Abstract
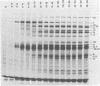
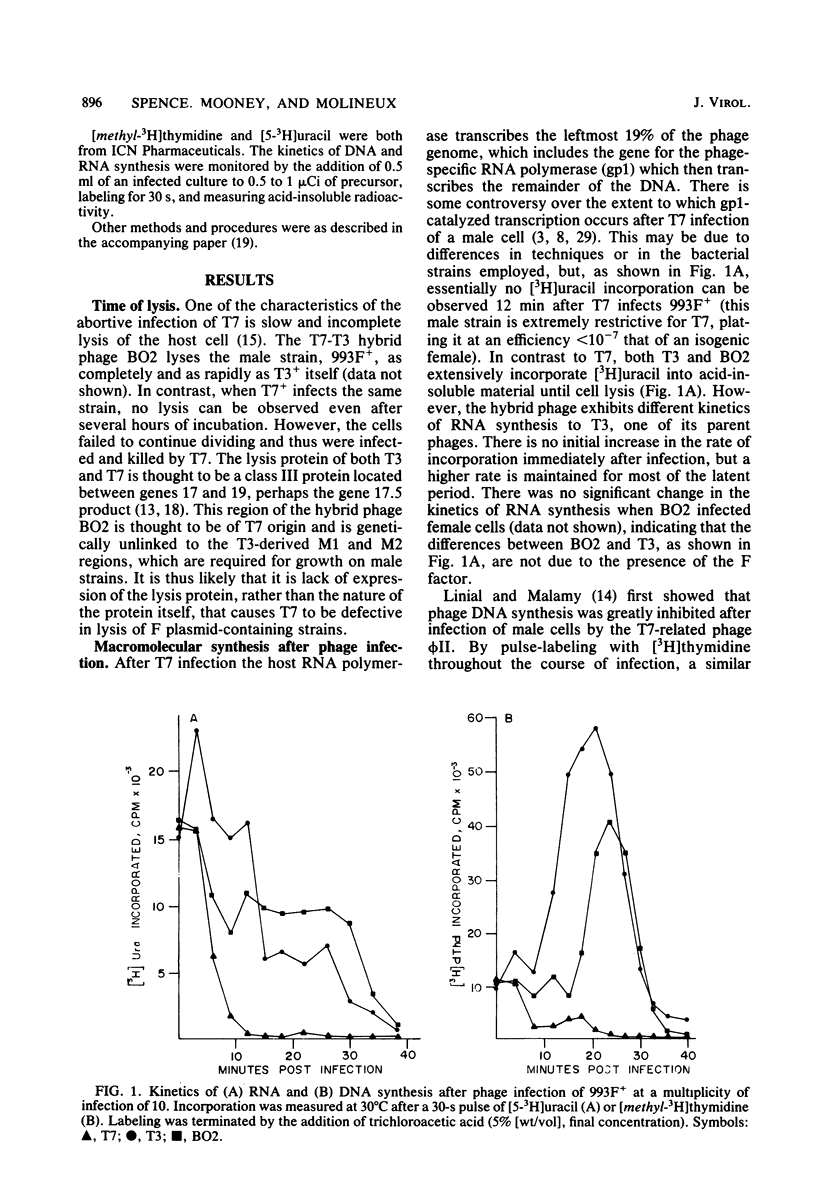
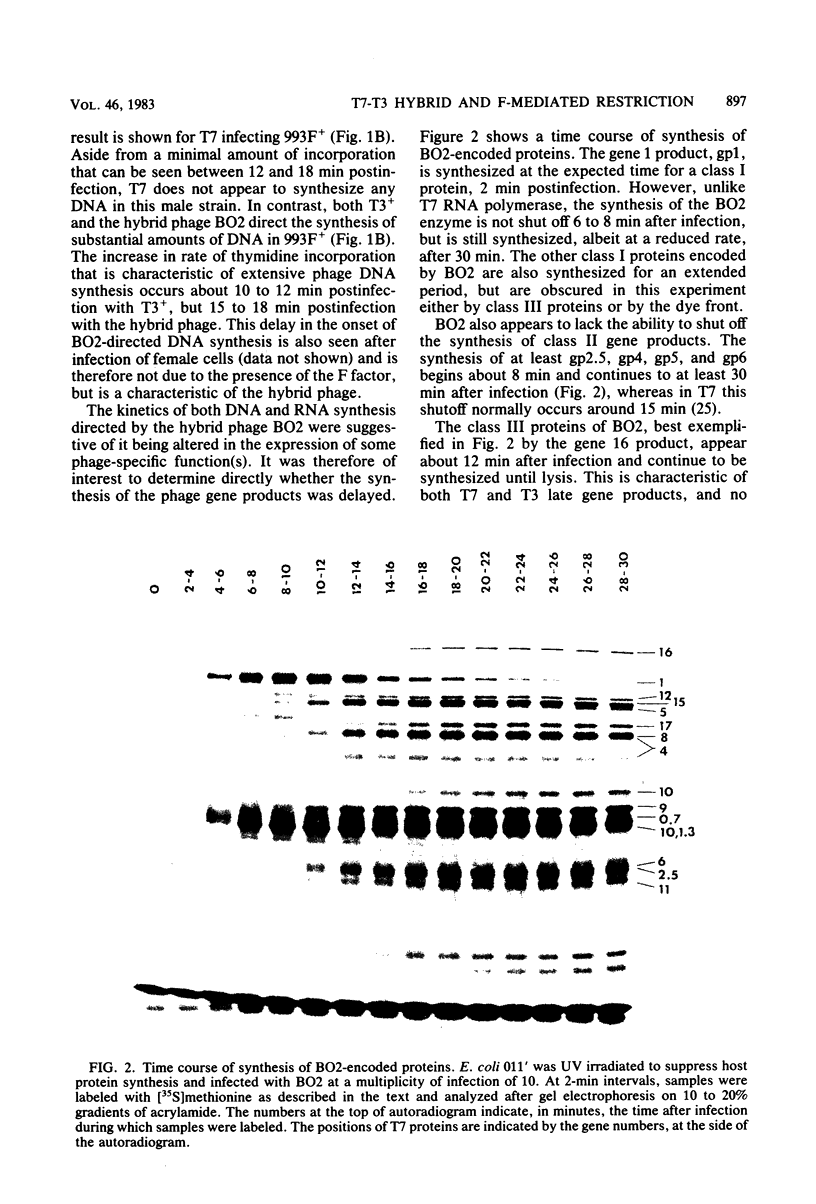
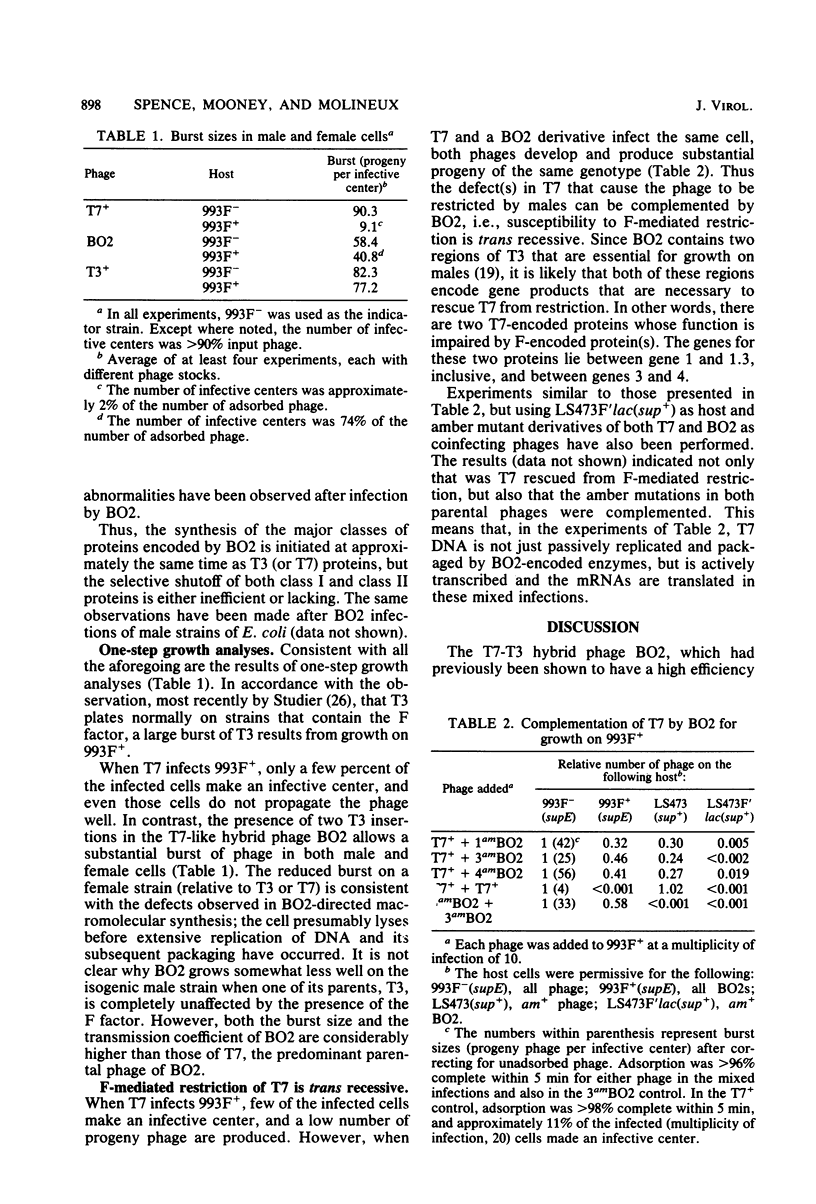
The T7-T3 recombinant phage BO2, whose isolation and physical properties are described in the accompanying paper (Molineux et al., J. Virol. 46:881-894, 1983), has been characterized by physiological means after infection of male (F plasmid-containing) and female strains of Escherichia coli. Single-step growth analyses have shown that the hybrid phage gives a burst about two-thirds that of T7 or T3 on females and about one-half that of T3 on males. This reduced burst size can be correlated with altered kinetics of macromolecular synthesis, probably at the level of transcription. The T3 insertions of BO2, designated M1 and M2, that are essential for growth of the hybrid phage on male strains, have been shown to be trans acting and can rescue T7 from F-mediated restriction. The nature of the gene products encoded by the M1 and M2 regions and their role in overcoming the abortive infection of males by T7 are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg D. D., Mabie C. T., Malamy M. H. T7 protein synthesis in F-factor-containing cells: evidence for an episomally induced impairment of translation and relation to an alteration in membrane permeability. J Virol. 1975 Jan;17(1):94–105. doi: 10.1128/jvi.17.1.94-105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg D. D., Malamy M. H. Evidence for the presence of nontranslated T7 late mRNA in infected F'(PIF+) episome-containing cells. J Virol. 1974 Feb;13(2):378–385. doi: 10.1128/jvi.13.2.378-385.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J. R., Haselkorn R. Macromolecular synthesis in T7 infected F' cells. Virology. 1975 Sep;67(1):264–275. doi: 10.1016/0042-6822(75)90423-7. [DOI] [PubMed] [Google Scholar]
