Abstract
The evolutionarily conserved Sec61 protein complex mediates the translocation of secretory proteins into the endoplasmic reticulum. To investigate the role of Sec61p, which is the main subunit of this complex, we generated recessive, cold-sensitive alleles of sec61 that encode stably expressed proteins with strong defects in translocation. The stage at which posttranslational translocation was blocked was probed by chemical crosslinking of radiolabeled secretory precursors added to membranes isolated from wild-type and mutant strains. Two classes of sec61 mutants were distinguished. The first class of mutants was defective in preprotein docking onto a receptor site of the translocon that included Sec61p itself. The second class of mutants allowed docking of precursors onto the translocon but was defective in the ATP-dependent release of precursors from this site that in wild-type membranes leads to pore insertion and full translocation. Only mutants of the second class were partially suppressed by overexpression of SEC63, which encodes a subunit of the Sec61 holoenzyme complex responsible for positioning Kar2p (yeast BiP) at the translocation channel. These mutants thus define two early stages of translocation that require SEC61 function before precursor protein transfer across the endoplasmic reticulum membrane.
INTRODUCTION
The translocation of secretory proteins into the endoplasmic reticulum (ER) is mediated by the Sec61 protein complex (Matlack et al., 1998). In the yeast, Saccharomyces cerevisiae, this evolutionarily conserved complex consists of a 52-kDa membrane protein called Sec61p and two small, single-spanning membrane proteins called Sss1p and Sbh1p (Hartmann et al., 1994; Panzner et al., 1995). Sec61p is an essential protein that spans the membrane 10 times (Wilkinson et al., 1996). In yeast, secretory precursors can translocate into the ER co- or posttranslationally depending on their signal sequence (Ng et al., 1996). Sec61p is the major crosslinking partner for secretory proteins following both co- and posttranslational translocation pathways and thus is considered the main pore component (Müsch et al., 1992; Sanders et al., 1992; Mothes et al., 1994; Matlack et al., 1997).
In cotranslational translocation, which has been well studied in mammalian systems, nascent chain–ribosome complexes first interact with the signal recognition particle (SRP) in the cytosol. Upon interaction with the ER-localized SRP receptor, SRP dissociates from the nascent chain–ribosome complex, which is then targeted to the membrane-embedded Sec61p complex. The specificity of this process is ensured by the affinity of the ribosome for the Sec61p complex and by signal sequence–Sec61p complex interactions (Walter and Johnson, 1994; Jungnickel and Rapoport, 1995). During cotranslational translocation the ribosome forms a tight seal with the Sec61p complex (Liao et al., 1997). The driving force for membrane transfer of a precursor in cotranslational translocation is thought to be provided by the elongation of the nascent chain on the translating ribosome. BiP, a luminal Hsp70, is thought to assist in folding of emerging polypeptides and to provide additional gating of the pore (Hamman et al. 1998).
In yeast the Sec61p complex is also part of a larger set of ER membrane proteins known as the Sec complex, which together with Kar2p, the yeast BiP orthologue, mediates posttranslational translocation. The Sec complex consists of the Sec61p complex and the Sec62/63p complex. The Sec62/63p complex includes two essential transmembrane proteins called Sec62p and Sec63p and two nonessential proteins, Sec71p and Sec72p (Deshaies et al., 1991; Brodsky and Schekman, 1993; Panzner et al., 1995). Prepro-α-factor (ppαf), a secretory precursor that follows the posttranslational pathway, binds to the cytosolic side of the membrane in the absence of ATP. The docking site consists of the Sec complex proteins, and ppαf interacts with the Sec62p, Sec71p, and Sec72p subunits of the Sec62/63p complex at this stage (Lyman and Schekman, 1997; Matlack et al., 1997). The energy required for posttranslational translocation comes from the hydrolysis of ATP by Kar2p in the ER lumen. Kar2p binds specifically to the luminal DnaJ domain of Sec63p (Corsi and Schekman, 1997). This interaction requires ATP-hydrolysis and is essential for both the dissociation of precursors from the cytosolic docking site to initiate membrane transit as well as the release of secretory proteins from the pore into the lumen (Lyman and Schekman, 1995, 1997). Mechanistically the latter stage is better understood, because Kar2p binds secretory proteins on the luminal side of the translocon and thus promotes precursor movement directly (Sanders et al., 1992). At present the mechanism by which Kar2p regulates from the luminal side the release of precursors at the cytoplasmic docking site and their concomitant delivery into the pore is unclear, but it seems likely that this involves conformational changes of Sec complex proteins (Lyman and Schekman, 1997).
In addition to its roles in forward transport, the Sec61p complex was shown to be involved in the regulated removal of an integral membrane protein and the export of misfolded secretory proteins from the ER to the cytosol for degradation by the proteasome (Wiertz et al., 1996; Pilon et al., 1997; Plemper et al., 1997).
It is unclear to what extent Sec61p participates in the regulation of translocation. In favorable circumstances phenotypic analysis of different mutant alleles of a gene allows discrimination of separable functions of a protein. Thus far, only two mutant alleles of SEC61, sec61-2 and sec61-3, have been characterized as mutant proteins, and each encodes an unstable form of Sec61p that is degraded by the ubiquitin-mediated pathway of proteolysis (Sommer and Jentsch 1993; Biederer et al., 1996). However, compared with alleles of kar2, sec62, and sec63, these two sec61 mutations have a limited effect on translocation (Stirling et al., 1992). Last year, we reported the isolation of two cold-sensitive (Cs) alleles of sec61 that have strong defects both in forward and in reverse translocation across the ER membrane (Pilon et al., 1997). We set out to isolate additional alleles of SEC61 defective in translocation. Chemical crosslinking was used to analyze the nature of the translocation defect in the novel and previously isolated strains. Our results show that SEC61 function is required at two early but distinguishable stages before the transfer of a secretory precursor through the channel.
MATERIALS AND METHODS
Yeast Strains, Growth Conditions, and Plasmids
The strains used in this study are listed in Table 1. Media were purchased from Difco (Detroit, MI). Yeast cells were grown in YPD (1% yeast extract, 2% peptone, 2% dextrose) or synthetic media (SD) with the appropriate additions. Media for plates were supplemented with 2% agar. Standard techniques were used for genetic analysis of yeast strains (Sherman, 1991). Plasmids pRS426 (Christianson et al., 1992), YEp352 (Hill et al., 1986), and YCpLac111 (Gietz and Sugino, 1988) are published. The 2.4-kb HindIII–EcoRI fragment harboring the SEC61 sequence with an N-terminal 6-histidine tag was subcloned into YCplac111 to give pDQ1 (Pilon et al., 1997). A 2μ/URA3-based multicopy SEC61 plasmid was obtained by subcloning the large PvuI–PvuI fragment of pDQ1 into the PvuI sites of pRS426 replacing the part of that vector that contains the multiple cloning site (pMP12). pSEC61-wt was constructed by insertion of the 2.2-kb HindIII–EcoRI fragment of pCS15, which contains the original SEC61 clone (Stirling et al., 1992), into YCplac111.
Table 1.
Strains used in this study
| Strain | Genotype | Source/reference |
|---|---|---|
| RSY524 | mata, leu2,3-112, ade2-1, ura3-52 pep4-3, sec61-2 | Schekman lab |
| RSY607 | matα, ura3-52, leu2-3,112, pep4∷URA3, | Schekman lab |
| RSY633 | matα, can1-100, leu2-3,112, his3-11,15, trp1-1, ura3-1,ade2-1, sec61∷HIS3, [pDF40] | C. Stirling/Schekman lab |
| RSY926 | matα, ade2-101, his3Δ200, leu2Δ1, lys2-801, trp1Δ63, ura3-52, sec71∷LEU2 | Feldheim et al., 1993 |
| RSY1006 | matα, ade2-101, his3Δ200, leu2Δ1, lys2-801, trp1Δ63, ura3-52, sec72∷HIS3 | Feldheim and Schekman, 1994 |
| RSY1132 | matα, leu2-3,113, ura3-52, trp1-1, sec61-3 (CSY 150) | Stirling et al., 1992 |
| RSY1293 | matα, can1-100, leu2-3,112, his3-11,15, trp1-1, ura3-1,ade2-1, sec61∷HIS3 (pDQ1 [sec61-his6]) | Pilon et al., 1997 |
| RSY1294 | same as RSY1293 but sec61-32 | Pilon et al., 1997 |
| RSY1295 | same as RSY1293 but sec61-41 | Pilon et al., 1997 |
| RSY1296 | same as RSY1293 but sec61-86 | This study |
| RSY1297 | same as RSY1293 but sec61-7 | This study |
| RSY1298 | same as RSY1293 but sec61-8 | This study |
| RSY1299 | same as RSY1293 but sec61-10 | This study |
| RSY1300 | same as RSY1293 but sec61-11 | This study |
| RSY1301 | same as RSY1293 but sec61-16 | This study |
| RSY1302 | same as RSY1293 but sec61-22 | This study |
| RSY1303 | same as RSY1293 but sec61-23 | This study |
| RSY1304 | same as RSY1293 but sec61-24 | This study |
| RSY1428 | same as RSY1293 but SEC61 (pSEC61-wt) | This study |
| RSY1429 | same as RSY1293 but sec61-110 | This study |
| WCG4a | mata, leu2,3-112, ura3, his3, -11,15 | Hiller et al., 1996 |
Multicopy yeast vectors with a URA3 marker were used for the overexpression of Sec complex proteins in sec61 mutants. Plasmid pDF15 contains the SEC63 sequence as a 3.5-kb HindIII–HindIII fragment subcloned into YEp352 (Feldheim et al., 1992); pMP62 is the 1.7-kb EcoRI–HindIII fragment of pRD8a containing the SEC62 sequence (Deshaies and Schekman, 1990) cloned into pRS426. To construct pDF58, we subcloned the EcoRI–EcoRI fragment containing the SEC72 gene (Feldheim and Schekman, 1994) into YEp352. Plasmid pMP71 contains a 0.9-kb EcoRI–EcoRI fragment containing the SEC71 sequence (Feldheim et al., 1993) subcloned into pRS426. The 2.8-kb EcoRI–EcoRI fragment of p24 containing the SSS1 gene (Esnault et al., 1993) was cloned into pRS426 to give pMP51. YEpSEB1 contains the SBH1 sequence on a 2μ/TRP1 vector (Toikkanen et al., 1996). Western blot analysis of total cell lysates confirmed the overexpression of the respective Sec protein by each of these plasmids.
