Abstract
A 44-year-old businessman with a history of hypertension presents for evaluation with a report of being under stress at work and home, which has led to “unsatisfactory” sleep. Although there is some despondency, screening for depression is negative. His blood pressure is 158/98 mm Hg. Laboratory results include a mean corpuscular volume of 102 fl (normal range, 80 to 100), an alanine aminotransferase level of 60 U per liter (normal range, 7 to 41), an aspartate aminotransferase level of 45 U per liter (normal range, 12 to 38), and a γ-glutamyltransferase level of 110 U per liter (normal range, 9 to 58). His physician asks about alcohol consumption, and the patient admits that perhaps he drinks “more than he should,” since he often wakes up with a hang-over and arrives late to work. After weekend golf outings, he comes home intoxicated, leading to arguments with his wife and embarrassment in front of his children. He has been quietly wondering about the need to cut down or stop drinking and wants some advice. His physician discusses medication or a referral to an alcohol clinic for further evaluation. Naltrexone is proposed as a treatment option.
THE CLINICAL PROBLEM
Alcohol-use disorders, including alcohol abuse and dependence, affect 7 to 8% of Americans at any given time, or about 15 to 20 million adults.1 The World Health Organization estimates that alcohol dependence is the third leading cause of disease burden in developing countries worldwide.2 In the United States, alcohol-use disorders account for $185 billion in health care costs, lost wages, bodily injury, and property damage annually.3 It is estimated that 20 to 36% of patients in primary care practices drink excessively and that 40 to 50% of cases in trauma and burn units involve excessive alcohol use.4,5 Excessive alcohol use is the leading cause of preventable hypertension and significantly increases the risk of heart attack and stroke.6 Despite these statistics, even among patients who drink excessively and meet the criteria for an alcohol-use disorder, the problem frequently goes undetected and untreated.7
The sine qua non of alcohol dependence is “lack of control” over alcohol use, indicated by drinking more than intended or the inability to cut down or stop drinking. The most common age range for initial treatment of alcohol dependence is 35 to 45. However, the peak period for meeting alcohol-dependence criteria is a decade or more earlier.8 A typical patient who presents for treatment of alcohol dependence is drinking, on average, more than five drinks per day on about 50 to 80% of days (a standard drink is 5 oz [0.15 liter] of wine, 12 oz [0.35 liter] of beer, or 1.5 oz [0.04 liter] of liquor). Health risks increase when drinking exceeds two to three drinks per day. With heavier and more frequent drinking, patients might encounter work or family problems, engage in high-risk behavior, or have health or legal problems, impaired concentration, and sleep difficulty. Clinically significant symptoms of alcohol withdrawal occur in less than 20% of patients with alcohol dependence, but such symptoms are usually a sign of severe dependence.
PATHOPHYSIOLOGY AND EFFECT OF THERAPY
Alcohol is the causative agent for alcohol-use disorders. Its excessive use influences several neurochemical systems in the brain, including the γ-aminobutyric acid (GABA), glutamate, dopamine, and opiate systems.9 The former two systems are primarily involved with alcohol stimulation, sedation, and intoxication, as well as many symptoms of alcohol withdrawal. The latter two systems are involved with reinforcement, reward, some aspects of craving, sustained use of alcohol, and potential relapse after prolonged abstinence in the dependent person.