- Britton J. R., Haselkorn R. Permeability lesions in male Escherichia coli infected with bacteriophage T7. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2222–2226. doi: 10.1073/pnas.72.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S. L., Gorini L. A link between streptomycin and rifampicin mutation. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2084–2087. doi: 10.1073/pnas.72.6.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C. F factor-mediated inhibition of bacteriophage T7 growth: increased membrane permeability and decreased ATP levels following T7 infection of male Escherichia coli. J Mol Biol. 1975 Oct 15;98(1):45–59. doi: 10.1016/s0022-2836(75)80100-8. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Steitz J. A. F factor-mediated inhibition of bacteriophage T7 growth: analysis of T7 RNA and protein synthesis in vivo and in vitro using male and female Escherichia coli. J Mol Biol. 1975 Oct 15;98(1):31–43. doi: 10.1016/s0022-2836(75)80099-4. [DOI] [PubMed] [Google Scholar]
- DeMartini M., Halegoua S., Inouye M. Lysozymes from bacteriophages T3 and T5. J Virol. 1975 Aug;16(2):459–461. doi: 10.1128/jvi.16.2.459-461.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H., Glenn J., McCorquodale D. J. Inhibition of bacteriophage replication by extrachromosomal genetic elements. Microbiol Rev. 1981 Mar;45(1):52–71. doi: 10.1128/mr.45.1.52-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Nucleotide sequence from the genetic left end of bacteriophage T7 DNA to the beginning of gene 4. J Mol Biol. 1981 Jun 5;148(4):303–330. doi: 10.1016/0022-2836(81)90178-9. [DOI] [PubMed] [Google Scholar]