In Vitro Mutagenesis, Mutant Isolation, and Characterization
In vitro mutagenesis of SEC61 by hydroxylamine treatment was done as described before (Pilon et al ., 1997). For mutagenesis by PCR, we amplified the SEC61 coding sequence in pDQ1 using primers that hybridize to vector sequences outside of the coding sequence. The sequence of the primers was primer 1, 5′-CTT GTT ACC CGG CGC GGC AG-3′; and primer 2, 5′-GCC AGG GTT TTC CCA GTC ACG-3′. A published protocol using Taq polymerase (Boehringer Mannheim, Mannheim, Germany) was used, except that DMSO and manganese were omitted to improve the yield (Leung et al., 1989). The extent of mutagenesis was estimated by DNA sequencing of a 400-bp region of six separately cloned PCR fragments. One clone had no base changes, four clones had one base change each, and one clone contained three base changes. pDQ1 was digested with XbaI and EcoRI, and the gapped vector was isolated from low-melting-point agarose gels using the Wizard kit (Promega, Madison, WI). The LiAc method was used to cotransform equal amounts of gapped vector and PCR product into yeast strain RSY633. Colonies that had obtained circular plasmid by in vivo recombination were selected on SC-leucine plates (Muhlrad et al., 1992). Restriction enzyme analysis of 18 randomly selected clones revealed that 17 of 18 plasmids had a restriction pattern identical to pDQ1, showing that correct recombination of gapped vector and PCR product had occurred with high frequency. Plasmid shuffling, replica plating to identify mutants, and the isolation of plasmids from cells exhibiting a conditional growth defect were done as described before (Pilon et al., 1997). The SEC61 sequence of the isolated plamids was subcloned into the pDQ1 vector using the XbaI site, which overlaps with codons 6–8 of wild-type SEC61, and the EcoRI site, which is located 0.2-kb downstream of the coding region. A unique StuI site that overlaps with codon 235 of the coding sequence was used to subclone separately the 5′ and 3′ regions of sec61 mutant DNA. RSY633 was again transformed with these plasmids and subjected to plasmid shuffling to produce the sec61 mutant strains used in this study. Multicopy plasmid versions of the sec61 mutants were obtained by replacing the 2.2-kb HindIII–EcoRI fragment of pMP12 with the respective mutant DNA.
Antibodies, Immunoblotting, Pulse Labeling, and Immunoprecipitations
Antisera raised against ppαf (Wuestehube and Schekman, 1993), Kar2p, carboxypeptidase Y (CPY) and Sec71p (Feldheim et al., 1993), Sec72p (Feldheim and Schekman 1994), Sec61p (Stirling et al., 1992), Sec62p (Deshaies and Schekman, 1990), Sec63p DnaJ domain (Feldheim et al., 1992), and Sss1p (Esnault et al., 1993) have been published. Specific antiserum against dipeptidyl-aminopeptidase B (DPAPB) was a generous gift from Dr. T. Stevens (University of Oregon, Eugene, OR). Sbh1p antibody was a kind gift from Dr. E. Hartmann (Max Delbruck Centrum, Berlin, Germany). Immunodetection of proteins transferred to nitrocellulose was done by the ECL method (Amersham, Arlington Heights, IL) following the manufacturer’s protocol. Quantitative immunodetection of proteins blotted to nitrocellulose was done using 35S-labeled protein A (Amersham). Serial dilutions of protein extracts from wild-type cells were used to ensure that detection was in the linear range. Pulse labeling of yeast cells and immunoprecipitation were done as described before (Pilon et al., 1997), except that uracil was omitted from the cultures described in Figure 9.
Figure 9.
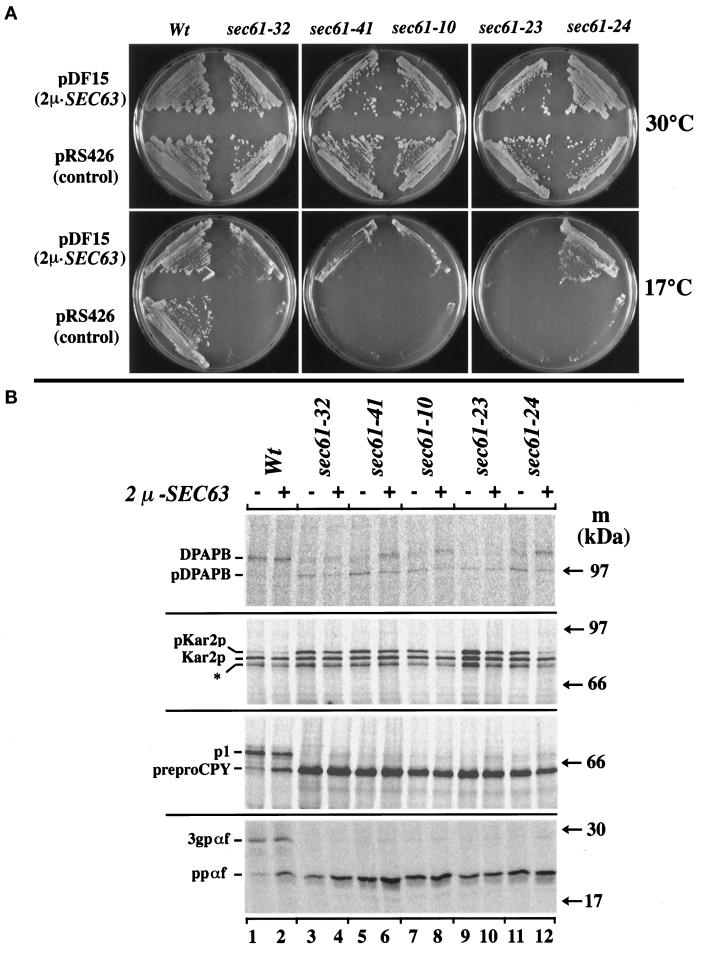
Overexpression of SEC63 partially suppresses the cold sensitivity of a subset of sec61 mutant strains by enhancing translocation. (A) Strains with the indicated plasmids were streaked onto SC plates minus uracil and incubated at 30°C for 3 d or 17°C for 5 d. pDF15 is a 2μ vector with the full-length SEC63 sequence. pRS426 is a multicopy vector used as a control. (B) Indicated strains carrying either pRS426 control plasmid (−) or the 2μ-SEC63 plasmid pDF15 (+) were pulse labeled at 17°C followed by immunoprecipitation as in Figure 2. The positions of precursor forms (pDPAPB, pKar2p, preproCPY, and ppαf), signal-cleaved, unglycosylated proteins (Kar2p and pαf), and signal-cleaved, glycosylated forms (DPAPB, p1, and 3 gpαf) are indicated. The band marked with an asterisk is unrelated.
In Vitro Assays
Microsomal membranes were prepared as described (Lyman and Schekman, 1995), except that spheroplasts were frozen at −80°C and thawed before homogenization. Membranes were stored in 20- to 75-μl aliquots at −80°C. In vitro–translated, 35S-labeled, wild-type ppαf was translocated into wild-type or mutant microsomes at 10 or 24°C in the presence of ATP and an ATP-regenerating system as described previously (Brodsky et al., 1993). Each 60-μl incubation contained 50 μg of microsomal protein. Reactions were analyzed by SDS-PAGE. Translocation efficiencies were obtained by determining the fraction of the added precursor that was fully glycosylated and protected against trypsin.
In vitro transcription and translation in the presence of [35S]methionine of wild-type and m3-mutant ppαf, partial purification of these proteins, and crosslinking assays using dithiobis-(succinimidylpropionate) (DSP; Pierce, Rockford, IL) to Sec complex proteins were performed as described by Lyman and Schekman (1997), except that for each immunoprecipitation 300,000 cpm of precursor was added to membranes (200 μg protein) in a 150-μl volume. To verify the specificity and determine the saturating concentration to be used, we titrated each antibody by performing immunoprecipitations on SDS-solubilized microsomes isolated from radiolabeled RSY1293 yeast cells followed by SDS-PAGE and autoradiography. For immunoprecipitation of Sec63p, we used affinity-purified antibodies raised against the luminal DnaJ domain of Sec63p.
ER-associated degradation (ERAD) of the nonglycosylated form of pro-α-factor (Δgpαf) (Mayinger and Meyer, 1993) was assayed as described before (McCracken and Brodsky, 1996; Pilon et al., 1997). Degradation reactions were incubated at 24°C for 30 min. At the end of the incubation, samples were precipitated with trichloroacetic acid and analyzed after electrophoresis on 18% polyacrylamide, 4 M urea SDS gels.
Octylglucoside extracts of microsomes were made and reconstitution into proteoliposomes was done as described by Brodsky et al. (1993). Kar2p was purified as described and was added to detergent extracts before reconstitution as 5% of total protein (Lyman and Schekman, 1997).
Fractionation of Sec Complex Proteins
Digitonin, obtained from Sigma (St. Louis, MO), was purified as described (Görlich and Rapoport, 1993). The fractionation of membrane proteins was adapted from Panzner et al. (1995). Briefly, microsomes (500 μg protein) were centrifuged at 10,000 × g and resuspended on ice in 100 μl of solubilization buffer (50 mM HEPES/KOH, pH 7.4, 400 mM KAc, 5 mM MgAc, 10% [wt/vol] glycerol, 0.05% [vol/vol] β-mercaptoethanol) containing the following protease inhibitors: 5 μg/ml leupeptin, 0.5 μg/ml pepstatin, 1 mM amino-benzamidine, 2.5 μg/ml chymostatin, and 0.1 mM PMSF. After the addition of 400 μl of solubilization buffer containing 3.75% (wt/vol) digitonin, the incubation samples were mixed by vortexing and incubated on ice for 30 min before centrifugation at 60,000 rpm in a Beckman TLA100.3 (Beckman Instruments, Palo Alto, CA) rotor for 30 min at 4°C. The pellet was processed to analyze the ribosome attached membrane proteins (RAMPs) as described below. The supernatant fraction was added to 100 μl of a suspension of concanavalin A (Con-A)-Sepharose (Pharmacia, Piscataway, NJ) equilibrated in 50 mM HEPES/KOH (pH 7.4), 10% (wt/vol) glycerol, 0.05% (vol/vol) β-mercaptoethanol, 1% (wt/vol) digitonin, and protease inhibitors. After incubation at 4°C for 1 h the beads were recovered by centrifugation at 2500 × g. The supernatant fraction was cleared from any remaining beads at 12,000 × g (free fraction). The Con-A beads were washed three times with 1 ml of equilibration buffer. To obtain the RAMP fraction, the first high-speed pellet fraction was dissolved in 50 mM HEPES/KOH (pH 7.8), 1 M KAc, 17.5 mM MgAc, 2.5% (wt/vol) digitonin, 1 mM puromycin, 0.2 mM GTP, 5 mM dithiothreitol, and protease inhibitors. After one 30-min incubation on ice and one 30-min incubation at 30°C the RAMPs were recovered in the supernatant after centrifugation at 100,000 × g for 30 min at 4°C. Equal aliquots of each fraction were analyzed by SDS-PAGE and immunoblotting with the indicated antibodies.
General Methods
Proteins radiolabeled with 35S in dried gels or on blots were detected and quantified using a STORM 850 PhosphorImager (Molecular Dynamics, Sunnyvale CA).
