Abstract
Styrene is widely used as one of the most important industrial materials for the production of synthetic rubbers, plastic, insulation, fiberglass, and automobile parts. Inhaled styrene has been reported to produce respiratory toxicity in humans and animals. Styrene oxide, a reactive metabolite of styrene formed via cytochrome P450 enzymes, has been reported to form covalent bonds with proteins, such as albumin and hemoglobin. Among all of the amino acids, cysteine is the most reactive amino acid to be modified by electrophilic species. The purpose of this study is to develop polyclonal antibodies for the detection of styrene oxide cysteinyl protein adducts. Two immunogens were designed, synthesized, and used to induce polyclonal antibodies in rabbits. Immune responses were observed from the raised antibodies by antiserum dilution tests. Competitive ELISA demonstrated that the resulting antibodies specifically recognized the styrene oxide-derived N-acetylcysteine adduct. Western blot results showed that the antibodies recognize styrene oxide-modified albumin. The binding was found to depend on the amount of protein adducts blotted and hapten loading in protein adducts. No cross reaction was observed from the native protein. Competitive Western blots further indicated that these antibodies specifically recognized styrene oxide cysteinyl–protein adducts. Immunoblots revealed the presence of several bands at a molecular weight ranging from 50 to 80 kDa in rat nasal mucosa treated with styrene. In conclusion, we successfully raised polyclonal antibodies to detect styrene oxide-derived protein/cysteine adducts.
Introduction
Styrene is an important industrial intermediate for the production of plastics and resins (1). It is also a widespread environmental contaminant to which human populations are exposed. Acute exposure to styrene was found to cause respiratory tissue injury in both animals and humans (2). Associated symptoms include obstructive pulmonary changes, lung ventilation disorder, nasal secretion, and nose irritation (3–6). In both humans and animals long-term exposed to styrene, increased incidences of cancer (7–11), chromosome aberrations, and sister chromatin exchange (SCEs) (12–15) have been reported. The potential carcinogenicity and genotoxicity of styrene have been associated with its reactive metabolite, styrene 7,8-oxide because this electrophilic species is the major metabolite of styrene in rats, mice, and humans via cytochrome P450. Toxicological studies have shown that this electrophile can attack nucleophilic sites on macromolecules, such as DNA and protein, to form adducts both in vitro and in vivo (16, 17). Among the proteins, albumin and hemoglobin are the only two proteins that have been studied intensively. GC/MS methods coupled with different techniques, such as Edman degradation (18–20), macromolecule hydrolysis (21), and Raney nickel reduction (22–24), have been used in these studies. Albumin and hemoglobin styrene oxide adducts have been identified both in vitro and invivo. These adducts have also been detected in styrene exposed workers. A dose–response relationship between these adducts and styrene exposure was observed in these exposed workers (25, 26). However, little knowledge about the interaction of styrene oxide with cellular proteins is available. The impact of cellular protein modification by styrene oxide on the observed toxicity of styrene remains unknown.
Immunochemical approaches have been successfully developed to identify target cellular proteins modified by reactive metabolites of many compounds, such as acetaminophen (27), halothane (28), bromobenzene (29), naphthalene (30), and trichloroethylene (31). This technique has provided a tool to investigate the toxicological importance of protein modification in experimental animals and exposed humans. In order to detect styrene oxide-modified cellular proteins, we raised polyclonal antibodies for the immunochemical detection of proteins modified by styrene oxide. These antibodies were shown to selectively recognize the styrene moiety. Styrene-oxide-modified proteins in styrene-treated rat nasal mucosa were detected by using these antibodies. It is anticipated that this method will facilitate the investigation of styrene-induced cytotoxicity and help us understand the relationship between cellular protein modification and styrene toxicity.
Experimental Procedures
Chemicals and Instruments
Styrene (99+%), styrene oxide (99+%), bovine albumin, N-acetylcysteine, Me2SO, and tris(2-carboxyethyl)phosphine (TCEP1) were obtained from Sigma-Aldrich (St. Louis, MO). Keyhole limpet hemocyanin (KLH) and N-succinimidyl (3(2)-pyridyldithio)propionate (SPDP) were purchased from Pierce (Rockford, IL). Immunoassays were performed with 96-well microtiter plates obtained from Nunc (Maxisorb, Roskilde, Denmark). The absorbances were read with a microplate reader (VERSAMax, Molecular Devices, Sunnyvale, CA). The curve fitting was accomplished with SigmaPlot. Western Blots were performed on an Invitrogen Xcell surelock electrophoresis system (Invitrogen, Carlsbad, CA). Structure identification was performed by both a 300-MHz NMR spectrometer (Variant Associates, Palo Alto, CA) and a LC-MS/MS system including an Agilent 1100 HPLC pump system interfaced with a Sciex API 2000 tandem quadrupole mass spectrometer (Applied Biosystems, Foster City, CA). A reverse phase C18 chromatography column (250 × 4.6 mm, Alltech, Deerfield, IL) was used for HPLC analysis and synthetic compound purification.