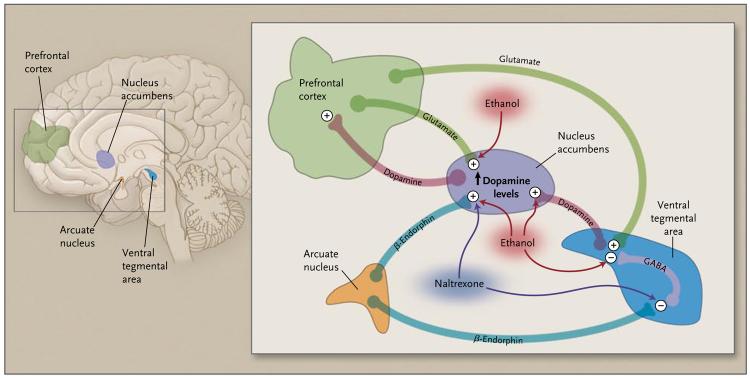
Acute alcohol use stimulates neurons in the ventral tegmental area of the brain, inducing the release of dopamine in the nucleus accumbens, an area of the brain that mediates reward, pleasure, and the assignment of salience to important environmental stimuli (Fig. 1). This effect may be enhanced by environmental cues that are associated with alcohol ingestion. Using brain-imaging techniques, investigators have shown that the nucleus accumbens of persons with alcoholism, but not social drinkers, is activated by such alcohol cues.10,11
Figure 1. Neurochemical Circuits Involved in Alcohol Dependence and Craving.
When an alcohol-dependent person consumes alcohol, dopamine is elevated in the nucleus accumbens. One mechanism of this elevation is the release of β-endorphin, which stimulates dopamine release either directly (in the nucleus accumbens) or indirectly (in the ventral tegmental area) by inhibiting the activity of γ-aminobutyric acid (GABA) neurons, thereby alleviating the blockade on dopamine cells. Naltrexone reverses both of these actions.
Several other neuronal pathways that are influenced by alcohol modulate this dopaminergic pathway. Low doses of alcohol stimulate the glutamate system, which enhances dopaminergic activity and leads to arousal and increased energy. In contrast, higher doses of alcohol inhibit glutamate and augment GABA neurotransmission, suppressing dopaminergic activity and leading to sedation, among other effects. These pathways are further modulated by endogenous opioid-like protein neurotransmitters, enkephalins and β-endorphin (Fig. 1). Such “endogenous opioids” are thought to be released during alcohol intoxication and may contribute to alcohol addiction by inhibiting GABA pathways and enhancing dopaminergic signaling.
Naltrexone is an agent that blocks opioid receptors, particularly the μ-opioid receptor. Use of this agent in animal models leads to a reduction of dopamine levels in the nucleus accumbens12-14 and a reduction in alcohol intake. The effect of environmental cues and associated alcohol craving can also be blocked by pretreatment with opioid-receptor antagonists in both animals and humans.14,15 In clinical laboratory settings, naltrex-one has been shown to decrease alcohol use as well.16-18 The confluence of these data has suggested that blocking opioid receptors with naltrexone might be one potential treatment approach to reduce alcohol use (by reducing the reward or pleasure of drinking) and to maintain abstinence (by reducing craving induced by environmental stimuli).
CLINICAL EVIDENCE
A number of randomized, controlled trials have evaluated the effectiveness of naltrexone in the management of alcohol dependence with the use of a variety of therapeutic end points. Many of these studies have included some form of behavioral intervention as an adjunct to medication.
Several of the largest such trials were multisite studies performed in the United States. A large clinical trial sponsored by the Department of Veterans Affairs19 enrolled 627 veterans with chronic, severe alcohol dependence. Naltrexone (at a dose of 50 mg daily), given for either 3 months or 12 months, was not significantly better than placebo in increasing the number of days until relapse or in reducing the percentage of drinking days or the number of drinks per drinking day.
On the other hand, the Combined Pharmacotherapies and Behavioral Interventions (COMBINE) study (ClinicalTrials.gov number, NCT00006206),20 which was conducted at 11 academic sites across the United States, enrolled 1383 patients with alcohol dependence and at least 4 days of abstinence. This trial showed that in patients who received medical treatment but not behavioral therapy, naltrexone (at a dose of 100 mg daily), given for 16 weeks, was more efficacious than placebo in increasing the percentage of days of abstinence (80.6% vs. 75.1%) and in reducing the risk of a heavy-drinking day (66.2% vs. 73.1%).
In the COMBINE study, investigators used an end point called “good clinical outcome,” which was defined as no more than 2 days of heavy drinking per week and drinking at or below a safe limit (14 drinks per week for men and 11 drinks per week for women) without significant alcohol-related problems during the last 8 weeks of the 16-week trial. In the group that received naltrexone, there was an absolute increase of approximately 15% in good clinical outcomes (73.7%, as compared with 58.2% in the placebo group; odds ratio in the naltrexone group, 2.16; 95% confidence interval, 1.46 to 3.20).
