Abstract
Tumour growth is dependent on angiogenesis, the key mediator of which is vascular endothelial growth factor-A (VEGF-A). VEGF-A exists as two families of alternatively spliced isoforms - pro-angiogenic VEGFxxx generated by proximal, and anti-angiogenic VEGFxxxb by distal splicing of exon 8. VEGF165b inhibits angiogenesis and is downregulated in tumours. Here, we show for the first time that administration of recombinant human VEGF165b inhibits colon carcinoma tumour growth and tumour vessel density in nude mice, with a terminal plasma half-life of 6.2 h and directly inhibited angiogenic parameters (endothelial sprouting, orientation and structure formation) in vitro. Intravenous injection of 125I-VEGF165b demonstrated significant tumour uptake lasting at least 24 h. No adverse effects on liver function or haemodynamics were observed. These results indicate that injected VEGF165b was taken up into the tumour as an effective anti-angiogenic cancer therapy, and provide proof of principle for the development of this anti-angiogenic growth factor splice isoform as a novel cancer therapy.
Keywords: VEGF, VEGF165b, Anti-angiogenesis, Cancer inhibition, Pharmacokinetics, Liver toxicity
1. Introduction
Vascular endothelial growth factor-A (VEGF) is the principal angiogenic promoter in most, if not all, cancers. VEGF is upregulated by hypoxia,1 and by over-expression of oncogenes in tumours,2 and stimulates the migration of endothelial cells, sprouting of blood vessels and generation of new vessels from existing vasculature in tumours (reviewed in3), resulting in sustained blood flow, oxygen supply and waste removal to the growing tumour. Anti-VEGF therapy has been hailed as the fourth line of cancer treatment alongside surgery, chemotherapy and radiotherapy, and antibodies to VEGF have shown clinical benefit in colorectal cancer, renal carcinoma, non-small cell lung cancer, breast cancer4,5 and are in further phase III clinical trials in other cancers.
VEGF is generated as multiple isoforms by alternative splicing of mRNA from 8 exons. Alternate splicing of exons 6 and 7 results in proteins with differing heparin binding affinity, and numbers of amino acids, such as VEGF165, VEGF121 and VEGF189, termed according to the number of amino acids encoded in the final secreted protein. Alternate splicing into the terminal exon, exon 8, gives rise to two families of isoforms, the VEGFxxxb and VEGFxxx isoforms. The VEGFxxxb family of isoforms, first identified in 2002,6 are generated by the use of a more distal 3′ splice acceptor site, and result in mRNA species that code for proteins of the same length as the VEGFxxx isoforms, but with different C-terminal six amino acids. Whereas the VEGFxxx isoforms (e.g. VEGF165 and VEGF121) are pro-angiogenic and are upregulated in tumours, the VEGFxxxb isoforms (e.g. VEGF165b and VEGF121b) are anti-angiogenic and downregulated in tumours.6,7 This anti-angiogenic activity is generated by receptor binding,7,8 but only weak receptor activation, and inhibition of downstream VEGFR2 signalling.7 Its activity has led to the hypothesis that VEGF165b may be a useful therapeutic tool in angiogenic conditions such as in tumour growth, or in neovascularisation associated with retinopathy9 such as in diabetes.
To determine whether VEGF165b has an appropriate pharmacokinetic profile for systemic anti-angiogenic therapy, we have investigated the clearance rates, toxicity, tumour uptake and circulating effects on blood pressure of VEGF165b injection in mice, and whether recombinant human VEGF165b exerts inhibitory effects on tumour growth in tumour-bearing mice.
2. Materials and methods
2.1. Production of recombinant protein
Recombinant human VEGF165b (rhVEGF165b), produced in Chinese Hamster Ovary Cells with correct glycosylation, dimerisation and receptor binding, was generated by Cancer Research Technologies, London, United Kingdom or PPS, Israel. Protein was analysed by MALDI-TOF mass spectrometry (Voyager DE-STR, Applied Biosystems, Foster City, CA). Endotoxins were removed by phase separation using Triton X-114 followed by endotoxin detection using Limulus Amebocyte Lysate performed by Cambrex (Cambrex Corporation). All the proteins were considered endotoxin free (endotoxin levels below 5 EU/ml of concentrated stock). Recombinant canine VEGF165 (rcVEGF165) or rcVEGF165b was produced and assayed for retained activity as previously described.8
2.2. Functional effects of recombinant VEGF 165 b
Human umbilical vein endothelial cells (HUVEC) were extracted from umbilical cords from caesarean sections (St. Michael’s Hospital, Bristol, UK). HUVECs were maintained in M200 supplemented with low serum growth factor supplements (Cascade Biologics, Portland, OR) in flasks coated with extracellular matrix proteins. Cells were used at passages 3-5. Sub-confluent HUVECs were serum starved for 7 h in M200 medium without supplement, and 100,000 serum starved cells were plated into collagen-coated 8 μm inserts (Millipore, Billerica, MA). Inserts were placed in 24-well plates with 500 μl of chemoattractant in M200 medium with 0.1% v/v FCS and 0.2% w/v BSA and incubated overnight at 37 °C to allow for migration. After incubation, inserts were washed, non-migrated cells were removed and migrating cells were stained in Mayer’s haematoxylin. Migrating cells were counted (10 fields per insert) and expressed either as a relative change to basal migration (media without chemoattractant) or% migration and plotted as average ± s.e.m. Experiments were performed in triplicate.