RESULTS
Isolation of Novel Cs sec61 Mutants Defective in Translocation
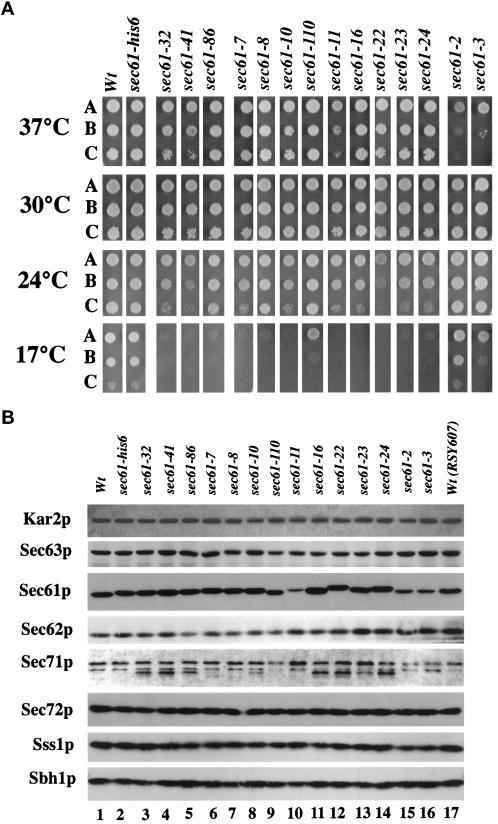
To obtain a better understanding of the role of Sec61p during protein translocation into the ER, we created new mutant alleles of the SEC61 gene. The SEC61 coding sequence was mutagenized in vitro both by hydroxylamine treatment and by error-prone PCR. We introduced the mutant sec61 coding sequences fused to an N-terminal 6-histidine tag into yeast by plasmid shuffling (Sikorski and Boeke, 1991). Initially 28°C was chosen as a permissive temperature for plasmid shuffling. Mutants that were either Cs, i.e., impaired for growth at 17°C, or temperature sensitive (Ts), i.e., impaired for growth at 37°C, were identified by replica plating onto YPD plates. In addition to the previously described sec61-32 and sec61-41 alleles, one new conditional sec61 allele was created by hydroxylamine mutagenesis, and nine additional alleles were generated by PCR mutagenesis. All strains expressing mutant Sec61 proteins exhibited strong Cs growth defects on rich medium (YPD; see Figure 1A), but when tested on minimal plates, only strains expressing sec61-32, sec61-41, sec61-10, sec61-11, sec61-23, and sec61-24 were unable to form colonies at 17°C, whereas the other strains formed small colonies. Growth of some of the strains expressing novel sec61 alleles was slightly impaired at 37°C, but all strains could form single colonies in the range from 24 to 37°C on both YPD and minimal plates (Figure 1A). For comparison the previously isolated chromosomal mutants sec61-2 and sec61-3 were included in this analysis. As reported before, sec61-2 was only Ts for growth, whereas sec61-3 was both Ts and Cs, although the Cs phenotype of sec61-3 was less pronounced (Figure 1A).
Figure 1.
(A) Cold sensitivity of sec61 mutant strains. Cells carrying plasmids with the indicated SEC61 alleles were grown to an OD600 of 1. Aliquots (10 μl) of each culture (A) and 10-fold (B) and 100-fold (C) dilutions were plated on YPD plates, which were incubated at the indicated temperatures for 3 d (37, 30, and 24°C) or 5 d (17°C). Strain sec61-his6 has the wild-type SEC61 sequence with an N-terminal 6-histidine repeat; the presence of the 6-histidine repeat had no influence on growth, expression, or translocation. The sec61-2 and sec61-3 strains are previously published chromosomal sec61 mutants; sec61-32, sec61-41, and sec61-86 were obtained by hydroxylamine mutagenesis; the other Cs alleles were obtained by PCR mutagenesis. (B) Sec complex protein levels in SEC61 mutant membranes. Microsomal membranes were isolated from the indicated strains and grown at the permissive temperature (30°C), and equal amounts of protein were separated by SDS-PAGE on 7.5–17.5% gradient gels. Proteins were analyzed by immunoblotting using specific antibodies for the indicated Sec proteins. The shift in electrophoretic mobility of Sec61p caused by the presence of the 6-histidine repeat can be seen. RSY607 carries a wild-type chromosomal copy of SEC61.
We sequenced the DNA of all novel alleles to determine the location of the mutations in the protein (see Table 2). All mutants obtained by hydroxylamine treatment had only a 1-bp change resulting in a single amino acid change, but all alleles obtained by PCR mutagenesis had multiple mutations, which led to multiple amino acid changes in these clones, except for sec61-24, which has a single amino acid change and two silent mutations (Table 2). The four single amino acid changes all map to predicted transmembrane domains 3 and 4 of Sec61p (Wilkinson et al., 1996). We used a unique restriction site in the middle of the SEC61 sequence and PCR techniques to subclone separately the 5′ and 3′ parts of some of the sec61 alleles carrying multiple mutations into pDQ1. This analysis revealed that the Gln to Arg mutation at amino acid position 156 in sec61-11 caused the Cs phenotype. The five C-terminal amino acid changes in this clone had no effect on colony formation at any temperature. Whereas the two C-terminal mutations of sec61-10 did not affect growth by themselves, cells expressing Sec61p with the single Phe to Ser change at position 92 were not viable at any temperature, because plasmid shuffling could never be completed for this clone. Thus in sec61-10 the C-terminal amino acid changes partially suppressed the mutation at position 92. Two mutants, sec61-16 and sec61-22, share a mutation with sec61-41 (see Table 2), and preliminary experiments showed that they also exhibited similar phenotypes; thus only sec61-41 was analyzed further.
Table 2.
Number and sequence of amino acid changes in novel sec61 alleles
| Clone | No. of amino acid changes | Sequence changes |
|---|---|---|
| sec61–32 | 1 | C150Y |
| sec61–41 | 1 | V134I |
| sec61–86 | 1 | G140D |
| sec61–7 | 7 | T87S, Q93K, L100S, L114F, E413D, A463T, V473A |
| sec61–8 | 3 | G128C, K226E, T454A |
| sec61–10 | 3 | F92S, F304S, E460G |
| sec61–11 | 6 | Q156R, L222V, Q398H, Y459F, F476Y, M480V |
| sec61–16 | 2 | V134I, N188I |
| sec61–22 | 4 | L80M, V134I, M248V, L342S |
| sec61–23 | 4 | L16N, I91T, A235V, Y265H |
| sec61–24 | 1 | L162P |
These changes are in addition to an N-terminal 6-histidine tag.
To determine whether the growth defects of any of the mutants were influenced by the presence of the histidine tag at the N terminus of Sec61p, we subcloned the coding sequences into a different vector to express untagged versions of the Sec61 mutant proteins. The histidine tag had no effect on the growth of cells expressing wild-type SEC61 (Figure 1A). In contrast, in the absence of the histidine tag none of the sec61 mutant strains exhibited measurable Cs growth defects, with the exception of the clone derived from sec61-10, which we termed sec61-110.
We next examined the expression of Sec complex proteins in the sec61 mutants by immunoblotting (see Figure 1B for Sec63p, Sec61p, and Sec71p). We quantified the amounts of Sec61p relative to Sec63p in each mutant and found that the expression level of Sec61p in most Cs strains was not significantly different from that in wild-type strains. The intensity of the Sec61p signal relative to Sec63p in microsomes from sec61-11 was much reduced, to ∼20% of wild-type levels; however, this was due to the sequence changes (see Table 2) in the 10 C-terminal amino acids that make up the epitope for the antibody that was used for the experiment in Figure 1B. By using a Sec61p antibody that recognizes an N-terminal epitope, the amounts of Sec61p in sec61-11 and wild-type membranes were judged to be the same. In contrast, the amount of Sec61p in Ts mutant sec61-2 was reduced to 40% of wild-type levels and in sec61-3 was reduced to ∼30% of wild-type levels, in agreement with previously published data (Sommer and Jentsch, 1993; Biederer et al., 1996). The steady-state levels of Kar2p, Sec62p, Sec72p, Sss1p, and Sbh1p were also not significantly different between these strains (Figure 1B). In several mutants an underglycosylated form of Sec71p could be detected (Figure 1B). The growth defects of none of the Cs sec61 mutants was overcome by overexpressing the mutant allele on a 2μ-based multicopy plasmid. This result confirms that Sec61 protein levels were not limiting growth of the Cs strains.
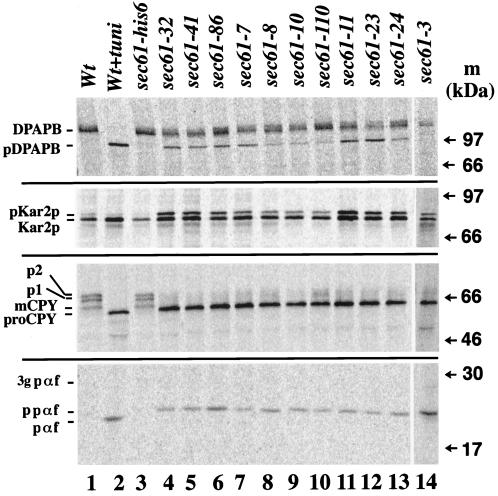
Translocation defects in the mutant strains were assessed by in vivo pulse labeling with [35S]methionine/cysteine at the permissive temperature of 30°C followed by immunoprecipitation of secretory proteins that undergo either cotranslational or posttranslational translocation (Figure 2). The precursor of the vacuolar membrane protein DPAPB is translocated cotranslationally, whereas the precursors of the vacuolar CPY and the mating pheromone α-factor are translocated posttranslationally (Ng et al., 1996). The precursor of Kar2p can use both pathways (Ng et al., 1996). Proteolytic processing of the signal sequence and N-glycolysation were used as indicators of translocation.
Figure 2.
Cs SEC61 mutant cells are deficient for protein translocation into the ER in vivo. Wild-type and mutant cells were pulse labeled with [35S]methionine/cysteine at 30°C for 15 min as described in MATERIALS AND METHODS, and secretory proteins were immunoprecipitated. The glycosylation inhibitor tunicamycin was present at 10 μg/ml in one culture of wild-type cells. The positions of precursor forms (pDPAPB, pKar2p, and ppαf), signal-cleaved, unglycosylated proteins (Kar2p, proCPY, and pαf), and signal-cleaved, glycosylated forms (DPAPB, p1, p2, mCPY, and 3 gpαf) are indicated.
