Abstract
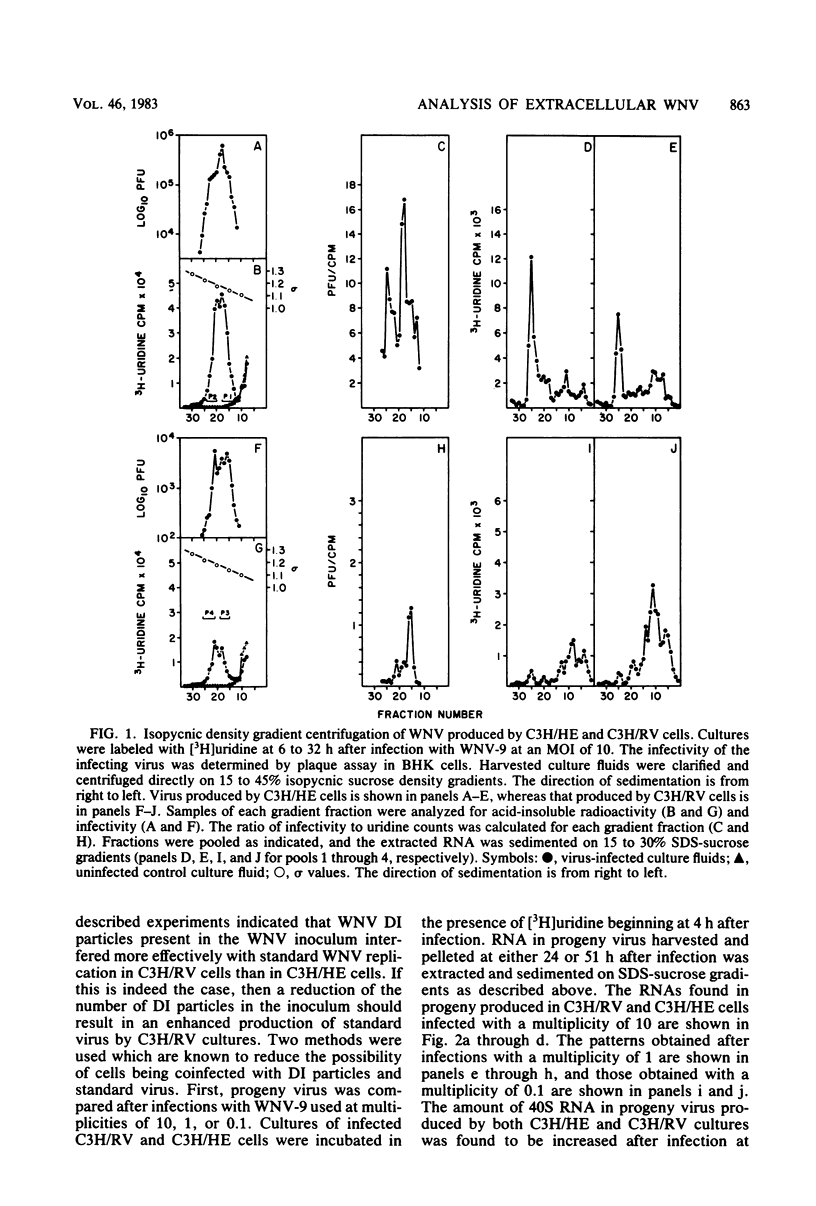
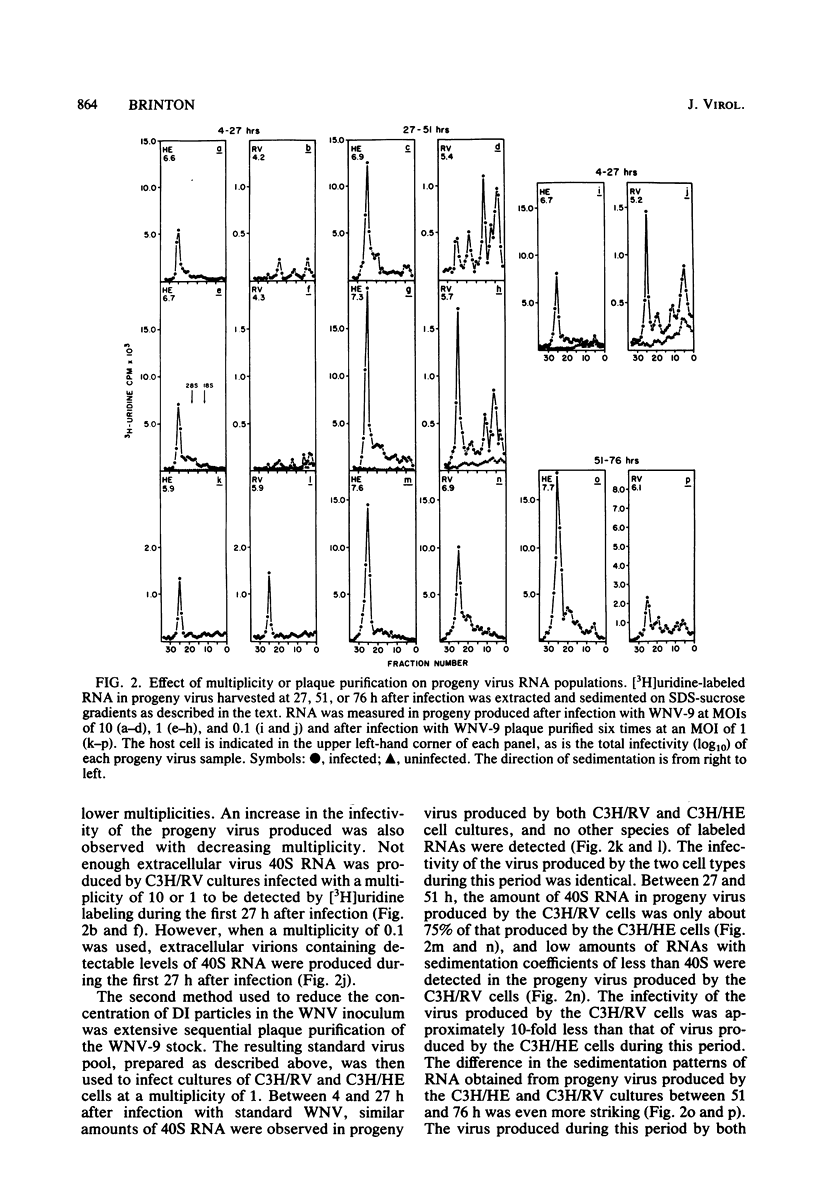
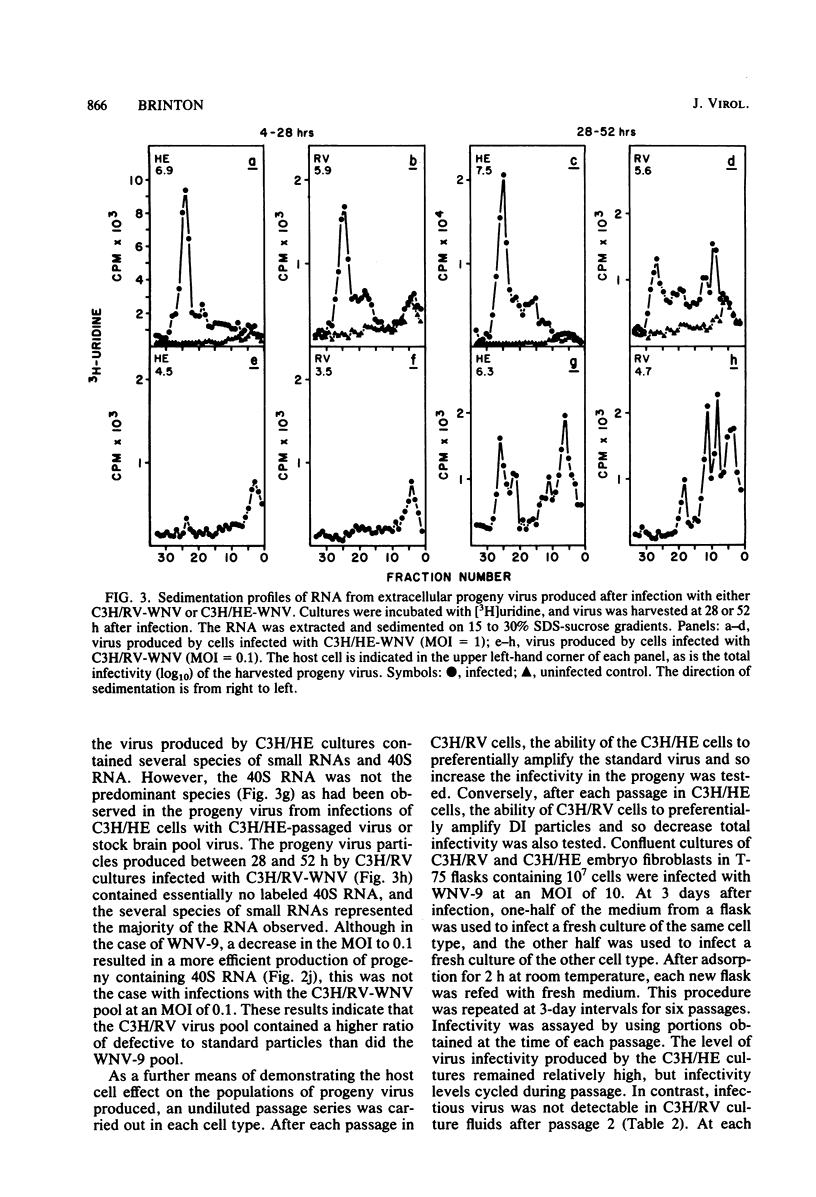
[3H]uridine-labeled extracellular West Nile virus (WNV) particles produced by cell cultures obtained from genetically resistant C3H/RV and congenic susceptible C3H/HE mice were compared by sucrose density gradient centrifugation as well as by analysis of the particle RNA. Defective interfering (DI) WNV particles were observed among progeny produced during acute infections in both C3H/RV and C3H/HE cells. Although only a partial separation of standard and DI particles was achieved, the DI particles were found to be more dense than the standard virions. Particles containing several species of small RNAs consistently constituted a major proportion of the total population of virus progeny produced by C3H/RV cells, but a minor proportion of the population produced by C3H/HE cells. Decreasing the multiplicity of infection or extensive plaque purification of the WNV inoculum decreased the proportion of small RNAs found in the progeny virus. The ratio of DI particles to standard virus observed in progeny virus was determined by the cell type used to grow the virus. The ratio could be shifted by passaging virus from one cell type to the other. Homologous interference could be demonstrated with WNV produced by C3H/RV cells but not with virus produced by C3H/HE cells. Continued passage of WNV in C3H/HE cells resulted in a cycling of infectivity. However, passage in C3H/RV cells resulted in the complete loss of infectious virus. Four size classes of small viral RNA, with sedimentation coefficients of about 8, 15, 26, and 34S, were observed in the extracellular particles. A preliminary analysis of these RNAs by oligonucleotide fingerprinting indicated that the smaller RNAs were less complex than the 40S RNA and differed from each other. The data are consistent with the conclusion that WNV DI particles