Synthesis. Styrene Mercapturic Acid I (2-(Acetylamino)-3-(2-hydroxy-1-phenylethylthio)propanoic Acid)
(Scheme 1). Styrene mercapturic acid I was synthesized as a mixture with styrene mercapturic acid II (Scheme 1). N-Acetylcysteine (826 mg, 5.1 mmol) was dissolved in 15 mL of 200 mM sodium phosphate buffer (pH 9.2). Styrene oxide (434 mg, 3.6 mmol) dissolved in 1.5 mL of Me2SO was added to the N-acetylcysteine solution. After stirring at room temperature for 14 h, the resulting solution was washed with ethyl acetate (10 mL × 3) and then acidified to pH 2.0 by 6.0 N HCl. The acidic solution was extracted with ethyl acetate (15 mL × 3), and the resulting extracts were pooled. The organic solvent was removed by rotary evaporation, and compound purification was conducted on a C18 reverse phase HPLC column (4.6 × 250 mm) eluted with solvent A (100% H2O) and solvent B (100% acetonitrile). Both solvents contained 0.05% trifluoroacetic acid. The structure of the purified compound was confirmed by both mass spectrometry and NMR. 1H NMR (CDCl3): δ 1.99 (s, 3H), 2.78 (m, 2H), 3.83 (m, 2H), 4.08 (m, 1H), 4.61 (m, 1H), 7.32 (m, 5H). ESI-MS: m/z 283.1.
Scheme 1.
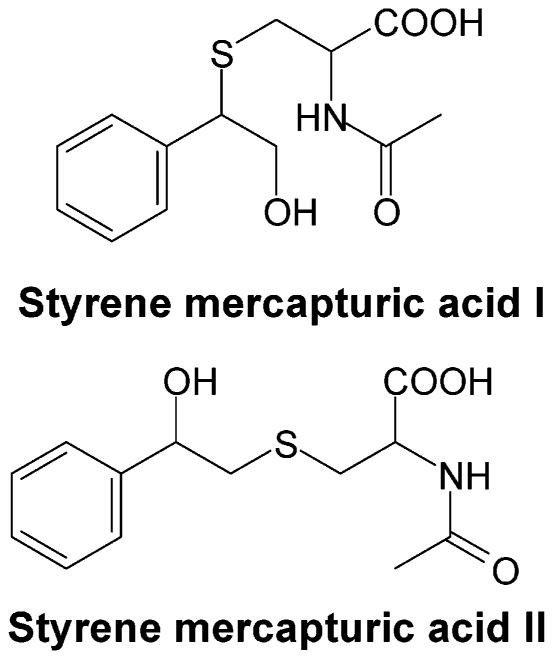
Styrene Mercapturic Acid II (2-(Acetylamino)-3-(2-hydroxy-2-phenylethylthio)propanoic Acid)
(Scheme 1). Styrene mercapturic acid II mixed with styrene mercapturic acid I was synthesized by the same procedure described above for the synthesis of styrene mercapturic acid I. 1H NMR (CDCl3): δ 1.95 (s, 3H), 2.86 (m, 2H), 2.98 (m, 2H), 4.51 (m, 1H), 4.78 (m, 1H), 7.32 (m, 5H). ESI-MS: m/z 283.1.
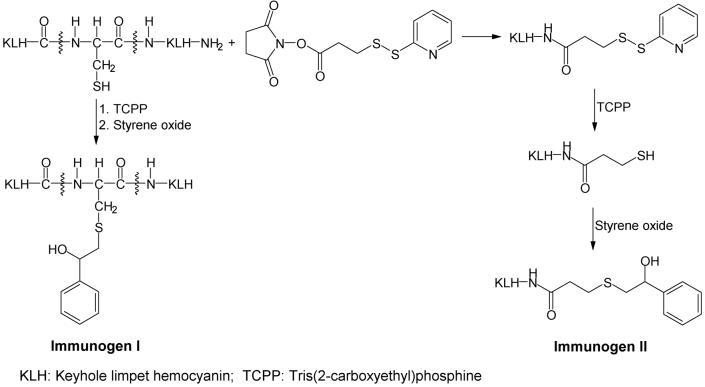
Keyhole Limpet Hemocyanin–Styrene Oxide Adduct (Immunogen I)
Immunogen I (Scheme 2) was synthesized as described by Zheng and co-workers (30) with slight modifications. A solution (1.0 mL) containing KLH (10 mg/mL) in phosphate buffer was added to 1 mL of 8 M guanidine solution. After adding TCEP (13.3 mg), the pH of this solution was adjusted to 6, and the reduction was performed under nitrogen while stirring for 3.5 h at room temperature. Styrene oxide (11.7 mg) was dissolved in 0.7 mL of Me2SO and added to the above reaction mixture. The pH of the reaction mixture was adjusted to 9.5 by adding dilute sodium hydroxide solution, and conjugation was conducted overnight under nitrogen while stirring. The next day, the reaction mixture was dialyzed three times against 1.0 L of deionized water and dried by lyophilization.
