Abstract
The Slump Test is used as a fast, low-cost diagnostic tool in the evaluation of leg and back pain disorders. The purpose of this study was to identify the normative sensory responses to the Slump Test in asymptomatic subjects. Eighty-four subjects were tested using a standardized procedure by the same examiner to ensure consistency. Prevalence, intensity, location, and nature of responses at each stage of the Slump Test [Slumped Sitting (SS), Knee Extension (KE), Ankle Dorsiflexion (AD), and Cervical Extension (CE)] were recorded. Of the subjects, 97.6% reported a sensory response during the Slump Test. Prevalence of responses increased significantly from 29.8% at SS to 94% at KE and decreased significantly from 97.6% at AD to 65.5% at CE. Median intensity of responses increased significantly from 0/10 at SS, through 4/10 at KE, to 6/10 at AD, and then decreased significantly to 2/10 at CE. At SS, responses were located at the back or neck, but during the subsequent stages, responses were located most commonly in the posterior thigh, knee, and calf. In terms of nature, a number of different descriptors were used, the most common being “stretch,” “tight,” and “pull.” Approximately 80% of subjects reporting a response had complete or partial relief of this response following cervical extension, indicating that the normal response to the Slump Test may be considered a neurogenic response. This normative data may be used as a reference point when using the Slump Test in the examination of leg and back pain disorders.
Key Words: Slump Test, Neural Tissue, Sensory Response, Normative Data
Neural tissue has been identified as a possible source of a wide variety of signs and symptoms in recent years. Neural tissue provocation tests are used in clinical examination to identify mechanically sensitive neural tissue as a potential source of pain. These tests consist of a series of passive movements designed to assess the mechanics and physiology of neural tissue1. A test is considered positive if symptoms can be reproduced, if responses on the involved side differ from the uninvolved side, and if symptoms are altered by additional movements, which further increase mechanical load on the neural tissue2. An abnormal response to such tests may implicate neural tissue as a source of symptoms.
The Slump Test has become widely advocated as a neural tissue provocation test for assessment of patients with spinal and lower limb pain. The test requires the subject to assume a “slumped” position of thoracolumbar and cervical flexion, and increasing mechanical stress is imparted on the nervous system as the knee is extended and the ankle is dorsiflexed3–5. The anatomical distance over which the neural tissue must travel is increased progressively throughout the test until cervical extension is performed following the ankle dorsiflexion stage. Cervical extension, by shortening the anatomical distance over which the neural tissue must travel, may relieve any symptoms of the earlier test stages. Throughout the procedure, pain (or other sensations), available range of movement, and muscle response are monitored. While non-neural structures such as subcutaneous connective tissues, skin, blood vessels, and fascia6,7 may also be placed under increasing loads during neural tissue testing, Coppieters et al8 demonstrated that successive stages of the Slump Test did not alter the perception of experimentally induced muscle pain (i.e., non-neural pain). This finding provides some validation for use of the Slump Test in the examination of neural structures.
Despite its widespread use, relatively little research in relation to the sensory responses of the Slump Test has been published. Literature pertaining to the sensory response has focused on prevalence and location9,10 or effect of cervical position11. However, the nature and intensity of sensory responses has not been reported to date. Furthermore, information regarding the stage of first onset of responses and prevalence of responses at each stage is limited. Without such normative data for comparative purposes, analysis of patient responses may prove difficult for the clinician. It would be expected that the Slump Test would be positive in subjects with a sciatic nerve pain disorder and negative in subjects with pain of non-neural origin. However, because a number of other tissues are also subjected to stress during the Slump Test, sensations may be experienced. Therefore, even in the cases of “normal” neural tissue, there may be responses. Once these normal responses to the Slump Test are known, an abnormal test response may be identified. This study aimed to obtain normative data for the Slump Test by investigating the sensory responses of asymptomatic subjects. It is hoped that this gathered data may be used as a reference point to aid the clinician in the examination and diagnosis of neural tissue pain disorders.
Methodology
Inclusion and Exclusion Criteria
Volunteers who were over 18 years of age at the time of testing and able to understand and speak English were included in the study. Those with a history of back or leg problems, any current back or leg pain, or any physical limitations to performing the test were identified through verbal questioning and excluded.
Subjects
Ninety-one subjects volunteered to take part in the study in response to advertisements placed on notice boards at Trinity College Dublin. Seven subjects were excluded from the study: four due to chronic lower back problems, two due to age restrictions, and one due to cervical pain. Thus, 84 subjects were tested.