interfere more effectively with standard virus replication and are amplified more efficiently in C3H/RV cells than in congenic C3H/HE cells. The relevance of these findings to the further understanding of genetically controlled resistance to flaviviruses is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron M. H., Baltimore D. Purification and properties of a host cell protein required for poliovirus replication in vitro. J Biol Chem. 1982 Oct 25;257(20):12351–12358. [PubMed] [Google Scholar]
- Barrett A. D., Crouch C. F., Dimmock N. J. Assay of defective-interfering semliki forest virus by the inhibition of synthesis of virus-specified RNAs. J Gen Virol. 1981 Jun;54(Pt 2):273–280. doi: 10.1099/0022-1317-54-2-273. [DOI] [PubMed] [Google Scholar]
- Boulton R. W., Westaway E. G. Togavirus RNA: reversible effect of urea on genomes and absence of subgenomic viral RNA in Kunjin virus-infected cells. Arch Virol. 1977;55(3):201–208. doi: 10.1007/BF01319906. [DOI] [PubMed] [Google Scholar]
- Brinton M. A., Arnheiter H., Haller O. Interferon independence of genetically controlled resistance to flaviviruses. Infect Immun. 1982 Apr;36(1):284–288. doi: 10.1128/iai.36.1.284-288.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton M. A. Characterization of West Nile virus persistent infections in genetically resistant and susceptible mouse cells. I. Generation of defective nonplaquing virus particles. Virology. 1982 Jan 15;116(1):84–98. doi: 10.1016/0042-6822(82)90405-6. [DOI] [PubMed] [Google Scholar]
- Darnell M. B., Koprowski H. Genetically determined resistance to infection with group B arboviruses. II. Increased production of interfering particles in cell cultures from resistant mice. J Infect Dis. 1974 Mar;129(3):248–256. doi: 10.1093/infdis/129.3.248. [DOI] [PubMed] [Google Scholar]