A few meta-analyses and a Cochrane review (all conducted before the publication of the COMBINE study) have systematically evaluated the accumulated data on the efficacy of naltrexone.21-25 Typically, the studies have shown that oral naltrexone was superior to placebo in preventing relapse to heavy drinking after an initial abstinence period and in increasing the percentage of abstinence days.
A long-acting, injectable form of naltrexone has also been evaluated in a large, multisite, randomized, controlled trial.26 In this study, persons with alcoholism were randomly assigned to receive one of two doses of intramuscular naltrexone (190 mg or 380 mg) or matching placebo. Injections were given monthly for up to 6 months, along with a form of structured medical treatment.27 The higher dose of naltrexone was found to reduce the number of heavy-drinking days by about 25% overall, with men doing signif than women. The beneficantly better icial effect of naltrexone was markedly greater among subjects who were abstinent for at least 4 days before randomization.26,28
CLINICAL USE
Therapeutic options for alcohol dependence range from brief interventions (such as counseling and education) performed by health care professionals through specialty counseling programs to drug therapies29,30 for more severe or chronic dependence. The Food and Drug Administration (FDA) has approved several medications to treat alcohol dependence, including disulfiram, acamprosate, and naltrexone. In addition, topiramate has also been shown to be potentially effective, although it has not been approved by the FDA for the treatment of alcohol dependence.31,32 In many cases, the optimal regimen may require a combination of therapeutic interventions or a trial of successive management approaches.
The ideal patient for naltrexone therapy would be a person who has moderate-to-severe alcohol dependence — for example, a person who drinks on more than 50% of days, consumes more than five drinks a day, and has alcohol-related problems. Such a person has probably failed in attempts to quit drinking but has a relatively high motivation to be abstinent or at least to try abstinence for a while. A good indication of this motivation is the ability to abstain from drinking for several days before starting naltrexone. The patient should always be asked to attempt to abstain for several days or be withdrawn from alcohol with medical assistance (benzodiazepines or anticonvulsants) before the drug is prescribed.
It is imperative that hepatic-enzyme tests (and perhaps tests of γ-glutamyltransferase and carbohydrate-deficient transferrin, if available)33-35 be obtained to establish baseline biomarker levels of drinking, along with urine screening for drugs of abuse, before the initiation of naltrexone treatment. Naltrexone is relatively contraindicated in patients who have liver-enzyme levels that are four to five times above the upper limit of the normal range. In addition, naltrexone, due to its blockade of brain opiate receptors, should not be used in patients who are dependent on opiates or those needing opiates for relief of chronic pain.
The typical starting dose of naltrexone is 25 mg for several days, with a subsequent increase to 50 mg per day over approximately 1 week. The drug should be taken after a meal, since nausea and vomiting are more likely to occur if the drug is taken while fasting. If abdominal symptoms occur, a reduction in the dose or maintenance of a lower dose with symptomatic treatment (e.g., with bismuth subsalicylate) may be effective. Tests of hepatic enzymes (and possibly of γ-glutamyltransferase and carbohydrate-deficient transferrin) should be obtained about a month after the initiation of treatment. Such tests can be repeated monthly during a 4-month course of treatment with naltrexone. Common adverse events are nausea, vomiting, headache, and fatigue. Most side effects are mild and self-limiting and usually occur only during initial therapy.
Treatment with daily oral naltrexone should last for at least 3 to 4 months. If the patient becomes completely abstinent during the last several months of treatment, naltrexone can be stopped, and monthly monitoring should continue during the next 4 to 6 months. If an increase in craving occurs or drinking resumes, naltrexone can be restarted. If sporadic heavy drinking occurs during the first 3 to 4 months of treatment, then continued naltrexone treatment for a prolonged period (chronic treatment model) should be considered. If the patient continues to drink heavily in the face of naltrexone treatment, the dose can be increased to 100 mg, or the use of long-acting injectable naltrexone (380 mg administered intramuscularly once every month) can be considered to rule out noncompliance as a cause of treatment failure. In such cases, long-term use of oral or injectable naltrexone should be prescribed and administered, and consultation with an alcohol-treatment specialist should be considered.