To determine the concentration at which 50% of the migratory response was inhibited, (IC50), increasing amounts of VEGF165b were added (1-100 ng/ml) with or without the optimal concentration of 40 ng/ml of VEGF165. For stability tests, recombinant VEGF165b was incubated under sterile conditions at 37 °C for 1 or 2 weeks, whereafter the retained inhibitory migratory response was assayed.
2.3. Angiogenesis assay
Extracellular matrix gel solution was prepared according to manufacturer’s instructions (Chemicon International, Temecula CA). Thirty microlitre gel solution was transferred to each well of pre-cooled 8-well culture slides (Falcon, BD, Oxford, Oxfordshire, UK) and incubated at 37 °C to solidify. VEGF165, VEGF165b and the combination were placed on one side of the gel to 1 nM final concentration. Human Microvascular Endothelial Cells, HMVEC, were serum starved for 3 h with EBM-2 (Clonetechs, Lonza), and 10,000 cells were seeded onto the gel in 100 μl EBM-2 basal media and incubated at 37 °C for 6 h. Gels were fixed in 4% w/v para-formaldehyde/PBS pH 7.4 for 5 min and washed twice with PBS. F-actin fibres were stained with Alexa 488 phalloidin for 1 h (Molecular Probes, dilution 1:200 in PBS/0.5% v/v Triton) and 10 min with Hoechst 33342 (5 μg/ml PBS/0.5% v/v Triton). Gels were washed twice with PBS/0.5% v/v Triton, twice with PBS and mounted with vectashield (Vector Laboratories Burlingame, CA). Images were taken on a Leica DM RB fluorescence microscope for structure analysis counting branch points, sprouts and closed polygons over the entire area of the well.
2.4. Injections of recombinant human VEGF 165 b into LS174t tumour-bearing mice
LS174t colon carcinoma cells (2 × 106) in 200 μl sterile PBS were injected into the nape of the neck of nude mice and injected with rhVEGF165b subcutaneously every day or twice weekly (in 200 μl 0.9% w/v NaCl), starting 24 h after the injection of tumour cells (prophylactic) or when tumours reached a diameter of 4-5 mm (therapeutic). Bi-weekly intraperitoneal injection was carried out in mice 4 d after the injection of 2 × 106 LS174t tumour cells as above. Xenotransplanted tumours were measured by calliper every day, and tumour volume was calculated according to (length × width × [length + width]/2). Mice were culled by cervical dislocation and organs and tumours were removed. Tumour vessel density was counted in 10 random fields (magnification 40x) in haematoxylin and eosin stained 6 μm frozen sections from tumours. Vessel presence was confirmed by staining by blocking in 5% v/v goat Ig for 30 min, 2 μg/ml PECAM-1 antibody (Santa Cruz, sc-1505) or 1 μg/ml Flk-1 (Santa Cruz sc-6251) overnight, 2 μg/ml anti-goat or anti-mouse biotin antibody (Vector Laboratories) for 1 h followed by avidin-biotinylated enzyme complex (ABC, Vector Laboratories) for 30 min followed by DAB substrate (Vector Laboratories). Sections were examined using a Nikon Eclipse E400 microscope and photos were captured using Nikon Eclipse Net software. Area of necrosis was scored in similar way as above by circling necrotic area, calculated from the lighter staining pattern of the haematoxylin and eosin, compared to whole tumour area using Open Lab.
2.5. Animal injections and collection of samples
C57/Bl6 mice (body weight >20 g) were kept on a heating mat under anaesthesia using 500 μg/kg medetomidine hydrochloride (Domitor, Pfizer, Tadworth, Surrey, UK) and 50 mg/kg ketamine (Vetalar V, Pharmacia, Tadworth, Surrey, UK) for intravenous injection and cardiac puncture according to UK Home Office regulations. Local anaesthetic cream (EMLA, Astra Zeneca, Macclesfield, Cheshire, UK) was added to the tail when collecting blood from the tail vein, and each mouse was bled (max 100 μl) only once every 24 h. For each time point at least 3 mice were used and for experiments less than 24 h, multiple mice groups were used with 3 mice in each group. During intraperitoneal and subcutaneous injections, mice were not anaesthetised but monitored for discomfort and pain. Blood was collected under general anaesthesia, as above either through the tail vein or through cardiac puncture into EDTA vacutainers (BD, Oxford, Oxfordshire, UK) and centrifuged at 3,000 rpm at 4 °C for 10 min, and plasma was transferred to a fresh tube and stored at -20 °C.
Mini pumps (Alzet, Kent, UK) with infusion rate of 208 ng/h(5 μg/24 h) for 14 d were implanted subcutaneously into C57/Bl6 mice under isofluorane anaesthesia. Mice were then sutured using Mersilk 6.0 (Ethicon, Johnson-Johnson, Ascot, Berkshire, UK). Mice were given an intramuscular injection of 50 μg/kg Temgesic (Schering-Plough, Hertfordshire, UK). Recombinant protein was diluted in sterile 0.9% w/v NaCl, and injection volumes were 100-200 μl as appropriate for the injection site. Mice were culled by cervical dislocation or exsanguinated under anaesthesia and organs were removed and snap-frozen in liquid nitrogen.