Procedure
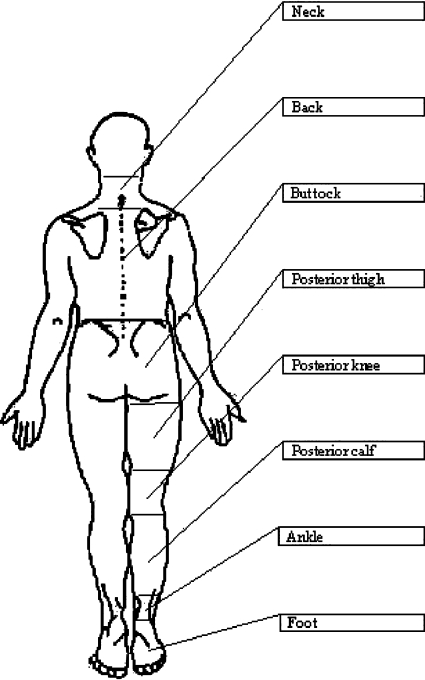
The left lower limb was used for all subjects. It was assumed that because subjects were asymptomatic, responses would be similar bilaterally. Standardized verbal instructions were given by the examiner to explain the test to each subject. Each subject was asked to sit on the plinth with his or her knees together and as far back as possible to ensure a standardized starting position. Any sensation or response was recorded in the starting position, at the four stages of the Slump Test [slumped sitting/thoracolumbar and cervical flexion (SS), knee extension (KE), ankle dorsiflexion (AD) and cervical extension (CE)], and again when the subject returned to a comfortable sitting position (Figure 1). At each stage of the test, nature, location, and intensity of any sensory response were recorded. To replicate the clinical scenario (where patients may use any descriptor in characterizing the nature of a response) and to avoid bias, subjects were allowed to describe the nature of any response in their own words rather than being given a list from which to choose. To determine location of responses, subjects were shown a body diagram (Figure 2) with eight regions clearly outlined (1 neck, 2 back, 3 buttock, 4 posterior thigh, 5 posterior knee, 6 posterior calf, 7 ankle, and 8 foot). At each stage of the test, subjects were asked to identify the location of each response according to this body diagram. To determine intensity, subjects were asked to rate the intensity of the response on a verbal analogue scale of 0 to 10.
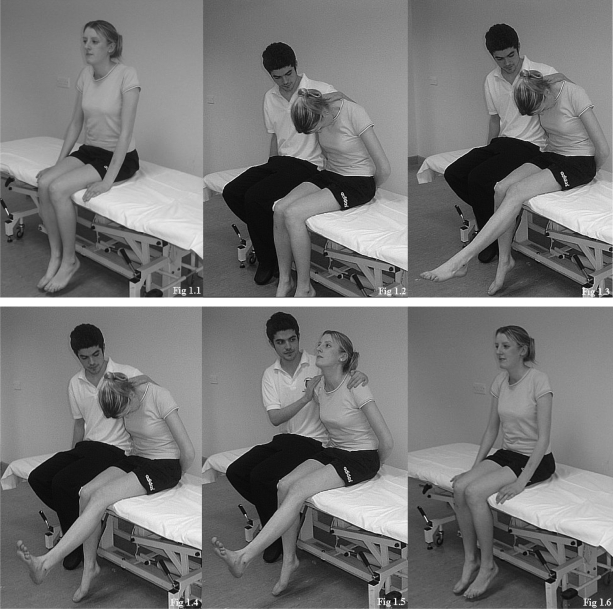
Fig. 1.
Slump Test—testing procedure.
Fig. 2.
Body chart used to identify location of response.
The procedure was divided into four stages: SS—the subject was asked to put her hands behind her back, to slump at the mid- and lower back, and to tuck her chin into the chest, while the examiner placed his hand at the cervicothoracic junction to monitor cervical position (Figure 1.2); KE—while maintaining the above position, the subject was asked to extend the left knee until full extension was reached (Figure 1.3); AD—the subject was then asked to dorsiflex the left ankle (Figure 1.4); CE—the subject was asked to maintain the lower limb position while the examiner removed his hand from the cervicothoracic junction and the subject extended the neck (Figure 1.5). The subject was then asked to assume a comfortable sitting position and any residual responses were recorded (Figure 1.6). The examiner practised the test procedure several times on one subject to increase the consistency in test application prior to data collection.