DPAPB is a type II membrane protein with an N-terminal signal anchor sequence. Upon translocation into the ER the protein is core glycosylated to form the mature protein that is seen in wild-type and sec61-his6 cells (Figure 2, top panel, lanes 1 and 3). In cells pretreated with the glycosylation inhibitor tunicamycin, unglycosylated protein accumulated (Figure 2, top panel, lane 2). In sec61 mutant cells a fraction of DPAPB accumulated as cytoplasmic precursor (Figure 2, top panel). Cells expressing sec61-8, sec61-10, sec61-110, and sec61-3 were only moderately defective for DPAPB translocation into the ER (Figure 2, top panel).
The precursor of Kar2p was efficiently translocated and processed in wild-type and sec61-his6 cells producing the mature form (Figure 2, second panel, lanes 1 and 3). Because Kar2p is not a glycoprotein, tunicamycin treatment did not influence Kar2p maturation (Figure 2, second panel, lane 2). In the sec61 mutants an increased amount of precursor form remained in the cytoplasm; sec61-8, sec61-10, sec61-110, and sec61-3 again displayed the weakest defects in translocation.
The signal sequence of CPY is cleaved upon translocation into the ER, resulting in proCPY, which is core glycosylated to p1CPY; after transport to the Golgi complex outer chain mannose residues are added (p2CPY); in the vacuole p2CPY is proteolytically processed to mature CPY (mCPY; Stevens et al., 1982). The p1, p2, and mCPY proteins were immunoprecipitated from wild-type and sec61-his6 cells (Figure 2, third panel, lanes 1 and 3). Tunicamycin pretreatment of the cells inhibited core glycosylation and transport to the vacuole and thus led to the accumulation of proCPY in the ER (Figure 2, third panel, lane 2). All sec61 mutants accumulated a protein with a lower electrophoretic mobility than proCPY, consistent with the molecular weight of the untranslocated precursor (Figure 2, third panel, lanes 4–14).
Upon translocation into the ER the signal sequence of ppαf is cleaved off (pαf), and the protein acquires core glycosylation at three asparagine residues (3 gpαf). In wild-type and sec61-his6 cells 3 gpαf was not detected under the labeling conditions used, because the protein was efficiently transported to the Golgi complex, where it was processed to smaller peptides (Figure 2, bottom panel, lanes 1 and 3). Tunicamycin treatment led to the accumulation of signal-cleaved pαf in the ER (Figure 2, bottom panel, lane 2). All mutant cells accumulated the precursor form ppαf in the cytoplasm.
In summary, strains expressing the new Cs sec61 alleles accumulated cytoplasmic precursors of both co- and posttranslationally translocated proteins; at 30°C the sec61 mutants were more defective for import of the posttranslationally translocated CPY and α-factor precursors (Figure 2). At 17°C complete translocation blocks were also observed for DPAPB and Kar2p.
sec61 Mutants Are Defective Both in Forward and in Retrograde Translocation across the ER Membrane In Vitro
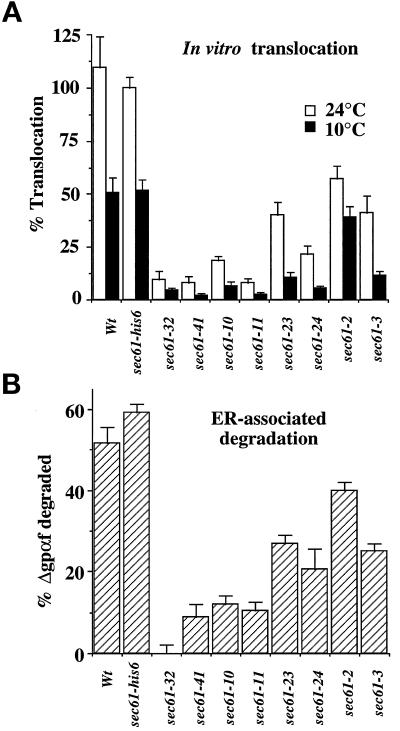
We next investigated the protein translocation defects in the sec61 mutants in cell-free assay systems using membranes from cells grown at the permissive temperature (30°C). We first analyzed the effects of the mutations on posttranslational protein import into the ER in vitro using an assay that measures the translocation of ppαf by the formation of fully glycosylated, membrane-enclosed 3 gpαf. The optimal temperature for in vitro translocation into ER-derived microsomes is 24°C (Pilon et al., 1997). We performed the assay at two temperatures, 24 and 10°C, to investigate whether the cold sensitivity of translocation was reproducible in vitro. The presence of the histidine tag in sec61-his6 did not influence translocation in vitro compared with the wild-type strain (Figure 3A). Microsomes from Cs sec61 mutants were strongly defective for translocation at 24°C (Figure 3A, open bars). Translocation was least affected in sec61-2 and sec61-3 membranes, which was remarkable given the low amounts of Sec61p in the ER of these cells (Figure 1B). At 10°C translocation into wild-type microsomes was reduced by only 50% relative to 24°C. In contrast, translocation into the Cs mutant membranes was negligible at 10°C (Figure 3A, filled bars). For sec61-2, which is the only sec61 mutant that has no Cs growth phenotype, lowering the temperature in the in vitro assay had only a limited effect on translocation.
Figure 3.
Sec61 mutant strains are defective both for ppαf translocation into the ER and export for degradation (ERAD) of an unglycosylated pαf form. (A) In vitro translocation. ppαf was incubated with membranes in the presence of ATP at the indicated temperature for 40 min. Translocation was measured by the formation of fully glycosylated, protease-protected pαf as described in MATERIALS AND METHODS. Each bar is the average of five experiments with SE. Translocation in sec61-his6 at 24°C was set at 100%. Approximately one-third of the added precursor was translocated in these membranes. (B) ERAD. The glycosylation site mutant pΔgpαf was translocated at 24°C for 50 min, and then the membranes were washed and reincubated with 6 mg/ml cytosol in the presence of ATP. The decrease in the amount of signal-cleaved, unglycosylated pro-α-factor (Δgpαf) over 30 min was quantified as described in MATERIALS AND METHODS. Each bar is the average of three experiments with SE.
Misfolded secretory and membrane proteins are not degraded in the ER lumen (a process formerly known as “ER degradation”) but, rather, are exported from the ER to the cytosol where they are degraded by proteasomes (Hiller et al., 1996; Werner et al., 1996; Wiertz et al., 1996). This process is now termed ERAD (McCracken and Brodsky, 1996). Using an in vitro assay that measures export from the ER and degradation of a mutant, unglycosylated form of pro-α-factor (Δgpαf) we have shown that sec61-41 and especially sec61-32 are deficient in this process (Pilon et al., 1997). As shown in Figure 3A, these mutants also have considerable defects in protein import into the ER. We asked whether any of our novel sec61 mutants were specifically defective for protein export from the ER. Because ERAD in vitro is very much reduced even in wild-type membranes below 20°C (Pilon et al., 1997), we performed the ERAD assays at 24°C. The results with only the more stringent alleles are shown in Figure 3B. Efficient ERAD was observed for both wild-type and sec61-his6 membranes. All sec61 mutants tested were clearly defective for ERAD in vitro (Figure 3B); in general the magnitude of the ERAD defect correlated with the defect in protein import into the ER in the mutants (Figure 3, compare A and B). Only sec61-32 was fully defective for ERAD, as reported before (Pilon et al., 1997). In addition to the experiments shown in Figure 3, we prepared membranes from all our novel sec61 mutant strains both with and without the N-terminal histidine tag and compared in vitro protein import into the ER and ERAD, but we were unable to identify sec61 alleles specifically defective in misfolded protein export from the ER.
Early Interactions of Secretory Precursor with Its Receptor in the ER Membrane Are Affected in sec61 Mutants
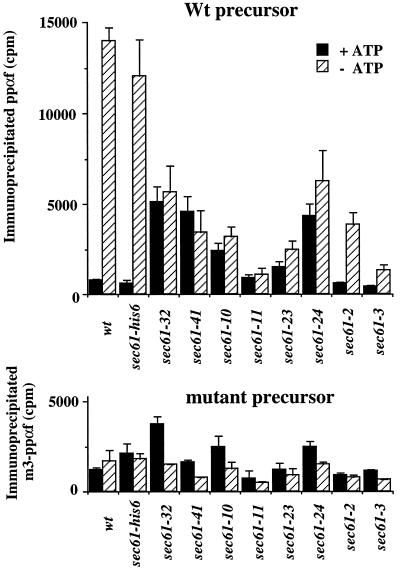
We further investigated the nature of the posttranslational protein import defects into the ER of our sec61 mutant strains in vitro. Posttranslational protein import into the ER proceeds through discrete stages: ATP-independent binding to a receptor complex consisting of at least Sec62p, Sec71p, and Sec72p; ATP-dependent and Kar2p/Sec63p-mediated transfer from receptor to the Sec61 pore; and Kar2p-mediated translocation through the Sec61 pore followed by release into the lumen of the ER (Sanders et al., 1992, Lyman and Schekman, 1995, 1997; Matlack et al., 1997). Receptor binding can be assayed by chemical crosslinking of the precursor to receptor proteins in the absence of ATP, followed by immunoprecipitation of specific receptor proteins of which Sec72p is the most prominent crosslinking partner for ppαf (Lyman and Schekman, 1997). A lack of transfer from the receptor should lead to increased crosslinking of ppαf to receptor proteins even in the presence of ATP, whereas trapping of the precursor inside the pore should lead to increased crosslinking to Sec61p in the presence of ATP. We incubated membranes from different mutants with ppαf in both the absence and presence of ATP followed by crosslinking and immunoprecipitation of Sec61p and Sec72p. As a control for nonspecific binding we used the m3-mutant of ppαf, which has a greatly reduced translocation efficiency because of a mutation in the signal sequence (Allison and Young, 1989). The most dramatic effects were obtained when the interaction with Sec72p was investigated (Figure 4). In agreement with previously published data (Lyman and Schekman, 1997) ppαf was efficiently crosslinked to Sec72p in wild-type membranes in the absence of ATP. This interaction depended on the presence of an intact signal sequence (Figure 4, compare A and B). Crosslinking in membranes containing wild-type or 6-histidine-tagged Sec61p (sec61-his6) gave similar results (Figure 4). The sec61 mutants, however, differed from wild-type in their interaction with Sec72p (Figure 4). We observed two effects: crosslinking to Sec72p in the absence of ATP was reduced, and the ATP dependence of this crosslink was lost. Based on the extent of these effects, two classes of mutants were distinguished. In a first class of mutants, of which sec61-11, sec61-23, and sec61-3 were the most prominent, little if any signal sequence-specific precursor crosslinking to Sec72p was observed (Figure 4, compare A and B). In a second class of mutants, most prominently sec61-32, sec61-41, and sec61-24, crosslinking to Sec72p occurred, albeit with reduced efficiency. However, in striking contrast to wild-type membranes, even in the presence of ATP the precursor remained associated with Sec72p (Figure 4A). In sec61-2 membranes crosslinking to Sec72p was reduced but still ATP regulated. Relative to wild-type membranes, only small differences were observed for the sec61 mutant membranes in precursor crosslinking to Sec61p (see Figure 5 for wild-type, sec61-11, and sec61-32).