2.6. ELISA for VEGF165b or VEGF165 in plasma
An in-house ELISA specific for VEGF165b has been developed and described elsewhere.7 All the reagents were from R&D Systems (Abingdon, Oxfordshire, UK) if not otherwise stated. Briefly, immulon 2HB 96-well plates (Thermo Scientific, Basingstoke, UK) were coated with 0.8 μg/ml goat anti-hVEGF capture antibody and blocked with 5% w/v BSA followed by the addition of samples. Detection was done by the addition of biotinylated mouse monoclonal anti-VEGF165b antibody at 0.4 μg/ml followed by incubation with horseradish peroxidase-conjugated streptavidin and substrate development before being read at 450 nm and 570 nm for reference.
For rhVEGF165, rcVEGF165 and rcVEGF165b, a commercial panVEGF ELISA was used according to manufacturer’s protocol (Duoset VEGF ELISA, R&D Systems, Minneapolis, MN). Plasma samples were diluted 1:10 and run in duplicates or triplicates.
2.7. In vivo imaging of 125I-rhVEGF165b biodistribution
Nude mice were injected with LS174t tumours on the right hindleg. 125I-rhVEGF165b was generated using Iodogen™ tubes (Pierce Biotechnology Inc.) and purified with NAP-10 columns (GE Healthcare). Analysis by thin layer chromatography revealed >95% purity. 125I-rhVEGF165b (125I-rhVEGF165b/total 125I). 3.2 MBq (70 μg protein) was injected into the tail vein when tumours were >10 mm in diameter. Anaesthesia was maintained by 2% halothane during X-ray and scanning (NanoSPECT/CT, Bioscan, Washington, DC), after 40, 70, 120, 240 or 1440 min. For biodistribution studies, 0.100 MBq (3 μg) 125I-rhVEGF165b was injected into the tail vein, and mice were culled at 120 or 240 min. Organs and tissues of interest were excised and assessed using a gamma counter (LKB Wallac 1282 Compugamma CS, Wallac). Uptake was expressed as% injected dose/g tissue.
2.8. Toxicology analysis
Snap-frozen livers from nude mice injected daily with 5 or 10 μg VEGF165b for 14 d were embedded into O.C.T. compound (Sakura Finetek, Zoeterwoude, Netherlands), and 6 μm cryosections were fixed in ice-cold methanol and stained using conventional haematoxylin and eosin staining.
Liver enzyme function was analysed in plasma from C57/Bl6 mice daily injected with 5 μg VEGF165b 0.9% w/v saline (vehicle control) for 8 d. Plasma was collected and analysed by Langford Veterinary Diagnostics, Bristol, UK, for liver enzyme activity (ALT = alanine transaminase, ALP = alkaline phosphatase and GGT = gamma glutamyl transpeptidase).
2.9. SDS-PAGE and immunoblotting of tissue and cell lysate
Tissue lysate from mouse organs or cells was extracted using RIPA buffer (50 mM Tris, 150 mM NaCl, 1% v/v NP-40, 0.25% w/v Na-deoxycholate, 1 mM EDTA, 1 mM phenylmethylsulphonyl fluoride, 1 μg/ml of each of aprotinin, pepstatin and leupeptin) and homogenised using a polytron and spun at 12,000 rpm for 10 min at 4 °C. Lysates were separated on 7.5 or 15% SDS-PAGE, transferred to polyvinylidene fluoride membranes, blocked in 10% w/v dry milk, incubated with a biotinylated mouse monoclonal anti-human VEGF165b antibody (0.5 μg/ml) followed by horseradish peroxidase-conjugated streptavidin (1/500, R&D Systems), 200 ng/ml Flt-1 antibody (Santa Cruz, sc-316-G) and 2 ng/ml anti-goat HRP-conjugated antibody or 1:1000 VEGFR-2 antibody (Cell Signalling #2479) and 2 ng/ml anti-rabbit HRP-conjugated antibody for 1 h each. Membranes were developed using supersensitive West Femto Maximum Sensitive Substrate (Pierce Biotechnology, Cramlington, Northumberland).
2.10. Blood pressure measurement
C57/Bl6 mice (>20 g) were deeply anaesthetised with sodium pentobarbitone (80 mg/kg, i.p.) and maintained airflexic throughout. The left external jugular vein was cannulated for supplementary anaesthetic (20-25 mg/kg/h, i.v.) and trachea intubated for maintenance of the airways. The left carotid artery was cannulated to allow blood pressure monitoring. Core body temperature (36-37 °C) was maintained throughout by means of a heating blanket connected to a rectal temperature probe, via an automatic feedback control unit. Following a period of time to allow blood pressure stabilisation, saline (vehicle, 100 μl) or rhVEGF165b (5 μg/100 μl) was infused through the external jugular vein and effects on blood pressure were monitored continuously.
2.11. Statistical analysis
Statistical analysis was performed using GraphPad Prism software (version 3.0cx). Data are given as means ± s.e.m. or mean ± 95% confidence interval when stated. Half-lives were compared using 95% confidence intervals. One-way ANOVA followed by Bonferroni post-hoc test was used to compare migration, liver enzyme levels, tumour weight and structure formations. Unpaired t-test and non-linear regression were used for k-values, vessel density and IC50 calculation. One or two-way ANOVA was used to compare tumour growth in mice.