Scheme 2.
Thiolated KLH–Styrene Oxide Adduct (Immunogen II)
Immunogen II (Scheme 2) was synthesized by a slight modification of the method of Zheng and co-workers (30). SPDP (1.1 mg) dissolved in 50 μL of Me2SO was added to 1 mL of KLH solution in phosphate buffer (10 mg/mL). After stirring for 1.5 h at room temperature, TCEP (16.1 mg) was added to this mixture, and the pH of the solution was adjusted to 6. Reduction was performed under nitrogen for 3.5 h at room temperature. Styrene oxide (15.3 mg) was dissolved in 0.5 mL of Me2SO and added to the above reaction mixture. The pH of the reaction mixture was adjusted to 9.5 with dilute sodium hydroxide solution, and the reaction was performed overnight under nitrogen while stirring. The next day, the reaction mixture was dialyzed three times against 1.0 L of deionized water and dried by lyophilization.
Bovine Albumin–Styrene Oxide Adducts
Bovine albumin–styrene oxide adducts were synthesized as coating antigen for ELISA studies. Bovine albumin (20 mg), dissolved in 1 mL of PBS buffer, was mixed with 5.0 mg of styrene oxide in Me2SO. The reaction was conducted overnight while stirring. The next day, the reaction mixture was dialyzed three times against 1.0 L of deionized water and dried by lyophilization. A separate reaction was performed to synthesize bovine albumin–styrene oxide adducts as positive control for immunoblot analysis. Bovine albumin (55 mg/mL in pH 7.4 PBS buffer) was incubated with various concentrations of styrene oxide in Me2SO at room temperature overnight. The final concentrations of styrene oxide solution were 0.75, 3.75, and 7.5 mM. A similar process of dialysis and lyophilization was conducted as above.
Immunization of Rabbits
Female New Zealand white rabbits (Herbert's Rabbitry, Plymouth, CA) weighing 2.5–3.0 kg were immunized with the immunogen. The immunogen (100 μg) was dissolved in 0.5 mL of PBS buffer (pH 7.4) and emulsified with 0.5 mL of Freund's complete adjuvant. The rabbits were injected subcutaneously with the emulsion (1.0 mL/rabbit) at multiple sites in the back. After 4 weeks, these animals were boosted several times with a four-week interval by the same procedure (except that Freund's incomplete adjuvant was used instead of Freund's complete adjuvant). The rabbits were boosted until no further increase in antibody titer was observed.
Analysis of Titer
The titer of the serum obtained from the rabbits immunized by the immunogen was determined by measuring the binding of serial dilutions (1/200 to 1/12800) to microtiter plates coated with bovine albumin–styrene oxide adducts. Coating antigen solution (100 μL) in PBS buffer (200 mM phosphate-buffered saline solution at pH 7.4) containing bovine albumin–styrene oxide adducts (20 μg/mL) was added to each well of a 96-well microtiter plate. Plates were incubated at 4 °C overnight. The following day, the plates were washed three times by a PBST buffer (200 mM phosphate-buffered saline solution containing 0.02% Tween-20 at pH 7.4). After washing, 150 μL of 5% nonfat milk in PBST buffer was added to each well and incubated at room temperature for 1.5 h. After incubation, the wells were washed three times with PBST buffer. Then, the antiserum in PBST buffer at various dilutions was added (100 μL per well) to the plates and incubated at room temperature for 2 h. After washing in the same manner, 100 μL of anti-rabbit IgG-horseradish peroxidase solution in PBST buffer (1/12,000) was added to each well. The mixture was incubated for 1 h at room temperature. The plates were washed again as described previously. To each well, 100 μL of freshly prepared substrate solution containing 0.3 mM tetramethylbenzidine and 0.1 mM H2O2 in 0.1 M sodium acetate buffer (pH 5.5) was added and incubated for 30 min at room temperature. The colorimetric development was quenched by adding 50 μL of a 4 M sulfuric acid solution to each well. The absorbance at dual wavelengths (450–650 nm) was read.