- Darnell M. B., Koprowski H., Lagerspetz K. Genetically determined resistance to infection with group B arboviruses. I. Distribution of the resistance gene among various mouse populations and characteristics of gene expression in vivo. J Infect Dis. 1974 Mar;129(3):240–247. doi: 10.1093/infdis/129.3.240. [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Zabel P., Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. doi: 10.1016/0092-8674(80)90516-4. [DOI] [PubMed] [Google Scholar]
- GOODMAN G. T., KOPROWSKI H. Study of the mechanism of innate resistance to virus infection. J Cell Comp Physiol. 1962 Jun;59:333–373. doi: 10.1002/jcp.1030590313. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Breindl M. Factors involved in the generation and replication of rhabdovirus defective T particles. J Virol. 1976 Mar;17(3):805–815. doi: 10.1128/jvi.17.3.805-815.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Allen R. Host function-dependent induction of defective interfering particles of vesicular stomatitis virus. J Virol. 1978 Jan;25(1):202–206. doi: 10.1128/jvi.25.1.202-206.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Pettersson R. F., Keränen S., Lehtovaara P., Söderlund H., Ukkonen P. Multiple structurally related defective-interfering RNAs formed during undiluted passages of Semliki forest virus. Virology. 1981 Sep;113(2):686–697. doi: 10.1016/0042-6822(81)90197-5. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Keene J. D., Schubert M. The origins of defective interfering particles of the negative-strand RNA viruses. Cell. 1981 Oct;26(2 Pt 2):145–154. doi: 10.1016/0092-8674(81)90298-1. [DOI] [PubMed] [Google Scholar]
- Lehtovaara P., Söderlund H., Keränen S., Pettersson R. F., Käriäinen L. 18S defective interfering RNA of Semliki Forest virus contains a triplicated linear repeat. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5353–5357. doi: 10.1073/pnas.78.9.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C. J., Hughes T. P. The Inheritance of Susceptibility to Yellow Fever Encephalitis in Mice. Genetics. 