Figure 4.
SEC61 mutants display defects in the interaction of ppαf with Sec72p in vitro. Radiolabeled ppαf wild-type or m3 (signal sequence)-mutant precursor were incubated with the indicated membranes (300,000 cpm precursor and 200 μg microsomal protein for each immunoprecipitation) either in the presence (filled bars) or absence (hatched bars) of ATP, followed by crosslinking with DSP and immunoprecipitation with Sec72p antibodies. The crosslinking and coimmunoprecipitation efficiency of ppαf was determined by liquid scintillation counting. Each bar is the average of three experiments with SE.
Figure 5.
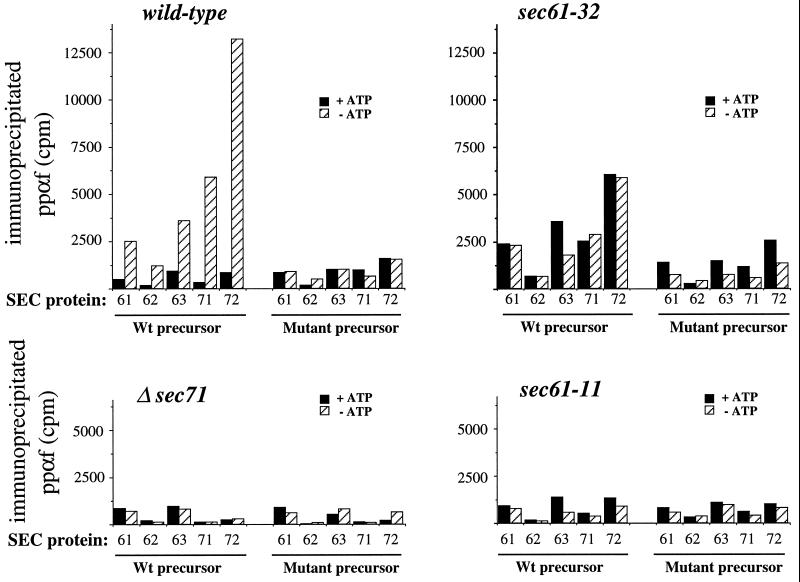
Sec61p, Sec62p, Sec63p, Sec71p, and Sec72p all interact with precursor proteins in the absence of ATP; these interactions are dependent on an intact signal sequence and are altered in sec61 mutant membranes. Radiolabeled ppαf wild-type or m3-mutant precursor (300,000 cpm and 200 μg microsomal protein per immunoprecipitation) were incubated with the indicated membranes either in the presence (filled bars) or absence (hatched bars) of ATP followed by crosslinking with DSP and immunoprecipitation using antibodies against the indicated Sec proteins. Each bar is the average of two separate experiments.
Lyman and Schekman (1997) showed that Sec72p is part of a composite precursor binding site of the Sec62/63p complex that also includes Sec71p and Sec62p, and that mutations in any of these proteins block interaction of the precursor with all members of this complex. We have shown here that mutations in Sec61p influence the binding of secretory precursors to the receptor complex protein Sec72p (Figure 4), suggesting that Sec61p is either a part of this complex or a regulator of it. Thus, we were prompted to reinvestigate which members of the Sec complex interact with ppαf in the absence of ATP in wild-type membranes, and whether these same proteins interact with precursor in the second class of sec61 mutants, which includes sec61-32. Furthermore, we asked whether binding to receptor complex proteins other than Sec72p was abolished in the first class of sec61 mutants, which includes sec61-11. To address these questions, we analyzed precursor crosslinking to all Sec complex proteins both in the presence and in the absence of ATP (Figure 5). A sec71 deletion strain in which binding to all receptor proteins was abolished was used as a control (Figure 5, Δsec71). In agreement with previously published data, ppαf was crosslinked to Sec complex proteins of wild-type membranes in a signal sequence–dependent manner only in the absence of ATP (Figure 5, wild-type). Crosslinking and immunoprecipitation was most efficient using Sec72p antibodies, followed by Sec71p antibodies, and the least efficient using Sec62p antibodies (Figure 5, wild-type). Although this was not reported previously (Lyman and Schekman, 1997), efficient and ATP-regulated crosslinks were also observed for Sec63p and to a lesser extent for Sec61p in wild-type membranes (Figure 5, wild-type). No specific precursor crosslinks were observed in immunoprecipitations with Sss1p or Kar2p antibodies. Similar results were obtained with the membranes from two different wild-type strains. Signal sequence–dependent crosslinks were not observed in membranes from a Δsec71 strain (Figure 5, Δsec71), suggesting that the interaction detected in wild-type membranes represented true binding to a receptor complex. Interestingly, in sec61-32 membranes ppαf was crosslinked to the same cohort of Sec proteins both in the absence and in the presence of ATP (Figure 5, sec61-32), suggesting that the precursor was in a similar environment in both cases. In addition, the relative amounts of crosslinking to individual Sec proteins in these membranes in the absence of ATP compares well with wild-type membranes (Figure 5, hatched bars). Comparable results were obtained with sec61-24 membranes. In contrast, in sec61-11 membranes all specific precursor crosslinks to Sec proteins were lost (Figure 5, sec61-11). Similar results were obtained with sec61-23 and sec61-3 membranes. We conclude that Sec61p is part of a large receptor complex that also includes Sec62p, Sec63p, Sec71p, and Sec72p. Mutations in SEC61 can lead to loss of receptor function or loss of the ATP-dependent precursor release from this complex.
Lack of Precursor Binding in sec61-11 Is Directly Due to a Loss of Sec61p Function
Given the two types of defect described above in precursor-docking site interaction, we were prompted to investigate whether the Sec complex was intact in these membranes. In wild-type membranes a characteristic fraction of Sec61p is found as part of the Sec complex, which includes the glycoprotein Sec71p (Deshaies et al., 1991). This complex remains intact upon solubilization of membranes in digitonin and binds to the lectin Con-A because of the presence of oligosaccharides on Sec71p (Panzner et al., 1995). To investigate whether this complex is present in sec61 mutant membranes, we fractionated membrane protein complexes after solubilization in digitonin. In agreement with previously published data, we found that ∼75% of the Sec61p in wild-type membranes solubilized in digitonin was found in the supernatant after high-speed centrifugation (Figure 6, wild-type, Sol. vs. Total). Sec63p and Sec72p were almost quantitatively solubilized in digitonin and found in the Con-A binding fraction (Figure 6, wild-type, Total vs. Con-A). Approximately half of the solubilized Sec61p was also found in the Con-A binding fraction and was thus part of the Sec complex (Figure 6, wild-type, Total vs. Con-A). The pellet fraction included Sec61p bound to ribosomes, which were sedimented in the high-speed centrifugation step. This fraction of Sec61p was partially released by puromycin and GTP treatment in high salt (Figure 6, wild-type, RAMP), but the recovery of Sec61p in this RAMP fraction varied from experiment to experiment because of difficulties in resuspension of the high-speed pellet. With sec61 mutant membranes the fractionation of Sec63p and Sec72p was essentially the same as for wild-type membranes (Figure 6). The fractionation of Sec61p was also unchanged in sec61-32, sec61-11, sec61-23, and sec61-24 membranes (Figure 6). As shown earlier (Figure 1B), sec61-3 membranes contain less Sec61p. The amount of free Sec61p in sec61-3 membranes was dramatically reduced, and most of the Sec61p in these membranes was found in the Con-A fraction. However, the Sec61p/Sec63p ratio in the Con-A fraction of sec61-3 was slightly reduced relative to wild-type membranes (Figure 6, sec61-3 Con-A).
Figure 6.
The Sec complex is intact in Cs sec61 strains. ER membrane proteins were fractionated after solubilization in digitonin. Total, total microsomal protein; Sol., digitonin-soluble protein recovered from the 100,000 × g supernatant; Free, fraction of digitonin-soluble proteins not binding to Con-A; Con-A, proteins binding to concanavalin A. Equal aliquots of each fraction were analyzed by SDS-PAGE and immunoblotting using the indicated antibodies in the first incubation. For Sec63p and Sec61p, [35S]protein A was used in the secondary incubation, and blots were exposed to a PhosphorImager. Sec72p was visualized using the ECL procedure. The band visible below Sec63p in the Sec63p immunoblots is unrelated.
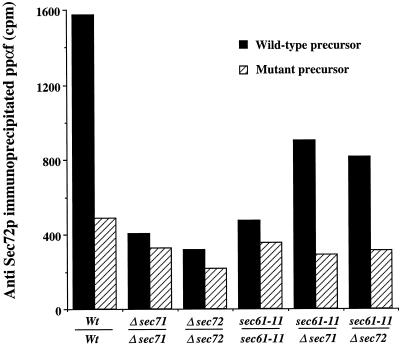
The reduced amounts of Sec61p in the Sec complex provide a direct explanation for the reduced precursor binding by sec61-3 membranes, if one assumes that Sec61p is an essential subunit of the receptor. In sec61-11 and sec61-23 membranes, however, which both displayed a strong defect in precursor binding (Figure 4), the Sec complex itself seemed intact (Figure 6, sec61-11 and sec61-23). An alternative possibility is that Sec61p function is required to assemble other members of the Sec complex before interaction with precursors, and that such assembly is defective in the absence of functional Sec61p. We reasoned that if the Sec62/Sec63p complex was intact in sec61-11 but simply lacked functional Sec61p, it should be possible to restore ppαf binding by providing wild-type Sec61p from a Δsec71 or Δsec72 strain (Lyman and Schekman, 1997). We took advantage of the observation that upon solubilization of membranes in the detergent octylglucoside Sec61p dissociates from a stable Sec63/Sec71/Sec72p subcomplex (Brodsky and Schekman, 1993). Functional Sec complex reassembles in reconstituted proteoliposomes upon removal of the detergent by dialysis (Brodsky et al., 1993). We analyzed ppαf binding to Sec72p, the most prominent crosslinking partner of the Sec complex in intact microsomes and in reconstituted membranes in the absence of ATP, and we used the m3-signal peptide mutant of ppαf as a control (Figure 7). Using reconstituted vesicles prepared from detergent extracts of wild-type membranes, we observed Sec72p crosslinking to ppαf (Figure 7, Wt/Wt, filled bar). The crosslinks were much reduced with m3-mutant ppαf (Figure 7, Wt/Wt, hatched bar). As expected, no specific crosslinking to Sec72p was observed in reconstituted vesicles from only a Δsec71 or a Δsec72 strain (Figure 7, Δsec71/Δsec71 and Δsec72/Δsec72). Proteoliposomes formed from a sec61-11 detergent extract were also inactive in specific ppαf binding to Sec72p (Figure 7, sec61-11/sec61-11). In contrast, mixing equal amounts of detergent extracts from sec61-11 and Δsec71 or Δsec72 restored the specific binding capacity of reconstituted proteoliposomes for ppαf (Figure 7, sec61-11/Δsec71 and sec61-11/Δsec72). These results indicate that Sec61p itself is required for ppαf binding to Sec72p, and that the defect in sec61-11 is not due to inactivation of other Sec proteins in this strain.