Competitive Enzyme-Linked Immunosorbent Assay
One hundred microliters of coating antigen bovine albumin–styrene oxide adducts in PBS buffer (20 μg/mL) was added to wells of a 96-well microtiter plate. The plates were incubated at 4 °C overnight. Serial dilutions of the competitor (styrene mercapturic acid) (0.1 to 10−11 mM) were prepared in PBST buffer. The resulting solution was mixed (1:1 v/v) with primary antisera (1/1000 dilution) in 5% nonfat milk dissolved in PBST buffer. The mixture was incubated at 4 °C overnight. The following day, the same procedure as in the titer analysis was followed. The absorbance at dual wavelength (450–650 nm) was read.
Western Blots
Protein bands were resolved by SDS–polyacryl-amide gel electrophoresis using pre-cast 10% Tris-glycine gels (Invitrogen, Carlsbad, CA) and then transferred to nitrocellulose membranes (Amersham International Plc, England). Before loading, a protein assay was conducted to ensure that an equal amount of protein was loaded. Blots were then blocked with 5% nonfat milk in TBS-Tween buffer (100 mM Tris-base buffer containing 154 mM NaCl and 0.5% Tween-20 at pH 7.4) for 1 h at room temperature. Blotted membranes were incubated with 1/1000 dilution of primary rabbit antiserum #1043 in TBS-Tween buffer with 5% nonfat milk in the absence or presence of styrene mercapturic acids at 4 °C overnight. The following day, after washing three times with 15 mL of TBS-Tween buffer, membranes were incubated with anti-rabbit IgG-horseradish peroxidase solution (1/3000 in TBS-Tween buffer with 5% nonfat milk) for 1.5 h at room temperature. After washing, protein bands were detected by chemiluminescence with an ECL detection kit (Amersham International Plc, England).
Study in Vitro
Freshly collected nasal mucosa from Sprague–Dawley rats were incubated in RPMI 1640 supplemented with FBS (10%) with styrene in Me2SO at 37 °C in a water bath for 2 h. The final concentration of styrene in the incubation was either 1 mM or 5 mM. The concentration of Me2SO in the incubation was 0.5% (v/v). After incubation, protease inhibitors were added, and the tissue was homogenized. The resulting homogenates were centrifuged (450g) at 4 °C for 15 min. Supernatants were collected and centrifuged (13,000g)at 4 °C for 40 min. The resulting supernatants were stored at −80 °C until needed.
Results and Discussion
Albumin and hemoglobin cysteinyl styrene oxide adducts have been identified in styrene-exposed workers. Dose–response relationships between these adducts and styrene exposure were observed (25, 26). These findings suggest that the identification of styrene oxide-modified cellular proteins could be a step to elucidate the mechanism of styrene-induced cytotoxicity. Western blotting and immunostaining techniques have been used as powerful tools to identify protein adducts by detecting the hapten moiety of protein adducts formed during the exposure of cellular proteins to a chemically reactive species. In order to develop a method, which can identify the target cellular proteins of styrene oxide, as a tool to better understand the relationship between the styrene oxide metabolite–cellular protein interaction and styrene-induced cytotoxicity, we developed polyclonal antibodies. Previous studies have shown that styrene oxide can covalently bind to a variety of nucleophilic sites in proteins, including sulfhydryl groups, histidine imidazole, aspartic, glutamic carboxylic groups, amino groups in lysine, and the N-terminal of the protein chain (16, 21, 32, 33). However, cysteine has been reported as the most reactive amino acid to be modified by styrene oxide (32, 34). Structural analysis of the interaction between hemoglobin and styrene oxide at the whole protein level also confirmed cysteine residues as the most active sites (35). Therefore, in our study, the polyclonal antibodies were developed to recognize the cysteinyl styrene oxide moiety of proteins modified by styrene oxide. First, immunogens (I and II) were designed and synthesized as shown in Scheme 2. For immunogen I, the free sulfhydryl groups of KLH were generated by reducing with TCEP before styrene oxide was added. For immunogen II, KLH was first thiolated by SPDP. The resulting disulfide product was reduced by TCEP to generate free sulfhydryl groups, which immediately reacted with styrene oxide. As shown in Scheme 2, a spacer was incorporated between carrier protein KLH and styrene oxide in immunogen II in order to make the hapten more accessible to the immune system.
Immunizations of rabbits were carried out with immunogen I and II. The titer of the serum obtained from the rabbits was determined by measuring the binding of serial dilutions (1/200 to 1/12,800) to microtiter plates coated with bovine albumin–styrene oxide adducts. The final absorbance was read at dual wavelength (450–650 nm). There were eight rabbits, and three gave the highest titers. Figure 1 shows the titration of antisera raised against immunogen II, and the information regarding antisera raised against immunogen I can be found in Supporting Information. Nevertheless, all of the antisera obtained resulted in appreciable immune response, although further work is needed to characterize the antisera as follows.