Ethics
Ethical approval was granted by the Faculty Ethics Committee of Trinity College, Dublin.
Data Analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS) version 14.0. Descriptive statistics were used to describe the prevalence, nature, and location of responses. Repeated measures analysis of variance (Friedman's test) was used to determine whether differences in prevalence and intensity of responses at the different stages of the Slump Test were statistically significant. Post-hoc pair-wise analysis was performed using Wilcoxon's signed ranks test to determine between which pairs of stages significant differences occurred.
Results
Subjects
The mean age of the group was 22 years (+/− 4.71). The age of the subjects ranged from 18 to 45 years. The majority of the subjects were female (84.5%). Data in relation to each stage of the test are detailed in Table 1. All percentages reported below refer to a total of 84 subjects, except where stated.
TABLE 1.
Responses at each stage of the Slump test. All percentages are based on a total of 84 subjects.
SS = Slumped Sitting, KE = Knee Extension, AD = Ankle Dorsif exion, CE = Cervical Extension, SD = Standard Deviation, Med. = Median, IQR = Inter-quartile Range.
| Intensity | |||||||
|---|---|---|---|---|---|---|---|
| Stage | Prevalence of Responses n(%) | Stage of First Onset n(%) | Mean (SD) | Med. (IQR) | Location n(%) | Nature n | (%) |
| SS | 25 (29.8) | 25 (29.8) | 3.2(1.8) n=25 | 0(0-2) | Back 14(16.7) | Stretch 10(11.9) | Discomfort 2(2.4) |
| Neck 11(13.1) | Tight 6(7.1) | Ache 1(1.2) | |||||
| Sharp 2(2.4) | Pull 1(1.2) | ||||||
| Strain 2(2.4) | Warm 1(1.2) | ||||||
| KE | 79 (94) | 54 (64.3) | 4.1(1.6) n=79 | 4(3-5) | Knee 34(40.1) | Stretch 30(35.7) | Tension 2(2.4) |
| Thigh 32(38.1) | Tight 22(26.2) | Tingling 2(2.4) | |||||
| Calf 11(13.1) | Pull 8(9.5) | Pain 1(1.2) | |||||
| Neck 1(1.2) | Strain 6(7.1) | Nervy 1(1.2) | |||||
| Ankle 1(1.2) | Discomfort 3(3.6) | Burn 1(1.2) | |||||
| Sharp 3(3.6) | |||||||
| AD | 82 (97.6) | 3 (3.6) | 5.5(1.8) n=82 | 6(4-7) | Knee 30(35.7) | Stretch 32(38.1) | Pain 3(3.6) |
| Calf 27(32.1) | Tight 21(25) | Discomfort 2(2.4) | |||||
| Thigh 23(27.4) | Pull 9(10.7) | Tension 2(2.4) | |||||
| Ankle 1(1.2) | Strain 4(4.8) | Nervy 1(1.6) | |||||
| Foot 1(1.2) | Tingling 4(4.8) | Burn 1(1.6) | |||||
| Sharp 3(3.6) | |||||||
| CE | 55 (65.5) | 0 | 3.7(1.9) n=55 | 2(0-4) | Calf 22(26.2) | Stretch 22(26.2) | Sharp 3(3.6) |
| Knee 18(21.4) | Tight 10(11.9) | Strain 2(2.4) | |||||
| Thigh 10(11.9) | Pull 7(8.3) | Tension 2(2.4) | |||||
| Foot 3(3.6) | Tingling 5(6) | Discomfort 1(1.6) | |||||
| Neck 1(1.2) | Pins and needles | ||||||
| Ankle 1(1.2) | 3(3.6) | ||||||
Prevalence
Of 84 subjects tested, 2 (2.4%) had no response throughout the Slump Test, while 82 (97.6%) reported a sensory response. Prevalence of sensory response increased from 29.8% at SS through 94% at KE to 97.6% at AD, but then decreased to 65.5% at CE (Table 1). Friedman's test demonstrated that the difference in prevalence of responses between the stages was statistically significant (p<0.001). Post-hoc pair-wise analysis of prevalence of responses showed that there was a significant difference between SS and KE (p < 0.001), no significant difference between KE and AD (p = 0.083), and a significant difference between AD and CE (p < 0.001).