Figure 7.
Secretory precursor interaction with the receptor component Sec72p in sec61-11 membranes can be partly restored by adding wild-type Sec61p from an sec71 or sec72 deletion strain. Detergent (octylglucoside) extracts of the indicated wild-type (Wt) or mutant membranes were prepared and mixed in equal amounts before reconstitution of proteoliposomes by dialysis as described in MATERIALS AND METHODS. Binding of wild-type and m3-mutant ppαf precursor to Sec72p in the proteoliposomes reconstituted from 200 μg of solubilized membranes was assayed as in Figure 4. Each bar is the average of two experiments.
ATP-mediated Transfer of Secretory Precursors to the Translocation Pore Is Blocked in sec61-32 and sec61-24 Membranes
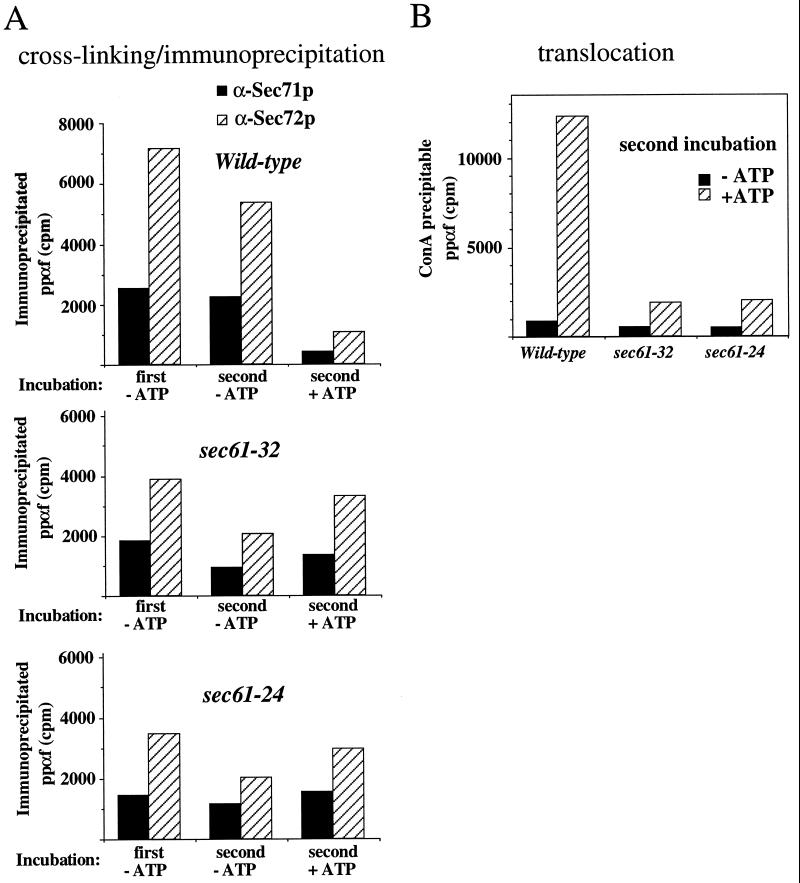
The observed crosslinking of secretory precursors to Sec proteins in the presence of ATP in sec61-32 and sec61-24 suggests that these membranes are defective in the Kar2p-mediated, ATP-dependent release of precursor from the receptor (Lyman and Schekman, 1997). To investigate this possibility directly, we first incubated membranes with precursor in the absence of ATP at 20°C to allow only binding but not translocation. After a 20-min incubation we split each reaction into three aliquots. One aliquot was analyzed directly for receptor binding (Figure 8A, first incubation), whereas the membranes in the other two aliquots were washed in buffer at 4°C to remove unbound ppαf. Subsequently, the washed membranes were resuspended in buffer in either the absence or presence of ATP. After a second incubation at 20°C for 20 min, receptor binding was assayed by analyzing crosslinking to Sec71p and Sec72p (Figure 8A, second incubation), and translocation was assayed by determining the amount of glycosylated protease-resistant α factor precursor (Figure 8B). Prebound precursor was efficiently released from Sec71p and Sec72p and translocated in wild-type membranes only in the presence of ATP (Figure 8, wild-type). In contrast, prebound precursor on sec61-32 and sec61-24 membranes was not significantly released from Sec71p and Sec72p, and only a low amount was translocated in the presence of ATP (Figure 8, sec61-32 and sec61-24). Instead, relative to the incubations without nucleotide, ATP seemed to stabilize the precursor at the receptor site. Thus sec61-32 and sec61-24 are defective in ATP-dependent release of secretory precursor from the receptor and its transfer to the translocation pore.
Figure 8.
Sec61-32 and sec61-24 membranes are defective in the ATP-mediated precursor release from the Sec complex and translocation through the Sec61p pore. ppαf precursor (300,000 cpm for each precipitation) was incubated with membranes at 20°C in the absence of ATP to allow interaction with the receptor complex. Incubations were then cooled on ice for 2 min, followed by centrifugation for 5 min at 15,000 × g to collect the microsomes. Membranes were washed in buffer and reincubated either in the presence or absence of ATP for 20 min at 20°C. After 20 min each reaction was divided into three aliquots, and receptor interaction (A) was assessed by analyzing crosslinking to Sec71p and Sec72p as outlined in Figure 4; translocation (B) was assessed by trypsin treatment followed by Con-A precipitation to determine the amount of protease-protected glycosylated pαf in the incubations. Note that because only a fraction of ppαf can be crosslinked and immunoprecipitated, the amounts of precursor in A represent only a portion of the molecules bound to the translocation machinery.
Overexpression of SEC63 Partially Suppresses the Cs Phenotype of a Subset of sec61 Mutants
Sec61p interacts with other proteins of the Sec complex, and such interactions may be perturbed by the sec61 mutations. Therefore we investigated whether growth defects in sec61 mutants could be overcome by overexpressing specific subunits of the complex. To avoid ambiguous results, we used only sec61 alleles with stringent growth phenotypes in this analysis. We tested suppression of the Cs phenotype by separately overexpressing Sec63p, Sec62p, Sec71p, Sec72p, Sss1p, and Sbh1p using multicopy vectors and Kar2p using a version of the gene under control of the GAL10 promoter. Strikingly, only overexpression of Sec63p alleviated the Cs growth defect of a subset of sec61 mutants, most notably sec61-24, and to a lesser extent sec61-32, sec61-41, and sec61-10 (Figure 9A). We determined by quantitative immunoblotting that Sec63p was overproduced approximately eightfold in each case. This level of Sec63p overexpression had no detectable effect on the growth of the wild-type strain (Figure 9A). In the sec61-23 strain, SEC63 overexpression led to slightly smaller colonies at the permissive temperature compared with the strain transformed with the multicopy control plasmid. In contrast, overproduction of Sec63p in sec61-11 impaired growth of this strain, and only very small colonies formed even at the permissive temperature (our unpublished results). As expected, Sss1p or Sbh1p overproduction but not Sec63p overproduction rescued the Ts phenotype of sec61-2 or sec61-3 (Esnault et al., 1994; Toikkanen et al., 1996).
To investigate whether the overexpression of SEC63 improved the performance of the translocation machinery in the sec61 mutants at the restrictive temperature, we performed pulse-labeling experiments (Figure 9B). The same secretory proteins that had been examined in Figure 2 were analyzed here by immunoprecipitation after labeling cells at 17°C. At this restrictive temperature sec61 mutants transformed with the control plasmid were defective for translocation of all precursors tested (Figure 9B, odd-numbered lanes). The four mutants whose growth defect was suppressed by Sec63p overproduction translocated relatively more DPAPB and Kar2p precursors when overexpressing Sec63p (Figure 9B, lanes 4, 6, 8, and 12); suppression of the translocation defect was most clearly seen in sec61-24. For CPY and α-factor, very little suppression of the translocation defect was observed (Figure 9B, bottom panels). Surprisingly, Sec63p overproduction in the wild-type strain led to an increase in cytoplasmic preproCPY and ppαf (Figure 9, lanes 2). Overproduction of Sec63p did not suppress the translocation defects in sec61-23 (Figure 9B, lane 9 vs. lane 10).
DISCUSSION
In this study we have characterized, both genetically and biochemically, a novel set of Cs mutants in sec61 that define two early stages of translocation (see Figure 10). The Cs alleles can be grouped into two classes based on both the stage at which translocation is blocked in vitro and on genetic interactions with SEC63. In contrast to the previously isolated Ts sec61 alleles, which affect the stability of Sec61p, the Cs mutant genes encode stable proteins that are assembled into the Sec complex in ER membranes. The growth defects of the Cs sec61 mutants are not overcome by overexpressing the mutant alleles on a multicopy plasmid. Together these results indicate that the growth defects of the Cs strains are due to a lack of Sec61p function and not reduced expression of the mutant protein. Conversely, the Cs mutants are not suppressed by overexpression of SSS1 or SBH1, which encode two other subunits of the Sec61p trimer, or by mutations in genes involved in ubiquitin- and proteasome-mediated protein degradation, whereas these genes and mutations suppress the Ts mutants sec61-2 and sec61-3 (Esnault et al., 1994; Biederer et al., 1996; Toikkanen et al., 1996).
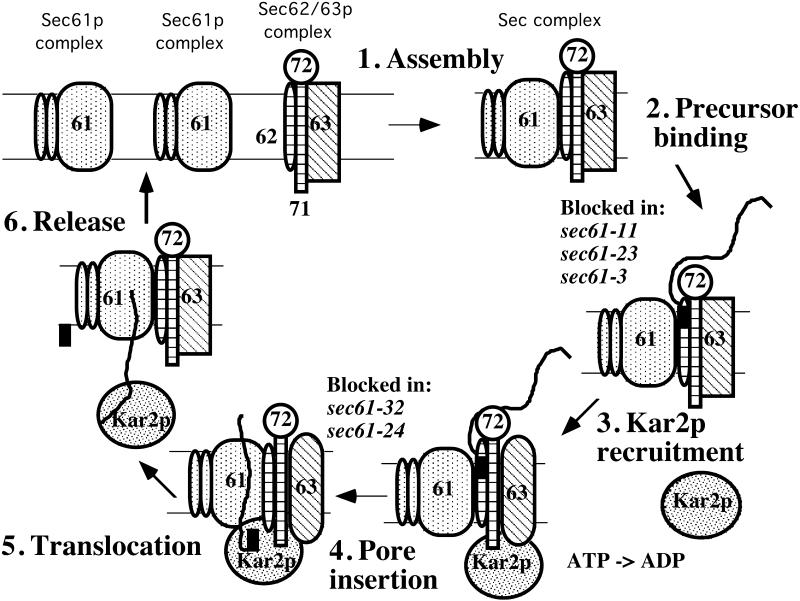
Figure 10.