Figure 1.
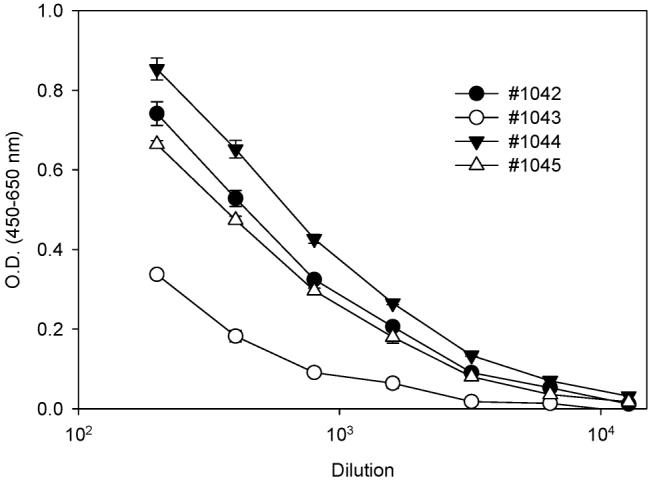
Titration tests of antisera raised against immunogen II. Ninety-six-well microtiter plates were coated with bovine albumin–styrene oxide adducts (20 μg/mL) and blocked with 5% nonfat milk. The resulting plates were incubated with individual antisera with serial dilutions, followed by the sequential addition of secondary antibody and substrate. The absorbance at dual wavelengths (450–650 nm) was read. The values are expressed as the mean ± SD from triplicate assays.
Styrene mercapturic acids I and II (see Scheme 1 for structures) as a mixture were synthesized to further characterize the antisera. The reaction of styrene oxide with N-acetyl cysteine produced a mixture of mercapturic acids I and II. Semiquantification by 1H NMR indicates an approximate 2:1 mole ratio of the mercapturic acid I/II mixture obtained in the conjugation reaction. This is consistent with a reported ratio of styrene mercapturic acid esters (65 vs 35) generated when styrene oxide reacted with the N-acetylcysteine ester (36). We assumed that the conjugation of cysteinyl residues of proteins with styrene oxide gave a similar ratio of its positional isomers in the preparation of the immunogens as well as the coating antigen. Thus, we used the positional styrene mercapturic acid mixture for the following studies.
In order to ensure that the immune response observed in the titration tests was caused by the styrene moiety, a competitive ELISA was conducted. In this study, the antisera (1/1000) were preincubated with a serial dilution of the synthetic styrene mercapturic acid positional mixture (Scheme 1) as the competitor. The preincubated antisera and competitor mixture was used for ELISA with styrene oxide–albumin adducts as the coating antigen. The optical density measured at 450 nm minus 650 nm was plotted against the logarithm of the various concentrations of the competitor. Although all of the antisera caused an immune response in titration tests, competition was only observed from antisera #1043 and #1044. These results are shown in Figure 2. The resulting sigmoid curves were fitted by a four-parameter logistic equation. The IC50 for antiserum #1043 and #1044 were 33.2 and 500 nM, respectively.
Figure 2.

Analysis of styrene mercapturic acids in a competitive ELISA using antiserum #1043 (A) and #1044 (B) raised against immunogen II. Ninety-six-well microtiter plates were coated with bovine albumin–styrene oxide adducts (20 μg/mL) and blocked with 5% nonfat milk. The resulting plates were incubated with corresponding antisera pretreated with serial dilutions of competitor styrene mercapturic acids (0.1 to 10−11 mM), followed by the sequential addition of antibody and substrate. The absorbance at dual wavelengths (450–650 nm) was read. The values are expressed as the mean ± SD from triplicate assays.
These results indicate that the absorbance observed from the other antiserum may be caused by nonspecific binding, only antisera #1043 and #1044 contain antibodies specific for the styrene moiety. These antibodies can recognize the cysteine-derived styrene oxide-modified protein. It is interesting that both antisera recognizing analyte styrene mercapturic acids were raised against immunogen II and that the antisera raised against immunogen I failed to recognize the analyte. This may be explained by the spacer used for immunogen II possibly making the styrene oxide adduct more visible by the immune system. Because of its lower IC50, antiserum #1043 was selected for further study.