Naltrexone should not be prescribed without some sort of supportive counseling or medical management. The medical-management approach that was used in the COMBINE study included an initial 45-minute visit with a health care professional (physician, nurse, or physician assistant), followed by visits of 15 or 20 minutes once a week for 2 weeks, then every 2 weeks over 10 weeks, and then again after 1 month.36,37 This regimen includes an emphasis on alcohol education, motivation toward abstinence, referral to Alcoholics Anonymous if such attendance is acceptable to the patient, assessment of medication adherence, review of side effects, and laboratory testing. Failure of this approach would trigger a referral to specialized alcohol counseling.
It is advisable for patients taking naltrexone to carry a card detailing their use of this agent that can be shown to health care providers. Because naltrexone blocks opioid receptors, it can render opiate analgesics ineffective, which may be of practical clinical importance, especially in an emergency setting.
In the United States, the cost of a 1-month supply of oral naltrexone (at 50 mg daily) is approximately $205 for the branded drug or $130 for the generic agent.38 The cost of a single dose (380 mg) of injectable naltrexone, for which no generic form is currently available, is $869.39 Additional costs of therapy include the costs of blood tests and office visits for monitoring, as well as expenses associated with specialized behavioral therapy.
ADVERSE EFFECTS
Most of the adverse events occurring early in treatment with naltrexone are gastrointestinal, including nausea, vomiting, and abdominal pain or discomfort. These side effects, along with headache and fatigue, made up the majority of reports in clinical trials.20,40,41 Such effects, usually mild in intensity, occurred in up to 30% of patients.
Hepatotoxicity that is associated with naltrexone has been reported, especially in obese patients who were receiving high doses of the drug (100 to 300 mg daily). These reports led to an FDA-mandated black-box warning, which is included in the package insert. In a safety study involving persons with alcoholism, 50 mg of naltrexone daily was not associated with substantial hepatotoxicity.40 In the COMBINE study, elevations in liver-enzyme levels of more than five times the upper limit of the normal range occurred in 11 of 614 patients receiving naltrexone at a dose of 100 mg daily. Most of these elevations normalized when the medication was stopped. No serious sequelae were reported, although some of the study subjects were lost to follow-up.
Naltrexone can cause immediate and severe withdrawal in patients who are physically dependent on opiates. A clinical review of opiate use is mandatory, and opiate-drug screening should be considered before naltrexone is prescribed. Patients with an anticipated future need for opiates (such as those expecting to undergo elective surgery) may not be good candidates for naltrexone. Oral naltrexone must be stopped 48 to 72 hours before opiate analgesia. For patients receiving injectable naltrexone, opiate blockade may last for a month or more. Opiate antagonism may be over-ridden with high opiate doses to provide acute opiate analgesia, but such management requires close monitoring by qualified personnel, since respiratory depression may be sudden and life-threatening.
AREAS OF UNCERTAINTY
It is not clear whether, for best results, patients with a diagnosis of alcohol dependence should be abstinent before taking naltrexone. However, the weight of the evidence suggests that such abstinence is the most judicious approach.28 The optimal length of treatment is also open to question. Most data20,42,43 suggest that 3 to 4 months of treatment can be efficacious but that many patients will relapse to heavy drinking within months to a year after the discontinuation of treatment.42,44,45 Longer courses of treatment or a staged approach to treatment may be attempted, although such approaches should be considered in consultation with an alcohol-treatment specialist.
Another area of uncertainty is whether the use of relapse-prevention therapy that is provided by an addiction counselor might work better with naltrexone therapy than would a medical-management approach alone. Although the COMBINE study did not support such an approach, others43,46 have advocated this form of management. It is possible that any kind of compliance enhancement, whether with the use of drugs (such as long-acting, injectable naltrexone) or counseling (such as medical management focused on compliance), may be equally beneficial. Data from the COMBINE study and the Long-Acting Injectable Naltrexone Study (ClinicalTrials.gov number, NCT00156923)26 and others47 are consistent with that idea.