Cs sec61 mutants define two early stages of posttranslational translocation. The trimeric Sec61p complex consists of Sec61p and two smaller proteins, Sbh1p and Sss1p. The Sec61p complex and Sec62/Sec63p complex assemble to form the heptameric Sec complex (1), which binds precursor at a cytoplasmic docking site (2). After Kar2p recruitment and ATP hydrolysis (3), precursor is released from the docking site and inserts into the pore (4). Kar2p directly promotes the translocation (5) and release of precursors into the lumen (6). The drawing does not represent all the protein–protein interactions known to occur.
We used chemical crosslinking in conjunction with immunoprecipitation to analyze the association of ppαf with individual Sec complex proteins. Mild conditions of crosslinking were used to diminish the possibility of indirect coimmunoprecipitation of ppαf and members of the Sec complex. However, precipitation of some of the less efficiently crosslinked products (e.g., Sec61p and Sec63p) may be mediated by an indirect contact with ppαf. We interpret the reduction in ppαf binding in sec61 mutants to be the consequence of a change in the translocation machinery caused by the sec61 mutation. However, this reduction could also be due to the presence of endogenous precursors bound to the receptor sites on isolated membranes. We consider this possibility unlikely, because membranes isolated from cells displaying a similar in vivo defect in translocation differ vastly in their capacity to bind ppα in vitro (e.g., compare sec61-32 and sec61-11 in Figures 2 and 4). In addition, translocation in vivo in sec61-10 is less severely impaired than in sec61-32 and sec61-24, yet sec61-10 membranes bind less ppαf in vitro.
The first class of Cs sec61 mutants, which includes sec61-11 and sec61-23, is defective in docking of precursor proteins onto the cytosolic face of the Sec complex. This characteristic is shared with the phenotype of sec62-1 and Δsec71 and Δsec72 strains (see Figure 5; Lyman and Schekman, 1997). It is likely that Sec61p directly interacts with the precursor, because ppαf is crosslinked in an ATP-inhibited manner to Sec61p in wild-type membranes, and these crosslinks are not observed in sec61-11 mutant membranes (Figure 5). The docking site also includes Sec63p in addition to Sec62p, Sec71p, and Sec72p, which are the previously identified components of this site (Lyman and Schekman, 1997). The interaction of ppαf with Sec63p was observed in our study and not before (Lyman and Schekman., 1997), most likely because of the use of a different Sec63 antibody preparation. The lack of precursor binding to sec61-11 membranes is due to the absence of functional Sec61p in the Sec complex and could be restored by providing wild-type Sec61p in a reconstitution experiment. Preprotein Sec complex interactions in the presence of ATP have previously been observed with solubilized Sec complex in the absence of Kar2p (Lyman and Schekman, 1997; Matlack et al,. 1997). Matlack et al. (1997) have shown that precursor docking to the ER membrane requires the assembly of the Sec61p complex and the Sec62/Sec63 complex into one unit. Our mutant analysis now demonstrates that the mere presence of Sec61p in this complex is not sufficient but that functional Sec61p is required.
The second class of sec61 mutants, which includes sec61-32 and sec61-24, allows interaction of a secretory precursor with the docking site on the cytoplasmic face of the Sec complex but is defective in the ATP-mediated release from this site, which in wild-type membranes leads to translocation. Precursors blocked at this stage interact with Sec61p and other Sec complex proteins. The phenotypes of the sec61-32 and sec61-24 mutants are very similar in this respect to those of kar2-203 and sec61-3 mutants, which are defective in releasing precursor from Sec61p at a stage before signal sequence cleavage or glycosylation of the precursor (Sanders et al., 1992; Lyman and Schekman, 1995, 1997; Matlack et al., 1997). The luminal Hsp70, Kar2p, hydrolyzes ATP and mediates the release of precursors from the docking site and concomitant insertion into the then-opened Sec61 pore (Sanders et al., 1992). Because Kar2p directly binds to the luminal DnaJ domain of Sec63p, a conformational change in Sec63p is proposed to trigger release of precursors from the docking site (Corsi and Schekman, 1997; Lyman and Schekman, 1997; Matlack et al., 1997). Our observation of a direct interaction of bound precursors with both Sec61p and Sec63p, and of the requirement of Sec61p function for precursor release from the binding site, suggests that Sec61p itself may also have to undergo a conformational change for this to happen. Interestingly, BiP, the mammalian orthologue of Kar2p, provides gating of the Sec61p complex in mammalian ER membranes (Hamman et al., 1998). We propose that sec61-32 and sec61-24 mutants are defective in pore opening toward the ER lumen, a decisive step in translocation that occurs in wild-type yeast cells when precursor binding initiates the interaction of Kar2p and Sec63p.
A striking observation in our study is that the growth of the first class of Cs sec61 mutants (sec61-11 and sec61-23) is sensitive to SEC63 overexpression. Membranes from these mutants fail to bind precursor and display no posttranslational translocation of ppαf. These mutants may adjust by adopting a more cotranslational path of translocation. The toxic effect of Sec63p overproduction in sec61-11 could well be due to the titration of components, most probably the mutant Sec61p itself, into an inactive Sec complex. In ER membranes part of the Sec61p complex is not attached to the Sec62/Sec63p complex or to ribosomes. In contrast, Sec63p seems almost quantitatively to be present in the Sec complex (Panzner et al., 1995; Figure 6), and its concentration may well be the limiting factor for Sec complex formation. Therefore Sec63p overproduction may sequester an essential fraction of mutant Sec61p that would otherwise be engaged in cotranslational translocation. An optimal Sec61p/Sec63p ratio may also be important in the wild type, because Sec63p overproduction resulted in some inhibition of translocation without affecting growth.
The second class of Cs sec61 mutants (sec61-32 and sec61-24) permits the first step in protein translocation, namely precursor docking onto the Sec complex, but is defective in proceeding to the next step, precursor release from the receptor and insertion into the pore. The partial suppression of this class of mutants by Sec63p overproduction may indicate that direct Sec61p/Sec63p interactions are involved in this step. Additional Sec63p may stabilize a more open state of the Sec61 channel, favoring the release and pore insertion of receptor site–bound precursors. Alternatively, suppression could result from the formation of more precursor-activated Sec complexes, allowing just enough translocation at the restrictive temperature for these cells to grow. Sec63p also functions in cotranslational translocation (Brodsky et al., 1995). Indeed, we found that the translocation defect of cotranslational cargo (e.g., pDPAPB and pKar2p) was more suppressed by Sec63p overproduction in sec61 mutants than the translocation defect of posttranslational substrates (e.g., pCPY and ppαf).
All of our mutants were defective in both secretory precursor import and in misfolded protein export from the ER. Most likely the defects in pore opening in protein translocation into the ER are accompanied by similar defects in retrograde transport. Presumably Sec61p interacts with different cofactor proteins that govern import and export, and therefore it should be possible to isolate alleles of sec61 that distinguish these processes. However, under normal growth conditions, misfolded secretory protein export from the ER is not essential; thus our selection for a Cs phenotype may exclude export-specific mutants. Compared with the Cs sec61 mutants ERAD was less defective in sec61-2 and sec61-3 membranes, which have reduced levels of Sec61p. The free fraction of Sec61p, Sec61p not bound to Sec complex or ribosomes, is most drastically reduced in the membranes of these Ts mutants, indicating that the amount of free Sec61p is not necessarily rate limiting to ERAD.
The N-terminal region of Sec61p seems to be particularly important to the function of the protein, and the addition of a histidine tag to the N terminus contributed to the observed phenotypes of sec61 mutants. Although the N-terminal 6-histidine tag did not affect the function of wild-type Sec61p, the Cs phenotype of the new sec61 alleles was lost without the tag. The possible functional importance of the N terminus of Sec61p was also observed by Wilkinson et al. (1996). Sec61p, which has 480 amino acids, has 10 transmembrane domains, each ∼20 amino acids in length (Wilkinson et al., 1996). In our mutants, of the 31 amino acid changes that we found, 17 were located in the 42% of the protein that makes up transmembrane domains, 11 mutations map to cytoplasmic regions, and 3 map to luminal loops. Strikingly, all his6-sec61 point mutants contained a single amino acid change in transmembrane domains 3 and 4, and the relevant mutation in sec61-11 also mapped to this region.
In contrast to the Ts Sec61 proteins, the Cs mutant proteins are metabolically stable. The location of mutations in hydrophobic regions may well contribute to the Cs phenotype of these strains, because hydrophobic interactions are adversely affected by low temperature (Baldwin, 1986). Protein translocation itself in Escherichia coli is Cs (Johnson and Beckwith, 1992). However, the strictly Ts phenotypes of kar2, sec62, sec63, and Δsec71 mutants suggests that cold sensitivity is a particular property of Sec61p.
Sec61p forms an aqueous channel in the ER membrane, whose opening and closing during protein translocation is tightly regulated (Hanein et al., 1996; Hamman et al., 1998). Sec61p could be a passive pore structure that is gated by other components, or it may have a more active role in channel creation and closure. The isolated Sec61p complex oligomerizes to form rings surrounding a 20-Å stain-filled pore. The assembly of these structures in synthetic membranes is enhanced by ribosomes or the Sec62/Sec63p complex (Hanein et al., 1996). Furthermore, the pore is in direct continuity with the polypeptide exit site on the ribosome (Beckmann et al., 1997). Whereas extrinsic factors such as the ribosome and BiP/Kar2p participate in pore regulation (Hamman et al., 1998), we suggest that the behavior of the two classes of mutant sec61 described in this report highlight a role of Sec61p in the transition from a closed to an open state.
ACKNOWLEDGMENTS
We thank Jaana Toikkanen and Sirkka Keränen (VTT Biotechnical Laboratory, Espoo, Finland) for the multicopy SBH1 plasmid, Enno Hartmann for Sbh1p antibodies, Dieter Wolf (University of Stuttgart, Germany) for yeast strains and a Der3 deletion plasmid, and Thomas Sommer (Max Planck Centrum, Berlin, Germany) for Ubc6, Ubc7, and Cue1 deletion strains. We are grateful for expert assistance from Bob Lesch with in vitro transcription and translation. We especially thank Jeff Brodsky and Susie Lyman for expert advice on in vitro translocation matters and crosslinking. We thank Edina Harsay, Sebastian Springer, Yuval Shimoni, and Mingyue Zhou for helpful comments on the manuscript and Jon Bertsch for help with computers. K.R. is a Senior European Fellow of The Wellcome Trust (042216). M.P. was supported by a fellowship from the Human Frontier Science Program and the Howard Hughes Medical Institute. R.S. is an investigator of the Howard Hughes Medical Institute and is supported by National Institutes of Health grant GM26755.