In an effort to evaluate the correlation between antibody binding and hapten loading in bovine albumin–styrene oxide adducts, these adducts with various hapten loading were synthesized by exposure of bovine albumin to styrene oxide at concentrations of 0.75, 3.75, and 7.5 mM, respectively. After excessive dialysis, the resulting bovine albumin–styrene oxide adducts were subjected to Western blots. Two micrograms of bovine albumin–styrene oxide adducts or native bovine albumin were loaded onto 10% Tris-glycine gels. After separation and transfer, the blotted membrane was incubated with antiserum #1043, followed by anti-rabbit IgG conjugated with horseradish peroxidase. The resulting antibody-incubated membrane was treated with an ECL chemiluminescence kit. As shown in Figure 3, a chemiluminescent band at 65 KDa was observed in the lanes loaded with the bovine albumin–styrene oxide adducts. In addition, exposure of albumin to styrene oxide at higher concentration produced greater chemiluminescence in the immunoblot, indicating that antibody recognition depends on the hapten loading of the protein adducts. As expected, no chemiluminescent band was observed in the lane loaded with the same amount of native albumin (Figure 3). This indicates that the antibodies are able to detect protein adducts derived from styrene oxide and show no cross-reaction toward the native protein (albumin).
Figure 3.
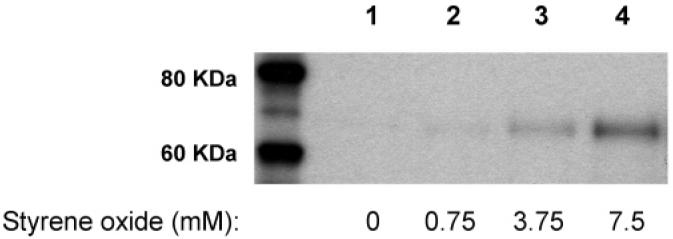
Immunochemical detection (antiserum #1043) of bovine albumin (55 mg) incubated with vehicle (lane 1), 0.75 mM styrene oxide (lane 2), 3.75 mM styrene oxide (lane 3), or 7.5 mM styrene oxide (lane 4). Each gel lane was loaded with 2.0 μg of the protein sample.
An additional immumoblot was performed to examine the concentration dependence of antibody binding. The albumin adducts prepared by an incubation of bovine albumin to styrene oxide at a concentration of 7.5 mM (see above) were mixed (diluted) with native albumin at ratios of 1:9 and 1:1 (albumin adducts/native albumin). The dilution ratios corresponded to the concentration of styrene oxide (0.75 and 3.75 mM vs 7.5 mM) in the reaction with bovine albumin for the preparation of the albumin styrene oxide adducts described above. The immumoblot analysis of protein adduct samples was processed in the same manner as that of the bovine albumin adducts prepared for the hapten loading study. As shown in Figure 4, a pattern in the density of chemiluminescence was observed similar to that found in the immunoblot of bovine albumin after exposure to styrene oxide at concentrations of 0.75, 3.75, and 7.5 mM (Figure 3), respectively. The albumin adducts without native albumin dilution produced the highest chemiluminescence, followed by those protein adducts diluted with 2- and 10-fold native albumin. This suggests a good quantitative immuno-response of the antibody to the protein adducts.
Figure 4.

Immunochemical detection of bovine albumin styrene oxide adducts (concentration dependence) by antiserum #1043. Bovine albumin (55 mg) was incubated with styrene oxide at a concentration of 7.5 mM. The resulting protein adducts were mixed with native bovine albumin at a mole ratio of 1:9 (lane 1) or 1:1 (lane 2). Lane 3 was loaded with the original protein adducts (without native albumin dilution). Each gel lane was loaded with 2.0 μg of the protein sample.
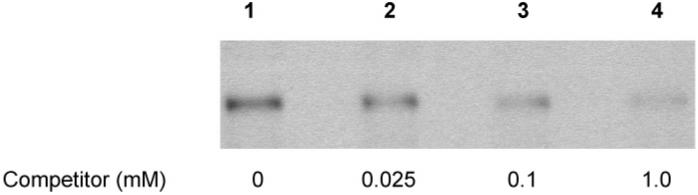
Competitive immunostaining was conducted to further determine the specificity of the antibody. The membrane blotted with the bovine albumin–styrene oxide adducts (exposed to 7.5 mM styrene oxide) was cut into four pieces. One was incubated with antiserum #1043 (1/1000), and the others were incubated with antiserum #1043 (1/1000) containing a series dilution of the styrene mercapturic acids at concentrations of 0.025, 0.1, and 1.0 mM. As shown in Figure 5, a slightly diminished chemiluminescence comparative to that of the vehicle treatment control was observed after exposure to the antibody in the presence of mercapturic acids at a concentration of 0.025 mM, and a significant decrease in chemiluminescence was found in the presence of 0.1 mM mercapturic acids. Furthermore, the presence of 1.0 mM mercapturic acids in the antibody incubation almost abolished chemiluminescence. This further supports the hypothesis that the binding of the antibodies to styrene oxide–protein adducts results from the immunorecognition of the cysteine residue modified by styrene oxide.
Figure 5.