Intensity
Mean intensity and standard deviation of reported responses at each stage of the Slump Test are detailed in Table 1. However, as not all subjects reported a response, to analyze intensity data of all subjects to determine whether differences in intensities at different stages of the Slump Test were significant, subjects with no response at a particular stage were assigned an intensity of 0. The Kolmogorv-Smirnov test confirmed that data were not normally distributed; hence, median intensities and inter-quartile ranges (IQR) for each stage are reported (Table 1). Median (IQR) intensity increased from 0(0–2) at SS through 4(3–5) at KE to 6(4–7) at AD, but this then decreased to 2(0–4) at CE. Friedman's test demonstrated that the difference in intensities between stages was statistically significant (p<0.001). Post-hoc pair-wise analysis of intensity showed that there were significant differences between SS and KE (p < 0.001), KE and AD (p < 0.001), and AD and CE (p < 0.001).
Slumped Sitting
Twenty-five (29.8%) subjects reported a sensory response during the first stage of the Slump Test. The median (IQR) intensity was 0(0–2), and this response was located in the back (n=14, 16.7%) or neck (n=11, 13.1%). This was most commonly described as “stretch” (n=10, 11.9%) or “tight” (n=6, 7.1%), while the terms “sharp,” “strain,” or “discomfort” were each used by 2 (2.4%) subjects and “ache,” “pull,” and “warm” were each used by 1 (1.2%) subject (Table 1).
Knee Extension
Fifty-four (64.3%) subjects reported first onset sensory response at KE, which, added to the 25 subjects with existing responses, gave a total of 79 (94%) subjects experiencing a response at this stage. Median (IQR) intensity was 4 (3–5) and responses were most commonly located at the knee (n=34, 40.1%), thigh (n=32, 38.1%), or calf (n=11, 13.1%). Nature was most commonly described as “stretch” (n=32, 38.1%), “tight” (n=22, 26.2%), or “pull” (n=8, 9.5%), while the terms “strain,” “discomfort,” “sharp,” “tension,” “tingling,” “pain,” “nervy,” and “burn” were used less commonly (Table 1).
Ankle Dorsiflexion
Three (3.6%) additional subjects reported first onset of sensory response at the AD stage, giving a total of 82 (97.6%) subjects who reported a response (new or existing) at this stage. Median intensity (IQR) was 6(4–7) with responses located most commonly at the knee (n=30, 35.7%), calf (n=27, 32.1%), or thigh (n=23, 27.4%). “Stretch” (n=32, 38.1%), “tight” (n=21, 25%), or “pull” (n=7, 8.3%) were the most commonly used descriptors, with the terms “strain,” “tingling,” “sharp,” “pain,” “discomfort,” “tension,” “nervy,” and “burn” used less commonly (Table 1).
Cervical Extension
No subjects reported new responses at CE, while 55 (65.5%) subjects reported responses persisting from an earlier stage. Thus, there was a 32.1% reduction in the number of subjects with a response between AD and CE. The majority of CE responses were located in the calf (n=22, 26.2%), knee (n=18, 21.4%), or thigh (n=10, 11.9%). The terms “stretch” (n=22, 26.2%), “tight” (n=10, 11.9%), and “pull” (n=7, 8.3%) were most commonly used to describe the nature of these symptoms, with “tingling,” “pins and needles,” “sharp,” “strain,” “tension,” and “discomfort” used less commonly (Table 1). Median (IQR) intensity at this stage was 2(0–4) and the effects of CE on intensity of response (of the 82 subjects with a response at AD) are detailed in Table 2. Of subjects experiencing a response at AD, 79.2% had complete or partial relief of this response at CE.
TABLE 2.
Effect of CE on intensity of responses (based on a total of 82 subjects with a response).