3. Results
3.1. Pharmacokinetic studies of circulating VEGF 165 b
Recombinant VEGF165b protein produced in CHO cells resulted in dimerisation and glycosylation of the protein (see Fig. 1A). The functionality of the protein was tested by VEGF165b-inhibition of VEGF165-mediated human umbilical vein endothelial cells (HUVEC) migration. Fig. 1B shows the migrated cells (relative to serum starved controls) induced under increasing concentrations of VEGF165b in the presence of 40 ng/ml VEGF165 (40 ng/ml = 1 nM). VEGF165b inhibited migration in a dose-dependent manner with an IC50 of approximately 1 nM (log IC50 =-9.00 ± 0.18, log mean ± 95% confidence interval, see Fig. 1B), similar to that observed with commercially produced protein or conditioned media.6,7 The produced VEGF165 b was stable, as incubation at 37 °C for two weeks did not alter the protein concentration or affect its activity (see Fig. 1C and inserted blot).
Fig. 1.
Recombinant human VEGF165b is dimeric, glycosylated, thermostable and inhibits rhVEGF165-mediated migration in endothelial cells. (A) MALDI-TOF mass spectrometry analysis of recombinant human VEGF165b produced in CHO cells and analysed by mass spectrometry showing that the protein forms dimers and is glycosylated. Monomer 19.9 kDa, glycosylated monomer 20.8 kDa, dimer 39.6 kDa and glycosylated dimer 41.7 kDa. (B) Serum starved human umbilical vein endothelial cells, HUVEC, were allowed to migrate through 8 μm pore inserts with increasing concentrations of rhVEGF165b (0-100 ng/ml) in the presence of 40 ng/ml rhVEGF165 for 18 h. Data plotted as percentage migration compared with no addition of rhVEGF165b (average ± SEM, one-way ANOVA p < 0.001, Bonferroni post-hoc test VEGF165 versus treatment, *p < 0.05, ***p < 0.001). IC50 for rhVEGF165b was 1 nM (log IC50 = -9.00 ± 0.18, log mean ± 95% confidence interval, non-linear regression). (C) rhVEGF165b was stable for more than two weeks in sterile buffer solution at 37 °C as confirmed by sustained inhibition of migration and western blot analysis (average ± SEM, one-way ANOVA p < 0.001, Bonferroni post-hoc test VEGF165 versus treatment, ***p < 0.001).
3.2. Pharmacokinetics and biodistribution
The pharmacokinetic parameters of VEGF165b were compared with conventional VEGF165. To analyse circulating levels of VEGF165b, mice were injected with recombinant protein, and plasma was collected at different time points and analysed by ELISA. Intravenous injections of rcVEGF165b into the tail vein of mice showed that VEGF165b (see Fig. 2A) had a circulating plasma half-life of 13 min, which was not significantly different from that for rcVEGF165 (see Fig. 2A, VEGF165 versus VEGF165b, p = 0.57, two-tailed Student’s t-test). VEGF165b was not detectable in the kidney after i.v. injection indicating that the removal from the plasma was not through excretion in the urine (data not shown), and therefore suggesting tissue uptake as the primary destination of the injected protein. Intraperitoneal (i.p. see Fig. 2B) or subcutaneous (s.c., see Fig. 2C) injection of 5 μg rhVEGF165b led to detectable levels of recombinant protein within 1 h after injection with a half-life of 4.0 h (i.p.) or 6.2 h (s.c.). Clearance rates of VEGF165b were compared with VEGF165. Injection of VEGF165 or VEGF165b showed no significant difference when delivered i.p. (see Fig. 2B, VEGF165 versus VEGF165b, p = 0.88, two-tailed Student’s t-test). Repeated s.c. injections every 24 h did not lead to accumulation of recombinant protein in the plasma (data not shown). A slow release of VEGF165b through infusion from an osmotic mini pump (0.25 μl/h, 5 μg/24 h) did not lead to accumulation of VEGF165b in the plasma, and no adverse effects were observed with this infusion of VEGF165b (data not shown).
Fig. 2.

rhVEGF165b is cleared at the same rate as rhVEGF165. After a single injection of 5 μg of recombinant protein per mouse either through the tail vein (A) intraperitoneal (B), or subcutaneously (C) into mice, blood was collected at different time points. Plasma samples were analysed by ELISA and plotted as normalised concentration (%). (A) Tail vein injections of recombinant canine VEGF165 or VEGF165b showed no difference in clearance rates (VEGF165 versus VEGF165b, p = 0.57, two-tailed Student’s t-test). (B) Intraperitoneal injection showed a slower clearance of both the proteins from the circulation. (C) Subcutaneous injection led to the slowest clearance from the circulation of VEGF165b.