Competitive immunostaining (antiserum #1043) of styrene oxide-modified bovine albumin (pretreated with 7.5 mM styrene oxide). The primary antibody was preincubated with the vehicle (lane 1), 0.025 mM (lane 2), 0.1 mM (lane 3), or 1.0 mM (lane 4) competitor styrene mercapturic acids. Each lane was loaded with 2.0 μg of the protein sample.
To examine the existence of cellular protein adducts derived by the styrene oxide metabolite and to determine whether these antibodies could detect such adducts, freshly collected rat nasal mucosa samples were incubated with 1 or 5 mM styrene in Me2-SO in RPMI 1640 supplemented with FBS (10%). The resulting protein samples were separated and blotted to nitrocellulose membranes. The membranes were incubated with antiserum #1043 (1/1000) and consecutively incubated with anti-rabbit IgG-horseradish peroxidase. The results are shown in Figure 6.
Figure 6.
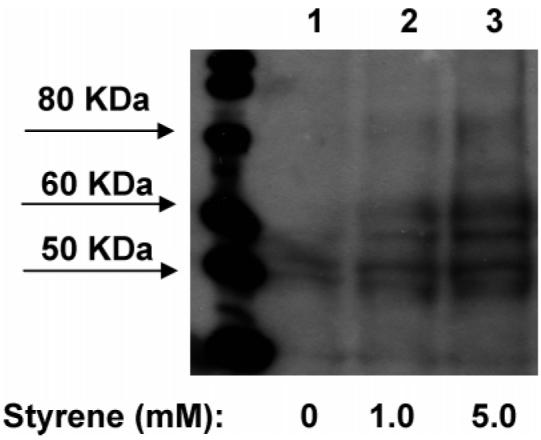
Western blot (antiserum #1043) of styrene oxide-modified protein samples obtained from rat nasal mucosa incubated with the vehicle (lane 1), 1 mM styrene (lane 2), or 5 mM styrene (lane 3). Each lane was loaded with 140 μg of the protein sample.
Several bands with molecular weight ranging from 50 to 80 kDa were detected from protein samples obtained from rat nasal mucosa incubated with 1 or 5 mM styrene. In the control lane, a 50 kDa band was also observed. However, this band was less dense compared to those of the styrene-treated samples. The results indicate the bioactivation of styrene to styrene oxide in situ and the covalent binding of styrene oxide metabolite to cellular proteins. However, the identities of the detected proteins are not yet known, and further studies will be conducted to elucidate these proteins.
In summary, we have successfully developed polyclonal antibodies to recognize cysteinyl styrene oxide–protein adducts. These antibodies are able to identify styrene oxide-modified proteins with little cross-reaction to native proteins. The specificity of these antibodies to detect the styrene moiety was confirmed by competitive ELISAs along with competitive Western blots. These antibodies could be used as a useful tool to detect cellular proteins modified by styrene oxide and to investigate the role of covalent binding in the mechanisms underlying styrene cytotoxicity.
Acknowledgment
We thank Fran Power-Charnitsky for her assistance in performing Western blots and her helpful suggestions. This work was supported by NIH Grant HL080226. Partial support was also provided by NIEHS Superfund Basic Research Program P42ES04699 and NIEHS Center for Environmental Health Science P30ES05707.
Footnotes
Supporting Information Available: Information regarding antisera raised against immunogen I. This material is available free of charge via the Internet at http://pubs.acs.org.
Abbreviations TCEP, tris(2-carboxyethyl)phosphine; KLH, keyhole limpet hemocyanin; SPDP, N-succinimidyl (3(2)-pyridyldithio)propionate; PBST, 200 mM phosphate-buffered saline solution containing 0.02% Tween-20 at pH 7.4; TBS-Tween, 100 mM tris-base buffer containing 154 mM NaCl and 0.5% Tween-20 at pH 7.4.