| Complete Relief n (%) | Partial Relief n (%) | No Change n (%) | Increased Intensity n (%) |
|---|---|---|---|
| 27 (32.9) | 38 (46.3) | 14 (17.1) | 3(3.7) |
CE = Cervical Extension
Discussion
Prevalence
This study was based on a sample of asymptomatic subjects, and so normal neural tissue was tested. The results of this study indicate that even when normal neural tissue is mechanically loaded during the Slump Test, responses are elicited. The vast majority (97.6%) of asymptomatic subjects reported a sensory response. Therefore, in the clinical setting, the mere reporting of a response by a patient is insufficient to merit a “positive” Slump Test. The response reported must be explored further to determine whether it is indicative of a positive finding or merely a normal response. Approximately 30% of subjects experienced a sensory response during the SS stage, while ≥ 94% of subjects experienced a response during the KE and AD stages of the Slump Test. Due to differences in data collection methods and slump testing procedures, comparing these findings with those of other studies needs to be done with caution. Yeung et al9 investigated the Slump Test response of both asymptomatic controls and a group of whiplash patients. Forty asymptomatic controls were asked to identify the areas where a sensory response, defined as a “pain, stretch, or discomfort” was felt. In slumped sitting with cervical flexion, 25% of controls reported no pain, 65% a single area of pain, and 10% two areas of pain. The difference between 75% of subjects reporting pain in the study by Yeung et al9 during SS compared to 30% in the current study may be due to the fact that Yeung et al defined sensory response, while in the current study subjects were free to use their own vocabulary to describe a response. This difference may also be explained by the fact that overpressure was used during the Slump Test in the study by Yeung et al but not in the current study.
Intensity
Median intensities at the successive four stages of the test were 0, 4, 6, and 2, respectively, as reported above. The fact that the intensity of responses increased significantly from SS through KE to AD is evidence that knee extension and ankle dorsiflexion lead to increasing mechanical loads being placed on the tissues. The moderate intensities at the KE and AD stages may be considered high for asymptomatic subjects, highlighting again the need for caution when interpreting the Slump Test in symptomatic individuals. The mere reporting of a moderate intensity response is not indicative of a positive test because this can be a feature of the normal response in asymptomatic subjects.
Location
All responses were located in the back or neck for the 29.8% of subjects who experienced a sensory response during SS, but for the subsequent three stages of the test, the vast majority of responses were located in the thigh, knee, or calf (Table 1). Yeung et al9 found that in 57.5% of controls, the predominant response in slumped sitting was in the mid-thoracic region. At the addition of knee extension, the main distribution of pain response was in the mid-thoracic and posterior thigh region. Ankle dorsiflexion provoked pain in the mid-thoracic area in 82.5% of controls, while posterior thigh pain was reported by 80% (left ankle) and 92.5% (right ankle) of controls. The high incidence of thoracic responses in that study9 may have resulted from the application of overpressure on the spine during slumped sitting. If overpressure is applied to the thoracic and cervical spine during the Slump Test, the increased load placed on the posterior spinal structures may be more likely to trigger a sensory response in the area under stress. As overpressure is quite a provocative procedure, there is a possibility of causing trauma with perhaps lasting neurological consequences. Shacklock12 expressed concern that many studies involve the application of overpressure yet do not examine the neurological function of the tissue after the testing procedure, while Coppieters et al13 warned that caution is required during testing to avoid neural complication.
Kuilart et al10 found that at the AD stage of the Slump Test, 66.7% of the subjects reported symptoms in the posterior knee, 35.7% in the posterior thigh, 33.3% in the posterior leg, and 14.2% in the combined cervical and thoracic region. The greater percentage of responses in the posterior knee at AD compared to the current study (35.7%) may be due to the fact that subjects in the study by Kuilart et al had perceived hamstring tightness, while asymptomatic subjects were used in the current study. The different samples may also explain why no subjects reported responses in the back or neck in the current study, compared to 14.2% in the Kuilart et al study, although this may also be due to the different methodologies; in the current study, responses were recorded after each stage of the Slump Test, while Kuilart et al only recorded responses at the AD and CE stages.
Nature
In terms of nature, “stretch,” “tight,” and “pull” were the most commonly used descriptors, although a number of other descriptors were used less commonly (Table 1). Further studies are required to determine the nature of sensory responses in a symptomatic population. It must be stated that the descriptors used may be a function of the local vocabulary—all subjects were recruited from notice board advertisements placed in Trinity College Dublin, and so it must be acknowledged that in other locations or countries different descriptors might be used.