- Fuchs E. The formation of bacteriophage T7 and T3 lysozymes from inactive precursors. FEBS Lett. 1977 Aug 1;80(1):27–29. doi: 10.1016/0014-5793(77)80399-2. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Schroeder C. Bacteriophage T3 and bacteriophage T7 virus-host cell interactions. Microbiol Rev. 1981 Mar;45(1):9–51. doi: 10.1128/mr.45.1.9-51.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Malamy M. H. Studies with bacteriophage phi II. Events following infection of male and female derivatives of Escherichia coli K-12. J Virol. 1970 Jan;5(1):72–78. doi: 10.1128/jvi.5.1.72-78.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAEKELAE O., MAEKELAE P. H., SOIKKELI S. SEX-SPECIFICITY OF THE BACTERIOPHAGE T7. Ann Med Exp Biol Fenn. 1964;42:188–195. [PubMed] [Google Scholar]
- McAllister W. T., Barrett C. L. Roles of the early genes of bacteriophage T7 in shutoff of host macromolecular synthesis. J Virol. 1977 Sep;23(3):543–553. doi: 10.1128/jvi.23.3.543-553.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister W. T., Wu H. L. Regulation of transcription of the late genes of bacteriophage T7. Proc Natl Acad Sci U S A. 1978 Feb;75(2):804–808. doi: 10.1073/pnas.75.2.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J. I., Ryo Y., Fujisawa H., Minagawa T. Mutation in bacteriophage T3 affecting host cell lysis. Virology. 1978 Aug;89(1):327–329. doi: 10.1016/0042-6822(78)90067-3. [DOI] [PubMed] [Google Scholar]
- Molineux I. J., Mooney P. Q., Spence J. L. Recombinants between bacteriophages T7 and T3 which productively infect F-plasmid-containing strains of Escherichia coli. J Virol. 1983 Jun;46(3):881–894. doi: 10.1128/jvi.46.3.881-894.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. T7 translational control mechanisms and their inhibiton by F factors. Nat New Biol. 1971 May 12;231(19):37–41. doi: 10.1038/newbio231037a0. [DOI] [PubMed] [Google Scholar]