REFERENCES
- Allison DS, Young ET. Mutations in the signal sequence of prepro-α-factor inhibit both translocation into the endoplasmic reticulum and processing by signal peptidase in yeast cells. Mol Cell Biol. 1989;9:4977–4985. doi: 10.1128/mcb.9.11.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin RL. Temperature dependence of the hydrophobic interaction in protein folding. Proc Natl Acad Sci USA. 1986;83:8069–8072. doi: 10.1073/pnas.83.21.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann R, Bubeck D, Grassuci R, Penczek P, Verschoor A, Blobel G, Frank J. Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex. Science. 1997;278:2123–2126. doi: 10.1126/science.278.5346.2123. [DOI] [PubMed] [Google Scholar]
- Biederer T, Volkwein C, Sommer T. Degradation of subunits of the Sec61p complex, an integral component of the ER membrane, by the ubiquitin-proteasome pathway. EMBO J. 1996;15:2069–2076. [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL, Goeckeler J, Schekman R. BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc Natl Acad Sci USA. 1995;92:9643–9646. doi: 10.1073/pnas.92.21.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL, Hamamoto S, Feldheim D, Schekman R. Reconstitution of protein translocation from solubilized yeast membranes reveals topologically distinct roles for BiP and cytosolic Hsc70. J Cell Biol. 1993;120:95–102. doi: 10.1083/jcb.120.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL, Schekman R. A Sec63p-Bip complex from yeast is required for protein translocation in a reconstituted proteoliposome. J Cell Biol. 1993;123:1355–1363. doi: 10.1083/jcb.123.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. Multifunctional yeast high copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Corsi AK, Schekman R. The luminal domain of Sec63p stimulates the ATPase activity of BiP and mediates BiP recruitment to the translocon in Saccharomyces cerevisiae. J Cell Biol. 1997;137:1483–1493. doi: 10.1083/jcb.137.7.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies RJ, Sanders SL, Feldheim DA, Schekman R. Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature. 1991;349:806–808. doi: 10.1038/349806a0. [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Schekman R. Structural and functional dissection of Sec62p, a membrane-bound component of the yeast endoplasmic reticulum protein import machinery. Mol Cell Biol. 1990;10:6024–6035. doi: 10.1128/mcb.10.11.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnault Y, Blondel MO, Deshaies RJ, Schekman R, Kepes F. The yeast SSS1 gene is essential for secretory protein translocation and encodes a highly conserved protein of the endoplasmic reticulum. EMBO J. 1993;12:4083–4094. doi: 10.1002/j.1460-2075.1993.tb06092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnault Y, Feldheim D, Blondel M-O, Schekman S, Kepes F. SSS1 encodes a stabilizing component of the Sec61 subcomplex of the yeast protein translocation apparatus. J Biol Chem. 1994;269:27478–24785. [PubMed] [Google Scholar]
- Feldheim D, Rothblatt J, Schekman R. Topology and functional domains of Sec63p, an endoplasmic reticulum membrane protein required for secretory protein translocation. Mol Cell Biol. 1992;12:3288–3296. doi: 10.1128/mcb.12.7.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim D, Schekman R. Sec72p contributes to the selective recognition of signal peptides by the secretory polypeptide translocation complex. J Cell Biol. 1994;126:935–943. doi: 10.1083/jcb.126.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim D, Yoshimura K, Admon A, Schekman R. Structural and functional characterization of Sec66p, a new subunit of the polypeptide translocation apparatus in the yeast endoplasmic reticulum. Mol Biol Cell. 1993;4:931–939. doi: 10.1091/mbc.4.9.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Sugino A. New yeast Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:627–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Görlich D, Rapoport TA. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993;75:615–630. doi: 10.1016/0092-8674(93)90483-7. [DOI] [PubMed] [Google Scholar]
- Hamman BD, Hendershot LM, Johnson AE. Bip maintains the permeability barrier of the ER membrane by sealing the luminal end of the translocation pore before and early in translocation. Cell. 1998;92:747–758. doi: 10.1016/s0092-8674(00)81403-8. [DOI] [PubMed] [Google Scholar]
- Hanein D, Matlack KES, Jungnickel B, Plath K, Kalies K-U, Miller KR, Rapoport TA, Akey CW. Oligomeric rings of the Sec61p complex induced by ligands required for protein translocation. Cell. 1996;87:721–732. doi: 10.1016/s0092-8674(00)81391-4. [DOI] [PubMed] [Google Scholar]
- Hartmann E, Sommer T, Prehn S, Görlich D, Jentsch S, Rapoport TA. Evolutionary conservation of components of the protein translocation complex. Nature. 1994;367:654–657. doi: 10.1038/367654a0. [DOI] [PubMed] [Google Scholar]
- Hill JE, Myers AM, Koerner TJ, Tzagaloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986;2:163–168. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Hiller MM, Finger A, Schweiger M, Wolf D. ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science. 1996;273:1725–1728. doi: 10.1126/science.273.5282.1725. [DOI] [PubMed] [Google Scholar]
- Johnson KJ, Beckwith J. The Cs mutations of Escherichia coli reflect the cold sensitivity of protein export itself. Genetics. 1992;133:763–773. doi: 10.1093/genetics/133.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel B, Rapoport TA. A posttargeting signal sequence recognition event in the endoplasmic reticulum membrane. Cell. 1995;82:261–270. doi: 10.1016/0092-8674(95)90313-5. [DOI] [PubMed] [Google Scholar]
- Leung DW, Chen E, Goeddel DV. A method for random mutagenesis of a defined DNA segment using a modified polymerase chain reaction. Technique. 1989;1:11–15. [Google Scholar]
- Liao S, Lin J, Do H, Johnson AE. Both luminal and cytosolic gating of the aqueous ER translocon pore are regulated from inside the ribosome during membrane protein integration. Cell. 1997;90:31–41. doi: 10.1016/s0092-8674(00)80311-6. [DOI] [PubMed] [Google Scholar]
- Lyman SK, Schekman R. Interaction between BiP and Sec63p is required for the completion of protein translocation into the ER of Saccharomyces cerevisiae. J Cell Biol. 1995;131:1163–1171. doi: 10.1083/jcb.131.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman SK, Schekman R. Binding of secretory precursor polypeptides to a translocon subcomplex is regulated by BiP. Cell. 1997;88:85–96. doi: 10.1016/s0092-8674(00)81861-9. [DOI] [PubMed] [Google Scholar]
- Matlack KES, Mothes W, Rapoport TA. Protein translocation: tunnel vision. Cell. 1998;92:381–390. doi: 10.1016/s0092-8674(00)80930-7. [DOI] [PubMed] [Google Scholar]
- Matlack KES, Plath K, Misselwitz B, Rapoport TA. Protein transport by purified yeast Sec complex and Kar2p without membranes. Science. 1997;277:938–941. doi: 10.1126/science.277.5328.938. [DOI] [PubMed] [Google Scholar]
- Mayinger P, Meyer DI. An ATP transporter is required for protein translocation into the yeast endoplasmic reticulum. EMBO J. 1993;12:659–666. doi: 10.1002/j.1460-2075.1993.tb05699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken AA, Brodsky JL. Assembly of ER-associated degradation in vitro: dependence on cytosol, calnexin and ATP. J Cell Biol. 1996;132:291–298. doi: 10.1083/jcb.132.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothes W, Prehn S, Rapoport TA. Systematic probing of the environment of a translocating secretory protein during translocation through the ER membrane. EMBO J. 1994;13:3973–3982. doi: 10.1002/j.1460-2075.1994.tb06713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad D, Hunter R, Parker R. A rapid method for localized mutagenesis of yeast genes. Yeast. 1992;8:79–82. doi: 10.1002/yea.320080202. [DOI] [PubMed] [Google Scholar]
- Müsch A, Wiedmann M, Rapoport T. Yeast Sec proteins interact with polypeptides traversing the endoplasmic reticulum membrane. Cell. 1992;69:343–352. doi: 10.1016/0092-8674(92)90414-8. [DOI] [PubMed] [Google Scholar]
- Ng DTW, Brown J, Walter P. Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J Cell Biol. 1996;134:269–278. doi: 10.1083/jcb.134.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzner S, Dreier L, Hartmann E, Kostka S, Rapoport TA. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell. 1995;81:561–570. doi: 10.1016/0092-8674(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Pilon M, Schekman R, Römisch K. Sec61p mediates export of a misfolded secretory protein from the endoplasmic reticulum to the cytosol for degradation. EMBO J. 1997;16:4540–4548. doi: 10.1093/emboj/16.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemper RK, Böhmler S, Bordallo J, Sommer T, Wolf DH. Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature. 1997;388:891–895. doi: 10.1038/42276. [DOI] [PubMed] [Google Scholar]
- Sanders SL, Whitfield KM, Vogel JP, Rose MD, Schekman R. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992;69:353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Boeke JD. In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. Methods Enzymol. 1991;194:302–318. doi: 10.1016/0076-6879(91)94023-6. [DOI] [PubMed] [Google Scholar]
- Sommer T, Jentsch S. A protein translocation defect linked to ubiquitin conjugation at the endoplasmic reticulum. Nature. 1993;365:176–179. doi: 10.1038/365176a0. [DOI] [PubMed] [Google Scholar]
- Stevens TB, Esmon B, Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982;30:439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Stirling CJ, Rothblatt J, Hosobuchi M, Deshaies R, Schekman R. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol Biol Cell. 1992;3:129–142. doi: 10.1091/mbc.3.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toikkanen J, Gatti E, Takei K, Saloheimo M, Olkkonen V, Söderlund H, de Camilli P, Keränen S. Yeast protein translocation complex: isolation of two genes SEB1 and SEB2 encoding proteins homologous to the Sec61B subunit. Yeast. 1996;12:425–438. doi: 10.1002/(SICI)1097-0061(199604)12:5%3C425::AID-YEA924%3E3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Walter P, Johnson AE. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10:87–119. doi: 10.1146/annurev.cb.10.110194.000511. [DOI] [PubMed] [Google Scholar]
- Werner ED, Brodsky JL, McCracken AA. Proteasome-dependent endoplasmic reticulum-associated protein degradation: an unconventional route to a familiar fate. Proc Natl Acad Sci USA. 1996;93:13797–13801. doi: 10.1073/pnas.93.24.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiertz EJHJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson BM, Critchley AJ, Stirling CJ. Determination of the transmembrane topology of yeast Sec61p, an essential component of the endoplasmic reticulum translocation complex. J Biol Chem. 1996;271:25590–25597. doi: 10.1074/jbc.271.41.25590. [DOI] [PubMed] [Google Scholar]
- Wuestehube LJ, Schekman R. Selection and screening for yeast secretory mutants. Methods Mol Genet. 1993;1:88–106. [Google Scholar]