Effect of Cervical Extension
The significant decrease in prevalence and intensity of responses from AD to CE is evidence that cervical extension reduces the mechanical load on the tissues. Similarly, Lew and Briggs11 (who investigated whether intensity of posterior thigh pain during the Slump Test was related to cervical spine position) found that of 22 normal subjects, 20 reported greater pain at the extreme of cervical flexion compared with extension, with a mean significant difference in pain levels of 39% between the two cervical positions. In another study, Kuilart et al10 investigated the prevalence and location of symptoms induced by the Slump Test in 42 asymptomatic subjects with perceived hamstring tightness and found that on cervical extension, 83.3% had complete or partial relief of symptoms. This is similar to the finding in the current study that 79.2% of subjects who had a sensory response to the Slump Test had either complete or partial relief of the response following cervical extension. That altering cervical position has such a profound effect on lower extremity symptoms confirms that these distal symptoms are not local in origin but rather due to changes in mechanical loading of the continuous nervous system as stated by other authors8,11. This indicates that the normal response to the Slump Test is indeed neurogenic.
Implications
The results of this study indicate that the Slump Test can elicit responses of significant intensity in asymptomatic subjects, responses that are located predominantly in the posterior aspect of the lower extremity, and that cervical extension partially or completely relieves the majority of evoked sensations. The underlying mechanism that produces these responses is normal and is not indicative of pathology. This is in accordance with other studies that investigated the responses of asymptomatic subjects to the Slump Test2,9,10.
In the clinical setting, this normative data should be considered when interpreting the Slump Test. A sensory response should be expected in the vast majority of subjects. This may be described in a variety of ways, most commonly by terms such as “stretch” or “tight” and will often be located in the posterior thigh, knee, or calf during the latter stages of the test. It may be of moderate intensity, which gradually increases through the first three stages and then decreases at the CE stage of the Slump Test. That a response of such magnitude can be produced in asymptomatic subjects is a sign of the provocative nature of the test, and so it should be applied with caution, particularly in cases of severe or highly irritable symptoms. Although such a response may appear significant to the clinician and the patient, the response must be viewed in the context of the entire clinical examination. Reproduction of presenting symptoms, differences compared to the contralateral asymptomatic limb, or significant deviations from the normative response may be deemed positive findings. A positive Slump Test implicates neural tissue as the source of symptoms. As found in this study, responses other than the presenting symptoms may simply be an artefact of the test, typical of the normal response in asymptomatic subjects; such responses must not be assumed to indicate a positive Slump Test. Bilateral comparison is advocated in interpreting this test2.
Limitations
A limitation of this study is that only one limb was tested. Further research is needed to determine whether a symmetrical response to the Slump Test is normal and whether the characteristic sensory responses reported in this study can be used to differentiate between asymptomatic and symptomatic subjects.
The results obtained from this study are representative of a subject base of 84 asymptomatic subjects. This was a significantly larger sample size than previous studies investigating normative data for the Slump Test9–11. Considering that the mean age of the sample population was 22 years and that the majority of participants were female (84.5%), it must be stated that the results obtained might have been different if the study were carried out using an older or more gender-balanced sample.
Significant variability exists between testing procedures among various studies. Lew and Briggs11 used a lower-limb fixation device to ensure that a repeatable starting position was assumed by subjects. However, in the clinical setting, fixation devices are not routinely used and so some variability in patient positioning may exist. Mechanical fixation methods were not employed for the current study, as it was thought that these laboratory conditions might not be applicable in clinical practice. To replicate the clinical setting, a procedure akin to that used in the clinical situation (verbal instructions and no additional equipment) was adopted. However, a limitation of this study is that a specific assessment of examiner reliability was not performed.
Findings in recent literature suggest that the order in which component movements of neural tissue provocation tests are introduced may influence the individual response12. There is considerable variation in the way in which these tests, including the Slump Test, can be performed. In many studies, knee extension acts as the terminal movement of the test14–16, while in others dorsiflexion is used9,10. Furthermore, cervical flexion may be introduced at various stages of the test. The effects of altering the sequence of movements of neural tissue provocation tests are currently unknown.
Conclusion
This study has gathered normative data in relation to sensory responses to the Slump Test in an asymptomatic sample. The study used a testing procedure that is easily replicated in a clinical setting and so may be useful for clinicians in the evaluation of neural tissue. The vast majority of subjects reported a response, typically described as “stretch,” “tight,” or “pull,” although a number of other descriptors were also used, albeit less frequently. All responses during SS were located in the neck or back, while the majority of responses during subsequent stages were located in the posterior thigh, knee, or calf. Median intensities were 0, 4, 6, and 2 for SS, KE, AD, and CE stages of the test, respectively. This increase in median intensity from SS through KE to AD, followed by a decrease at CE was statistically significant. Similarly, increased prevalence of responses from SS to KE, with a decrease in prevalence from AD to CE was statistically significant. Given the effect of CE on evoked lower limb sensory responses, this indicates that the normal response to the Slump Test can be considered neurogenic.
