration of the novel vascular-targeting agent, ZD6126, in patients with solid tumors. J Clin Oncol. 2006;24:1491–1498. doi: 10.1200/JCO.2005.02.7458. [DOI] [PubMed] [Google Scholar]
- Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, Kerbel RS. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–4346. [PubMed] [Google Scholar]
- Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: from promiscuity to surrogate marker and target identification. Nature Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- Browder T, Butterfield CE, Kraling BM, Marshall B, O’Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- Burris H, III, Rocha-Lima C. New therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathways. Oncologist. 2008;13:289–298. doi: 10.1634/theoncologist.2007-0134. [DOI] [PubMed] [Google Scholar]
- Ciarrocchi A, Jankovic V, Shaked Y, Nolan DJ, Mittal V, Kerbel RS, Nimer SD, Benezra R. Id1 restrains p21 expression to control endothelial progenitor cell formation. PLoS ONE. 2007;2:e1338. doi: 10.1371/journal.pone.0001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Galli R, Sergi LS, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci U S A. 2007;104:17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farace F, Massard C, Borghi E, Bidart JM, Soria JC. Vascular disrupting therapy-induced mobilization of circulating endothelial progenitor cells. Ann Oncol. 2007;18:1421–1422. doi: 10.1093/annonc/mdm367. [DOI] [PubMed] [Google Scholar]
- Franco M, Man S, Chen L, Emmenegger U, Shaked Y, Cheung AM, Brown AS, Hicklin DJ, Foster S, Kerbel RS. Targeted anti-VEGFR-2 therapy leads to short and long term impairment of vascular function and increases in tumor hypoxia. Cancer Res. 2006;66:3639–3648. doi: 10.1158/0008-5472.CAN-05-3295. [DOI] [PubMed] [Google Scholar]
- Furstenberger G, von Moos R, Lucas R, Thurlimann B, Senn HJ, Hamacher J, Bonebert EM. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy or primary breast cancer. Br J Cancer. 2006;94:524–531. doi: 10.1038/sj.bjc.6602952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Hudis CA. Clinical implications of antiangiogenic therapies. Oncology (Williston Park) 2005;19:26–31. [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth H, Helm W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312:1171–1175. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS, Kamen BA. Antiangiogenic basis of low-dose metronomic chemotherapy. Nature Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin D, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroep JR, Giaccone G, Voorn DA, Smit EF, Beijnen JH, Rosing H, van Moorsel CJ, van Groeningen CJ, Postmus PE, Pinedo HM, Peters GJ. Gemcitabine and paclitaxel: pharmacokinetic and pharmacodynamic interactions in patients with non-small-cell lung cancer. J Clin Oncol. 1999;17:2190–2197. doi: 10.1200/JCO.1999.17.7.2190. [DOI] [PubMed] [Google Scholar]
- Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, Fu L, Malik AK, Gerber HP, Ferrara N, Fuh G. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281:951–961. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- Miller KD, Sweeney CJ, Sledge GW., Jr Redefining the target: chemotherapeutics as antiangiogenics. J Clin Oncol. 2001;19:1195–1206. doi: 10.1200/JCO.2001.19.4.1195. [DOI] [PubMed] [Google Scholar]
- Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B, Kinzler KW, Lengauer C. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Strieter RM. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med. 2003;167:1676–1686. doi: 10.1164/rccm.200301-071OC. [DOI] [PubMed] [Google Scholar]
- Powell TM, Paul JD, Hill JM, Thompson M, Benjamin M, Rodrigo M, McCoy JP, Read EJ, Khuu HM, Leitman SF, Finkel T, Cannon RO., III Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25:296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]
- Prewett M, Huber J, Li Y, Santiago A, O’Connor W, King K, Overholser J, Hooper A, Pytowski B, Witte L, Bohlen P, Hicklin DJ. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii DC, Psaila B, Butler J, Jin DK, Lyden D. Regulation of vasculogenesis by platelet-mediated recruitment of bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2008;28:217–222. doi: 10.1161/ATVBAHA.107.151159. [DOI] [PubMed] [Google Scholar]
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- Schober A, Knarren S, Lietz M, Lin EA, Weber C. Crucial role of stromal cell-derived factor-1alpha in neointima formation after vascular injury in apolipoprotein E-deficient mice. Circulation. 2003;108:2491–2497. doi: 10.1161/01.CIR.0000099508.76665.9A. [DOI] [PubMed] [Google Scholar]
- Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, Rawn K, Voskas D, Dumont DJ, Ben-David Y, Lawler J, Henkin J, Huber J, Hicklin DJ, D’Amato RJ, Kerbel RS. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis: implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005a;7:101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, Hicklin DJ, Chaplin D, Foster FS, Benezra R, Kerbel RS. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- Shaked Y, Emmengger U, Man S, Cervi D, Bertolini F, Ben-David Y, Kerbel RS. The optimal biological dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005b;106:3058–3061. doi: 10.1182/blood-2005-04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b(+)Gr1(+) myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- Udagawa T, Puder M, Wood M, Schaefer BC, D’Amato RJ. Analysis of tumor-associated stromal cells using SCID GFP transgenic mice: contribution of local and bone marrow-derived host cells. FASEB J. 2006;20:95–102. doi: 10.1096/fj.04-3669com. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.