3.3. Biodistribution of intravenous VEGF 165 b
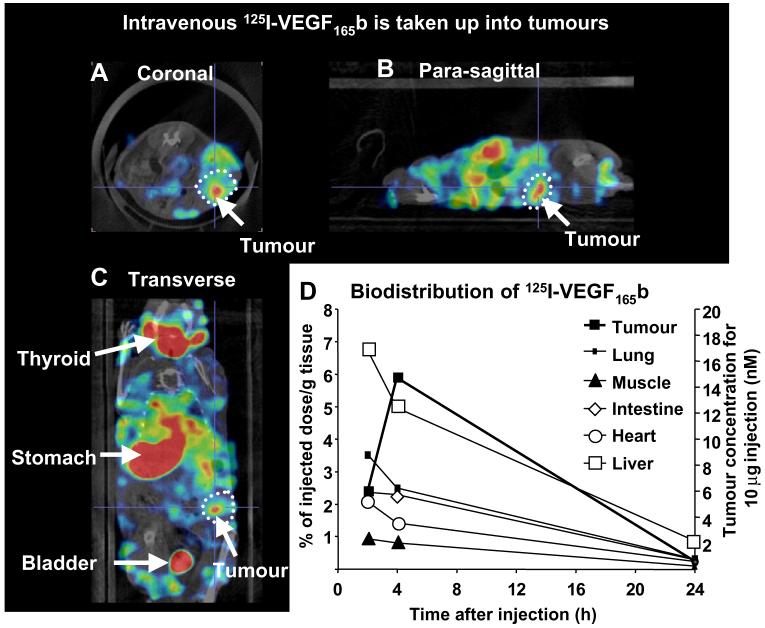
To determine how intravenously injected VEGF165b was distributed in vivo, 125I-rhVEGF165b was injected intravenously into tumour-bearing mice and imaged using high-resolution single photon emission computed tomography (NanoSPECT/CT). Radiolabelled VEGF165b was distributed quickly through the mouse and images acquired at 40 min post-injection showed accumulation in the organs of metabolism and secretion. Thereafter, the overall signal gradually declines due to deiodination of the radiolabelled protein. Fig. 3A-C show whole body transverse, coronal and sagittal sections through the tumour acquired by 70 min post-injection. Uptake of radioactivity can be seen in the tumour as well as in the abdominal tissues such as liver and intestines; however, the image is dominated by accumulation of radioiodine into the thyroid, stomach and bladder indicating rapid dissociation of the radiolabelled iodine attached to the VEGF165b. This was confirmed by the analysis of urine which showed that only 10% of the activity was retained by a 10 kDa cut-off dialysis column, indicating that most of the excreted radioiodine was not protein-bound. In contrast, the proportion of low molecular weight iodine in tumours was similar to that in plasma (50%, data not shown). Collection of tumour and other tissues and measurement of biodistribution indicated that approximately 6% of the total 125I-VEGF165b was found in the tumour at 4 h and was still detectable at 24 h (see Fig. 3D). Of the non-secretory organs, liver showed the highest uptake at 4 h.
Fig. 3.
In vivo distribution of 125I-rhVEGF165b. Tumour-bearing mice received an intravenous injection of 125I-rhVEGF165b and 3D imaged using NanoSPECT/CT. (A-C) Coronal, sagittal and transverse sections through the centre of the tumour after 70 min post-injection are shown. The tumour is circled and arrows indicate different organs. (D) Quantification of uptake into different organs and tissues over time. Data expressed as% in tissue relative to the total injected dose, per gram of tissue, or on the right hand axis the concentration that would be reached in the tumour if the pharmacokinetics were identical for a 10 μg intravenous dose of VEGF165b.
3.4. Recombinant VEGF 165 b reduces tumour growth
We have previously shown that over-expression of VEGF165b reduces tumour growth in at least five different tumour types7,10,11, but VEGF165b expression does not directly affect proliferation or apoptosis of LS174t cells.7 To determine whether recombinant human VEGF165b inhibits tumour growth, LS174t colon carcinoma tumour cells were implanted subcutaneously into nude mice and rhVEGF165b was injected s.c. daily (see Fig. 4). Daily s.c. injection of 5 μg rhVEGF165b reduced tumour growth compared with saline injections (vehicle) (see Fig. 4A, p < 0.05 on day 12, p < 0.01 on day 13, two-way ANOVA, Bonferroni post-hoc test, n = 6 per group). The mice did not exhibit any obvious adverse effects from the injections or tumours. Representative images of excised tumours on the day of culling illustrate that vehicle injection (see Fig. 4A, inserted picture) resulted in larger tumours than treatment with VEGF165b (see Fig. 4A, inserted picture). Analysis of tumour weights revealed a trend towards smaller tumours in VEGF165b treated than saline-treated animals (unpaired t-test p = 0.08, n = 6 per group, Fig. 4B), and the doubling time of the LS174t tumours treated with rhVEGF165b was significantly increased (p < 0.05, unpaired t-test vehicle versus rhVEGF165b, data not shown).
Fig. 4.