There is also uncertainty as to whether practitioners should use naltrexone as a first treatment option and, if so, under which circumstances. Several other forms of above-mentioned therapies may also be effective. One option is that of using a brief intervention (education and counseling by the primary physician) first, especially in cases of mild alcohol dependence, and then adding naltrexone (or perhaps other medications) with medical management or referral to specialized counseling as necessary. For cases of moderate-to-severe dependence, either specialized counseling or naltrexone with medical management might be used first, reserving other approaches for treatment failures.
GUIDELINES
In 1998, a Treatment Improvement Protocol on the use of naltrexone in alcohol dependence was published.48 This protocol provided detailed recommendations, including a discussion of eligibility for treatment, dosing regimens, and monitoring of patients. It emphasized the importance of concurrent psychosocial intervention and the concern about the risk of hepatotoxicity. In 2007, the American Psychiatric Association published a practice guideline on the treatment of substance abuse, including a section on alcohol dependence.41 It included a systematic review of the literature and concluded that “naltrexone may attenuate some of the reinforcing effects of alcohol, although data on its long-term efficacy are limited.” Finally, in 2005, the National Institute on Alcohol Abuse and Alcoholism published a clinician's guide entitled Helping Patients Who Drink Too Much, with revisions in 2007.49 This guide provides information for primary care practitioners on the screening and management of alcohol-use disorders. It includes a table comparing the commonly used drugs and session templates designed to assist in the conduct of office visits.
RECOMMENDATIONS
The case presented here is a fairly typical example of a heavy drinker who is mildly to moderately dependent on alcohol. Since his condition does not appear to be severe, the practitioner could offer brief advice to reduce or stop drinking with monitoring of blood tests and blood-pressure measurement during a period of 2 to 4 weeks. Obtaining spousal reports to confirm progress would be important as well.
If episodes of drinking and levels of liver enzymes and blood pressure do not substantially improve, referral to a specialized alcohol counselor could be offered. However, this patient is also a reasonable candidate for oral naltrexone or, on the basis of the preference of the patient or physician, long-acting injectable naltrexone. Treatment should begin at a dose of 25 mg daily, with an increase to 50 mg during the first week. During this treatment, the patient should be seen every 1 to 2 weeks for the first several months and then less frequently thereafter. Concomitant supportive counseling or active medical guidance, as described in the Clinical Use section, is an essential counterpart to medication. Spousal reports and blood tests are helpful to gauge progress and provide objective feedback. Treatment for at least 4 months is indicated, with follow-up monthly for up to a year. Treatment may be reinstituted at the first sign of relapse to heavy drinking or if craving increases. Once abstinence is maintained for a month or so, the need for antihypertensive medication can be reevaluated.
Acknowledgments
Dr. Anton reports receiving grant support from Pfizer, Ortho-McNeil, Bristol-Myers Squibb, and Hythiam, consulting fees from AstraZeneca, Axis-Shield, Eli Lilly, Intranasal Therapeutics, Sanofi-Aventis, Bristol-Myers Squibb, and Merck, and lecture fees from Cephalon and serving on scientific advisory boards for Sanofi-Aventis, Hythiam, Solvay, Novartis, and Johnson & Johnson.