1936 Mar;21(2):104–112. doi: 10.1093/genetics/21.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeve C. W., Trent D. W. Identification of Saint Louis encephalitis virus mRNA. J Virol. 1978 Feb;25(2):535–545. doi: 10.1128/jvi.25.2.535-545.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin A. B. Nature of Inherited Resistance to Viruses Affecting the Nervous System. Proc Natl Acad Sci U S A. 1952 Jun;38(6):540–546. doi: 10.1073/pnas.38.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A. A specific internal RNA polymerase recognition site of VSV RNA is involved in the generation of DI particles. Cell. 1979 Nov;18(3):749–757. doi: 10.1016/0092-8674(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Smith A. L. Genetic resistance to lethal flavivirus encephalitis: effect of host age and immune status and route of inoculation on production of interfering Banzi virus in vivo. Am J Trop Med Hyg. 1981 Nov;30(6):1319–1323. doi: 10.4269/ajtmh.1981.30.1319. [DOI] [PubMed] [Google Scholar]
- Stollar V. Defective interfering particles of togaviruses. Curr Top Microbiol Immunol. 1979;86:35–66. doi: 10.1007/978-3-642-67341-2_2. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Sedwick W. D., Plotkin S. A., Maes R. Cytopathic effect of rubella virus in RHK21 cells and growth to high titers in suspension culture. Virology. 1965 Oct;27(2):239–241. doi: 10.1016/0042-6822(65)90170-4. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Gross H. J. Studies on virus-specific nucleic acids synthesized in vertebrate and mosquito cells infected with flaviviruses. Virology. 1978 Sep;89(2):423–437. doi: 10.1016/0042-6822(78)90185-x. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Terminal sequences of the genome and replicative-from RNA of the flavivirus West Nile virus: absence of poly(A) and possible role in RNA replication. Virology. 1981 Sep;113(2):544–555. doi: 10.1016/0042-6822(81)90182-3. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]