Daily subcutaneous injection of rhVEGF165b reduces tumour growth in nude mice-bearing colon carcinoma tumours. (A) LS174t cells injected subcutaneously resulted in large, bloody tumours when treated daily with saline (inserted picture). Subcutaneous injection of rhVEGF165b, however, resulted in smaller tumours (inserted picture). (B) Weight of excised tumours, p = 0.08 unpaired t-test, n = 6 per group. (C) Subcutaneous injection of rhVEGF165b also inhibited established colon carcinoma tumours in nude mice. LS174t colon carcinoma cells were injected subcutaneously and treatment was started when tumours reached 4-5 mm in diameter (day 4 after implantation). Tumour growth was reduced in mice treated with 5 μg rhVEGF165b compared to vehicle control (p < 0.05 on day 11 after start of treatment, one-way ANOVA). (D) Tumours treated with VEGF165b showed significantly fewer blood vessels per unit area than control-injected tumours. Each point represents the mean of ten random analysed fields and 6 tumours per treatment were examined (**p < 0.01 unpaired t-test). (E-F) Immunohistochemistry staining of blood vessels in LS174t tumours using CD31/PECAM-1 (E) or VEGFR2 (F). Arrows indicate staining of the vessels. Inserted images are IgG control showing no non-specific staining. Scale bar = 100 μm. (G) Western blot illustrating the lack of VEGFR-1 or VEGFR-2 in LS174t cells. (H) 135,000 LS174t cells were treated with control (no VEGF165b), 0.25 nM or 1 nM VEGF165b and cell number counted with a haemocytometer after 24 or 48 h treatment. There was no effect of VEGF165b treatment on cell number (expressed relative to starting cell number).
The excised tumours were sectioned and stained to visualise blood vessel distribution (see Fig 4E for representative CD31/PECAM-1 vessel staining of tumour section). Analysis of the vessel density in 10 random fields in each tumour revealed a decreased number of vessels in rhVEGF165b-treated tumours (control versus rhVEGF165b 24 h post-injection, 2.9 ± 0.4 versus 0.9 ± 0.4, p < 0.01, control versus rhVEGF165b established tumours, 3.2 ± 0.5 versus 0.8 ± 0.1, unpaired t-test, n = 6 tumours per treatment 10 fields analysed per tumour, Fig. 4D). The level of necrosis was not different in the tumours (control versus rhVEGF165b, 29.1 ± 8.7% versus 32.3 ± 9.6%, p < 0.80, unpaired t-test). VEGFR-2 staining of tumours sections revealed expression of VEGFR-2 in the tumour vessels and not in the general tumour mass (see Fig. 4F-G) indicating an effect of VEGF165b on the endothelium rather than an anti-proliferative effect on the tumour cells. VEGFR-1 expression was also absent from the LS174t cells (see Fig. 4G), and recombinant VEGF165b did not affect LS174t cell growth in vitro (Fig. 4H).
To determine whether VEGF165b had a therapeutic role, we measured the effect of treatment of tumours with rhVEGF165b on the growth rate of established tumours. Two million colon carcinoma cells (LS174t) were injected subcutaneously into nude mice, and tumours were allowed to grow to a diameter of 4-5 mm (day 4) before daily injection of rhVEGF165b was initiated. Treatment with rhVEGF165b significantly reduced the growth rate in established tumours, and significance was reached on day 15 after 11 d of treatment (p < 0.05, one-way ANOVA n = 5 or 6, Fig. 4C). Again, sectioning and staining for blood vessels indicated a reduction in microvessel density in the tumours from VEGF165b-treated mice (see Fig. 4D).
To determine whether VEGF165b administration could reduce tumour growth at longer dosing intervals, we measured the effect of treatment of tumours by subcutaneous injection of 100 μg rhVEGF165b bi-weekly. Two million colon carcinoma cells (LS174t) were injected subcutaneously into nude mice, and tumours were allowed to grow to a diameter of 4-5 mm (day 4) before bi-weekly subcutaneous injection of 100 μg rhVEGF165b was initiated. Treatment with rhVEGF165b significantly reduced the volume of the established tumours compared with before injection on days 7 and 10 (p < 0.01, two-way ANOVA, p < 0.001, Bonferroni n = 8, Fig. 5A), and the volumes were significantly smaller than those treated with saline after 10 d (p < 0.01, Bonferroni). These data show that subcutaneous injections of recombinant VEGF165b reduce the growth in vivo of heterotopic colon carcinoma tumours.
Fig. 5.
Bi-weekly administration of VEGF165b inhibits tumour growth. (A) Subcutaneous injection of 100 μg rhVEGF165b bi-weekly resulted in the reduction of volume of established colon carcinoma tumours in nude mice. LS174t colon carcinoma cells were injected subcutaneously and treatment was started when tumours reached 4-5 mm in diameter (day 4 after implantation). 100 μg rhVEGF165b reduced tumour volume (**p < 0.01) compared with start of treatment and compared with saline treated (##p < 0.01). (B) Intraperitoneal injection of rhVEGF165b dose dependently reduced the tumour growth of colon carcinomas in nude mice. LS174t colon carcinoma cells were injected subcutaneously and the treatment was started after 4 d. VEGF165b treatment inhibited tumour growth compared with saline-treated tumours (*p < 0.05, ***p < 0.001 compared with saline, ###p < 0.001 compared with 75 μg on day 14). (C) Dose response curve for inhibition of tumours. The relationship between tumour volume at 10 d compared with control tumours and dose (μg bi-weekly) indicated that VEGF165b inhibited tumour growth with a mean (CI) IC50 of 48 (33-70) μg.