In this study, these normative responses were demonstrated in asymptomatic individuals. The results compel the clinician to recognize that sensory responses elicited during the Slump Test are not necessarily indicative of pathology. Rather, a positive Slump Test, i.e., reproduction of presenting symptoms or responses that differ significantly from the normative response, may be suggestive of a neural tissue pain disorder. Further research is necessary to determine whether characteristics of the response to the Slump Test in asymptomatic subjects can be used as a basis of comparison to accurately identify neural tissue involvement in symptomatic populations.
REFERENCES
- 1.Coppieters M, Stappaerts K, Janssens K, Jull G. Reliability of detecting “onset of pain” and “submaximal pain” during neural tissue provocation testing of the upper quadrant. Physiother Res Int. 2002;7:146–156. doi: 10.1002/pri.251. [DOI] [PubMed] [Google Scholar]
- 2.Butler D. The Sensitive Nervous System. Adelaide, Australia: Noigroup Publications; 2002. [Google Scholar]
- 3.Maitland GD. Negative disc exploration: Positive canal signs. Aust J Physiother. 1979;25:129–134. doi: 10.1016/S0004-9514(14)61220-4. [DOI] [PubMed] [Google Scholar]
- 4.Maitland GD. The slump test: Examination and treatment. Aust J Physiother. 1985;31:215–219. doi: 10.1016/S0004-9514(14)60634-6. [DOI] [PubMed] [Google Scholar]
- 5.Maitland GD. Vertebral Manipulation. 6th ed. London, UK: Butter-worths; 1986. [Google Scholar]
- 6.Gajdosik RL, LeVeau BF, Bohannon RW. Effects of ankle dorsiflexion on active and passive unilateral straight leg raising. Phys Ther. 1985;65:1478–1482. doi: 10.1093/ptj/65.10.1478. [DOI] [PubMed] [Google Scholar]
- 7.Barker PJ, Briggs CA. Attachments of posterior layer of lumbar fascia. Spine. 1999;24:1757–1764. doi: 10.1097/00007632-199909010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Coppieters MW, Kurz K, Mortensen TE, Richards NL, Skaret IA, McLaughlin ML, Hodges PW. The impact of neurodynamic testing on the perception of experimentally induced muscle pain. Man Ther. 2005;10:52–60. doi: 10.1016/j.math.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Yeung E, Jones M, Hall B. The response to the slump test in a group of whiplash patients. Aust J Physiother. 1997;43:245–252. doi: 10.1016/s0004-9514(14)60413-x. [DOI] [PubMed] [Google Scholar]
- 10.Kuilart K, Woollam M, Barling E, Lucas N. The active knee extension test and slump test in subjects with perceived hamstring tightness. Int J Osteo Med. 2005;8:89–97. [Google Scholar]
- 11.Lew PC, Briggs CA. Relationship between the cervical component of the slump test and change in hamstring muscle tension. Man Ther. 1997;2:98–105. doi: 10.1054/math.1997.0291. [DOI] [PubMed] [Google Scholar]
- 12.Shacklock MO. Improving application of neurodynamic (neural tension) testing and treatments: A message to researchers and clinicians. Man Ther. 2005;10:175–179. doi: 10.1016/j.math.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Coppieters M, Stappaerts KH, Staes FF, Everaert DG. Shoulder girdle elevation during neurodynamic testing: An assessable sign? Man Ther. 2001;6:88–96. doi: 10.1054/math.2000.0375. [DOI] [PubMed] [Google Scholar]
- 14.Goeken LN, Hof L. Instrumental straight leg raising: Results in healthy subjects. Arch Phys Med Rehabil. 1993;74:194–203. [PubMed] [Google Scholar]
- 15.Philip K, Lew P, Matyas TA. The intertherapist reliability of the slump test. Aust J Physiother. 1989;35:89–94. doi: 10.1016/S0004-9514(14)60499-2. [DOI] [PubMed] [Google Scholar]
- 16.Pahor S, Toppenberg R. An investigation of neural tissue involvement in ankle inversion sprains. Man Ther. 1996;1:192–197. doi: 10.1054/math.1996.0268. [DOI] [PubMed] [Google Scholar]