- Remes B., Elseviers D. Adenosine 5'-triphosphate leakage does not cause abortive infection of bacteriophage T7 in male Escherichia coli. J Bacteriol. 1980 Aug;143(2):1054–1056. doi: 10.1128/jb.143.2.1054-1056.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Richardson C. C. Genetic analysis of gene 1.2 of bacteriophage T7: isolation of a mutant of Escherichia coli unable to support the growth of T7 gene 1.2 mutants. J Virol. 1981 Jan;37(1):343–351. doi: 10.1128/jvi.37.1.343-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Richardson C. C. Processing of mRNA by ribonuclease III regulates expression of gene 1.2 of bacteriophage T7. Cell. 1981 Dec;27(3 Pt 2):533–542. doi: 10.1016/0092-8674(81)90395-0. [DOI] [PubMed] [Google Scholar]
- Silberstein S., Inouye M. Studies on the role of bacteriophage T7 lysozyme during phage infection. J Mol Biol. 1975 Jul 25;96(1):1–11. doi: 10.1016/0022-2836(75)90178-3. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Movva N. R. SAMase gene of bacteriophage T3 is responsible for overcoming host restriction. J Virol. 1976 Jul;19(1):136–145. doi: 10.1128/jvi.19.1.136-145.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Relationships among different strains of T7 and among T7-related bacteriophages. Virology. 1979 May;95(1):70–84. doi: 10.1016/0042-6822(79)90402-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H. Genetic and physical mapping of the late region of bacteriophage T7 DNA by use of cloned fragments of T7 DNA. J Mol Biol. 1981 Dec 15;153(3):503–525. doi: 10.1016/0022-2836(81)90405-8. [DOI] [PubMed] [Google Scholar]
- Young E. T., Menard R. C. Analysis of the template activity of bacteriophage T7 messenger RNAs during infection of male and female strains of Escherichia coli. J Mol Biol. 1975 Nov 25;99(1):167–184. doi: 10.1016/s0022-2836(75)80166-5. [DOI] [PubMed] [Google Scholar]