To determine whether intraperitoneal injection could inhibit tumour growth, two million colon carcinoma cells (LS174t) were injected subcutaneously into nude mice, and tumours were allowed to grow for 4 d before bi-weekly intraperitoneal injection of rhVEGF165b was initiated (Fig. 5B). Treatment with rhVEGF165b significantly and dose dependently inhibited the growth of the established tumours compared with saline injection on day 10 for 50, 75 and 100 μg VEGF165b (p < 0.001, two-way ANOVA, n = 8, Fig. 5B). The 100 μg dose resulted in a significantly greater inhibition of tumour volume on 14 d than the 75 μg dose (p < 0.001, two-way ANOVA, n = 8, Fig. 5B). Analysis of the dose response curve at day 10 showed that the IC50 for the tumour inhibition was 48 μg.
These results show that injections of recombinant VEGF165b reduce the growth in vivo of heterotopic colon carcinoma tumours.
3.5. VEGF 165 b reduces spreading and vessel formation of endothelial cells on extracellular matrix
To explore the effect of VEGF165b on microvascular cells, an in vitro angiogenesis assay was used. Human dermal microvascular cells were seeded on an extracellular matrix gel containing 1 nM VEGF165, 1 nM VEGF165b, a combination of 1 nM VEGF165 and 1 nM VEGF165b or media without growth factors or serum. As expected, cells stimulated with VEGF165 produced extended vessel structures (see Fig. 6A) quantified by increased branch points, sprouting structures and closed polygon structures (see Fig. 6C). VEGF165b or the combination of VEGF165 and VEGF165b reduced the cells ability to extend and form structures on the extracellular matrix (see Fig. 6B and C). This further indicates that VEGF165b has a direct effect on the endothelial cells along with reduced migration of HUVECs (see Fig. 1C) and reduction in tumour growth by reduction of the microvessel density (see Fig. 4D).
Fig. 6.
VEGF165b reduces vessel structures on matrigel. HMVECs were seeded on top of extracellular matrix gel for 6 h and F-actin fibres were visualised. Cells underwent extensive structure formation after challenge with VEGF165 (A) but showed rather poor structures with a combination of VEGF165 and VEGF165b (B), resulting in a significantly reduced number of structure elements like sprouts, branch points and closed polygons (C). Arrow points towards polygon structures.
3.6. Accumulation and toxicology analysis
VEGF165b was shown to accumulate in the liver (see Fig. 3D) and to determine whether VEGF165b had any negative effects on liver function, liver sections from mice receiving i.p. injections daily for 2 weeks were sectioned and stained. No apparent changes were seen in the morphology of the liver sections from mice injected with vehicle compared with mice injected with increasing amount of VEGF165b (see Fig. 7A) even though intact VEGF165b protein was found (see Fig. 7B). Mice were also closely monitored during the injection period by visual inspection, and no changes in behaviour, indications of inflammation or any other unusual observations were apparent. No significant difference was observed in the liver enzyme levels between i.p. VEGF165b injected and vehicle injected mice (see Fig. 7C, n = 3 mice per enzyme test, p > 0.05, one-way ANOVA), indicating that VEGF165b had no apparent adverse effects on liver function. Proteinuria was not seen after 100 μg bi-weekly i.p. injection (mean ± SEM urinary protein:creatinine ratios 32.6 ± 5.49 mg/mmol control compared with 32.6 ± 10.2 mg/mmol, VEGF165b).
Fig. 7.
No adverse effects of VEGF165b injections. (A) No toxic effects on liver morphology were seen after repeated i.p. injections of VEGF165b. Haematoxylin and eosin stained cryosections of livers excised from mice injected daily with saline vehicle, 5 μg or 10 μg rhVEGF165b for 14 d. No adverse or toxic effects or changes on the liver morphology were observed. Scale bars = 100 μm. (B) After intraperitoneal injection, the protein accumulated in the liver for the entire time of analysis (24 h). (C) No significant change in liver enzyme levels in plasma from C57/Bl receiving i.p. injections for 8 d with 0.9% w/v NaCl (vehicle) or 5 μg rhVEGF165b. ALT = alanine transaminase, ALP = alkaline phosphatase and GGT = gamma glutamyl transpeptidase (n = 3 mice per enzyme test, p > 0.05 one-way ANOVA). (D) Infusion of 5 μg rhVEGF165b through the external jugular vein led to no change in blood pressure measured through a cannulation of the carotid artery compared with saline injection (vehicle).
VEGF165 is known to rapidly induce hypotension after i.v. injection.12 Mice were injected with 5 μg rhVEGF165b i.v. and blood pressure was measured. No hypotension was observed (see Fig. 7D).
4. Discussion
The anti-angiogenic isoform of VEGF, VEGF165b, is a potent inhibitor of blood vessel growth in ocular angiogenesis9 as well as in models of VEGF165-induced angiogenesis.7 VEGF165b levels are high in normal, non-angiogenic tissues, forming a substantial proportion or the majority of VEGF isoforms (13and unpublished data). This family is downregulated or poorly expressed in angiogenesis-related conditions, both in physiological (e.g. in placenta14) and in pathological angiogeneses such as in tumours6,7, or diabetic retinopathy.13 As this is an endogenous anti-angiogenic isoform, the principle of restoring the balance of anti- versus pro-angiogenic isoforms in pathologies that are dependent on blood vessel growth is an attractive concept.