Footnotes
No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration . Results from the 2006 National Survey on Drug Use and Health: national findings. Office of Applied Studies; Rockville, MD: 2007. (NSDUH series H-32). DHHS publication no. SMA 07-4293. [Google Scholar]
- 2.Global status report: alcohol policy. World Health Organization; Geneva: 2004. [Google Scholar]
- 3.Updating estimates of the economic costs of alcohol abuse in the United States: estimates, update methods, and data. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000. [Google Scholar]
- 4.Fiellin DA, Carrington R, O'Connor PG. New therapies for alcohol problems: application to primary care. Am J Med. 2000;108:227–37. doi: 10.1016/s0002-9343(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 5.Lowenfels AB, Miller TT. Alcohol and trauma. Ann Emerg Med. 1984;13:1056–60. doi: 10.1016/s0196-0644(84)80070-0. [DOI] [PubMed] [Google Scholar]
- 6.Klatsky AL. Alcohol-associated hypertension: when one drink makes a difference. Hypertension. 2004;44:805–6. doi: 10.1161/01.HYP.0000146538.26193.60. [DOI] [PubMed] [Google Scholar]
- 7.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 8.Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004;74:223–34. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Koob GF, Roberts AJ, Schulteis G, et al. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- 10.Myrick H, Anton RF, Li X, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 11.Heinz A, Siessmeier T, Wrase J, et al. Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–9. doi: 10.1176/appi.ajp.161.10.1783. Erratum, Am J Psychiatry 2004;161:2344. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin D, Grant ER, Pohorecky LA. Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res. 1993;621:137–40. doi: 10.1016/0006-8993(93)90309-b. [DOI] [PubMed] [Google Scholar]
- 13.Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–71. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middaugh LD, Szumlinski KK, Van Patten Y, Marlowe AL, Kalivas PW. Chronic ethanol consumption by C57BL/6 mice promotes tolerance to its interoceptive cues and increases extracellular dopamine, an effect blocked by naltrexone. Alcohol Clin Exp Res. 2003;27:1892–900. doi: 10.1097/01.ALC.0000099264.36220.48. [DOI] [PubMed] [Google Scholar]
- 15.Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cueinduced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–75. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drobes DJ, Anton RF, Thomas SE, Voronin K. Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment-seeking alcoholics and social drinkers. Alcohol Clin Exp Res. 2004;28:1362–70. doi: 10.1097/01.alc.0000139704.88862.01. [DOI] [PubMed] [Google Scholar]
- 17.O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and acti-vates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- 18.Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology (Berl) 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- 19.Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–9. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- 20.Anton RF, O'Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 21.Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alcohol Alcohol. 2001;36:544–52. doi: 10.1093/alcalc/36.6.544. [DOI] [PubMed] [Google Scholar]
- 22.Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–41. [PubMed] [Google Scholar]
- 23.Bouza C, Angeles M, Muñoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–28. doi: 10.1111/j.1360-0443.2004.00763.x. Erratum, Addiction 2005;100:573. [DOI] [PubMed] [Google Scholar]
- 24.Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267–80. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- 25.Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005;1:CD001867. doi: 10.1002/14651858.CD001867.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Garbutt JC, Kranzler HR, O'Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–25. doi: 10.1001/jama.293.13.1617. Errata, JAMA 2005;293:1978, 2864. [DOI] [PubMed] [Google Scholar]
- 27.Pettinati HM, Volpicelli JR, Pierce JD, Jr, O'Brien CP. Improving naltrexone response: an intervention for medical practitioners to enhance medication compliance in alcohol dependent patients. J Addict Dis. 2000;19:71–83. doi: 10.1300/J069v19n01_06. [DOI] [PubMed] [Google Scholar]