This is the first time recombinant VEGF165b has been shown to inhibit tumour growth and reduce the growth of established colon carcinomas, and it indicates that VEGF165b may have a therapeutic role as an anti-cancer agent. Bi-weekly administration in mice, at doses similar to other anti-angiogenic therapies, is clearly effective. Systemic administration of VEGF165b will require sufficient protein for tumour accumulation to reach therapeutic values. Previous studies have shown that VEGF165 has a short circulating plasma half-life (25 min in mice15, 13.8-72 min in rats16,17,18) when given as a bolus i.v. injection, consistent with the experiments described here. However, continuous infusion of VEGF165 results in detectable circulating levels after up to 28 d15,17 Combined with the data described here, showing that the concentration of VEGF165b in the tumour was 6% of the injected dose, this suggests that the circulating plasma half-life of VEGF165b is less important than the tissue concentration. By using these levels as a guide, the level of VEGF165b that may be required to inhibit tumour growth when given i.v. in humans might be speculated to be at doses of the order of 0.1 to 1.4 mg kg-1 day-1 17. VEGF165 is known to be found in organs such as liver, kidney, spleen and lungs in humans19 and rats16 after intravenous or subcutaneous injections, respectively. A previous study with a single i.v. injection of VEGF165 into mice showed undetectable levels after 50 min, but was detected in the liver and kidney as measured by the accumulation of radioactive labelled VEGF165.15 Despite analysing kidneys and lungs, we only detected accumulation of intact rhVEGF165b in the liver. These data suggest that delivery of VEGF165b appears to be safe in animal models, that it is an effective anti-cancer agent and that further optimisation of the dosing strategy may be appropriate to determine the most effective therapeutic regimen in patients.
The current findings indicate that systemic administration of recombinant VEGF165b protein would be an effective therapeutic agent in cancer (e.g. by infusion, or subcutaneous or intraperitoneal injection).
Acknowledgements
The authors would like to thank Cancer Research Technologies, London, for generation of VEGF165b, Leslie Sage for technical assistance and extraction of HUVEC and Dr. Kate Heesom at the Department of Biochemistry, University of Bristol for mass spectrometry. This work was supported by a Cancer Research UK Development Grant (A5047), the British Heart Foundation (BB2000003 and BS06/005) and National Kidney Research Fund Grant R15/2/2003.
Footnotes
Conflict of interest statement
Prof. Bates and Dr. Harper are co-inventors on the patent describing VEGF165b as a potential therapeutic in cancer. There are no other conflicts of interest.
REFERENCES
- 1.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–5. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 2.Rak J, Mitsuhashi Y, Bayko L, et al. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55(20):4575–80. [PubMed] [Google Scholar]
- 3.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23(15):3502–8. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New Engl J Med. 2004;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 6.Bates DO, Cui TG, Doughty JM, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62(14):4123–31. [PubMed] [Google Scholar]
- 7.Woolard J, Wang WY, Bevan HS, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64(21):7822–35. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 8.Cebe Suarez S, Pieren M, Cariolato L, et al. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci. 2006;63(17):2067–77. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA. VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Mol Vis. 2006;12:626–32. [PubMed] [Google Scholar]
- 10.Varey AH, Rennel ES, Qiu Y, et al. VEGF165b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008;98(8):1366–79. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rennel E, Waine E, Guan H, et al. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br J Cancer. 2008;98(7):1250–7. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang R, Thomas GR, Bunting S, et al. Effects of vascular endothelial growth factor on hemodynamics and cardiac performance. J Cardiovasc Pharmacol. 1996;27(6):838–44. doi: 10.1097/00005344-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48(11):2422–7. doi: 10.1007/s00125-005-1951-8. [DOI] [PubMed] [Google Scholar]
- 14.Bates DO, MacMillan PP, Manjaly JG, et al. The endogenous anti-angiogenic family of splice variants of VEGF, VEGFxxxb, are down-regulated in pre-eclamptic placentae at term. Clin Sci (Lond) 2006;110(5):575–85. doi: 10.1042/CS20050292. [DOI] [PubMed] [Google Scholar]
- 15.Gabrilovich D, Ishida T, Oyama T, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92(11):4150–66. [PubMed] [Google Scholar]
- 16.Kim TK, Burgess DJ. Pharmacokinetic characterization of 14C-vascular endothelial growth factor controlled release microspheres using a rat model. J Pharm Pharmacol. 2002;54(7):897–905. doi: 10.1211/002235702760089009. [DOI] [PubMed] [Google Scholar]
- 17.Yang R, Bunting S, Ko A, et al. Substantially attenuated hemodynamic responses to Escherichia coli-derived vascular endothelial growth factor given by intravenous infusion compared with bolus injection. J Pharmacol Exp Ther. 1998;284(1):103–10. [PubMed] [Google Scholar]
- 18.Hsei V, Deguzman GG, Nixon A, Gaudreault J. Complexation of VEGF with bevaczumab decreases VEGF clearance in rats. Pharm Res. 2002;19(11):1753–6. doi: 10.1023/a:1020778001267. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Peck-Radosavljevic M, Kienast O, et al. Iodine-123-vascular endothelial growth factor-165 (123I-VEGF165). Biodistribution, safety and radiation dosimetry in patients with pancreatic carcinoma. Q J Nucl Med Mol Imaging. 2004;48(3):198–206. [PubMed] [Google Scholar]