- 28.O'Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent before treatment. J Clin Psychopharmacol. 2007;27:507–12. doi: 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- 29.Miller WR, Wilbourne PL. Mesa Grande: a methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction. 2002;97:265–77. doi: 10.1046/j.1360-0443.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- 30.Carroll KM. Integrating psychotherapy and pharmacotherapy to improve drug abuse outcomes. Addict Behav. 1997;22:233–45. doi: 10.1016/s0306-4603(96)00038-x. [DOI] [PubMed] [Google Scholar]
- 31.Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–51. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BA, Rosenthal N, Capece JA, et al. Improvement of physical health and quality of life of alcohol-dependent individuals with topiramate treatment: US multisite randomized controlled trial. Arch Intern Med. 2008;168:1188–99. doi: 10.1001/archinte.168.11.1188. [DOI] [PubMed] [Google Scholar]
- 33.Miller PM, Ornstein SM, Nietert PJ, Anton RF. Self-report and biomarker alcohol screening by primary care physicians: the need to translate research into guidelines. Alcohol Alcohol. 2004;39:325–8. doi: 10.1093/alcalc/agh070. [DOI] [PubMed] [Google Scholar]
- 34.Miller PM, Anton R. Biochemical alcohol screening in primary health care. Addict Behav. 2004;29:1427–37. doi: 10.1016/j.addbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Fleming M, Mundt M. Carbohydrate-deficient transferrin: validity of a new alcohol biomarker in a sample of patients with diabetes and hypertension. J Am Board Fam Pract. 2004;17:247–55. doi: 10.3122/jabfm.17.4.247. [DOI] [PubMed] [Google Scholar]
- 36.Medical management (MM) treatment manual. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2004. (COMBINE monograph series). DHHS publication no. (NIH) 04-5289. [Google Scholar]
- 37.Pettinati HM, Weiss RD, Dundon W, et al. A structured approach to medical management: a psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. J Stud Alcohol Suppl. 2005;15:170–8. doi: 10.15288/jsas.2005.s15.170. [DOI] [PubMed] [Google Scholar]
- 38.Williams SH. Medications for treating alcohol dependence. Am Fam Physician. 2005;72:1775–80. [PubMed] [Google Scholar]
- 39.New drug bulletin: naltrexone extended-release suspension for injection. Department of Pharmacy Services, University of Utah Hospital; Salt Lake City: 2007. Accessed July 21, 2008, at http://uuhsc.utah.edu/pharmacy/bulletins/NDB_126.pdf. [Google Scholar]
- 40.Croop RS, Faulkner EB, Labriola DF. The safety profile of naltrexone in the treatment of alcoholism: results from a multicenter usage study. Arch Gen Psychiatry. 1997;54:1130–5. doi: 10.1001/archpsyc.1997.01830240090013. [DOI] [PubMed] [Google Scholar]
- 41.Practice guideline for the treatment of patients with substance use disorders. Am J Psychiatry. (2nd ed.) 2007;164(Suppl):A5–A124. Also available at http://ajp.psychiatryonline.org/cgi/data/164/4/A58/ DC2/1.
- 42.O'Malley SS, Jaffe AJ, Chang G, et al. Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Arch Gen Psychiatry. 1996;53:217–24. doi: 10.1001/archpsyc.1996.01830030039007. [DOI] [PubMed] [Google Scholar]
- 43.Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156:1758–64. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- 44.Donovan DM, Anton RF, Miller WR, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence (the COMBINE Study): examination of posttreatment drinking outcomes. J Stud Alcohol Drugs. 2008;69:5–13. doi: 10.15288/jsad.2008.69.5. [DOI] [PubMed] [Google Scholar]
- 45.Anton RF, Moak DH, Latham PK, et al. Posttreatment results of combining naltrexone with cognitive-behavior therapy for the treatment of alcoholism. J Clin Psychopharmacol. 2001;21:72–7. doi: 10.1097/00004714-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Berglund M, Thelander S, Salaspuro M, Franck J, Andréasson S, Ojehagen A. Treatment of alcohol abuse: an evidence-based review. Alcohol Clin Exp Res. 2003;27:1645–56. doi: 10.1097/01.ALC.0000090144.99832.19. [DOI] [PubMed] [Google Scholar]
- 47.O'Malley SS, Rounsaville BJ, Farren C, et al. Initial and maintenance naltrexone treatment for alcohol dependence using primary care vs specialty care: a nested sequence of 3 randomized trials. Arch Intern Med. 2003;163:1695–704. doi: 10.1001/archinte.163.14.1695. [DOI] [PubMed] [Google Scholar]
- 48.Treatment Improvement Protocol (TIP) series. Department of Health and Human Services; Rockville, MD: 1998. Accessed July 21, 2008, at http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat5.chapter.51510. [Google Scholar]
- 49.Helping patients who drink too much: a clinician's guide: updated 2005 edition. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 2007. Accessed July 21, 2008, at http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf. [Google Scholar]