References
- 1.Kolstad HA, Juel K, Olsen J, Lynge E. Exposure to styrene and chronic health effects: mortality and incidence of solid cancers in the Danish reinforced plastics industry. Occup. Environ. Med. 1995;52:320–327. doi: 10.1136/oem.52.5.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bond JA. Review of the toxicology of styrene. Crit. Rev. Toxicol. 1989;19:227–249. doi: 10.3109/10408448909037472. [DOI] [PubMed] [Google Scholar]
- 3.Ohashi Y, Nakai Y, Ikeoka H, Koshimo H, Esaki Y, Horiguchi S, Teramoto K. Electron microscopic study of the respiratory toxicity of styrene. Osaka City Med. J. 1985;31:11–21. [PubMed] [Google Scholar]
- 4.Alarie Y. Sensory irritation of the upper airways by airborne chemicals. Toxicol. Appl. Pharmacol. 1973;24:279–297. doi: 10.1016/0041-008x(73)90148-8. [DOI] [PubMed] [Google Scholar]
- 5.Stewart RD, Dodd HC, Baretta ED, Schaffer AW. Human exposure to styrene vapor. Arch. Environ. Health. 1968;16:656–662. doi: 10.1080/00039896.1968.10665124. [DOI] [PubMed] [Google Scholar]
- 6.Chmielewski J, Renke W. Clinical and experimental studies on the pathogenesis of toxic effects of styrene. II. The effect of styrene on the respiratory system. Bull. Inst. Marit. Trop. Med. Gdynia. 1975;26:299–302. [PubMed] [Google Scholar]
- 7.Huff JE. Styrene, styrene oxide, polystyrene, and beta-nitrostyrene/styrene carcinogenicity in rodents. Prog. Clin. Biol. Res. 1984;141:227–238. [PubMed] [Google Scholar]
- 8.Maltoni C, Failla G, Kassapidis G. First experimental demonstration of the carcinogenic effects of styrene oxide; long-term bioassays on Sprague-Dawley rats by oral administration. Med. Lav. 1979;70:358–362. [PubMed] [Google Scholar]
- 9.Maltoni C. Early Results of the Experimental Assessments of the Carcinogenic Effects of One Epoxy Solvent: Styrene Oxide. In: Englung A, Ringen K, Mehlman MA, editors. Occupational Health Hazards of Solvents. Vol. 2. Princeton Scientific Publishers; Princeton, NJ: 1982. p. 97. [Google Scholar]
- 10.Jersey GC. Two Year Chronic Inhalation Toxicity and Carcinogenicity Study of Monomeric Styrene in Rats—Final Report. Chemical Manufacturers Association; 1978. CMA No.: Sty 1,2-Tox-Inh. [Google Scholar]
- 11.Conti B, Maltoni C, Perino G, Ciliberti A. Long-term carcinogenicity bioassays on styrene administered by inhalation, ingestion and injection and styrene oxide administered by ingestion in Sprague-Dawley rats, and para-methylstyrene administered by ingestion in Sprague-Dawley rats and Swiss mice. Ann. N.Y. Acad. Sci. 1988;534:203–234. doi: 10.1111/j.1749-6632.1988.tb30112.x. [DOI] [PubMed] [Google Scholar]
- 12.International Agency for Research on Cancer (IARC) IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Vol. 60. IARC; Lyon, France: 1994. Some Industrial Chemicals; pp. 233–320. [Google Scholar]
- 13.Fleig I, Thiess AM. Mutagenicity study of workers employed in the styrene and polystyrene processing and manufacturing industry. Scand. J. Work, Environ. Health. 1978;4:254–258. [PubMed] [Google Scholar]
- 14.Andersson HC, Tranberg EA, Uggla AH, Zetterberg G. Chromosomal aberrations and sister-chromatid exchanges in lymphocytes of men occupationally exposed to styrene in a plastic-boat factory. Mutat. Res. 1980;73:387–401. doi: 10.1016/0027-5107(80)90203-1. [DOI] [PubMed] [Google Scholar]
- 15.Maki-Paakkanen J, Walles S, Osterman-Golkar S, Norppa H. Single-strand breaks, chromosome aberrations, sister-chromatid exchanges, and micronuclei in blood lymphocytes of workers exposed to styrene during the production of reinforced plastics. Environ. Mol. Mutagen. 1991;17:27–31. doi: 10.1002/em.2850170105. [DOI] [PubMed] [Google Scholar]
- 16.Phillips DH, Farmer PB. Evidence for DNA and protein binding by styrene and styrene oxide. Crit. Rev. Toxicol. 1994;24:S35–S46. doi: 10.3109/10408449409020139. [DOI] [PubMed] [Google Scholar]
- 17.Byfalt Nordqvist M, Lof A, Osterman-Golkar S, Walles SA. Covalent binding of styrene and styrene-7,8-oxide to plasma proteins, hemoglobin and DNA in the mouse. Chem.-Biol. Interact. 1985;55:63–73. doi: 10.1016/s0009-2797(85)80120-4. [DOI] [PubMed] [Google Scholar]
- 18.Tornqvist M, Mowrer J, Jensen S, Ehrenberg L. Monitoring of environmental cancer initiators through hemoglobin adducts by a modified Edman degradation method. Anal. Biochem. 1986;154:255–266. doi: 10.1016/0003-2697(86)90524-5. [DOI] [PubMed] [Google Scholar]
- 19.Christakopoulos A, Bergmark E, Zorcec V, Norppa H, Maki-Paakkanen J, Osterman-Golkar S. Monitoring occupational exposure to styrene from hemoglobin adducts and metabolites in blood. Scand. J. Work, Environ. Health. 1993;19:255–263. doi: 10.5271/sjweh.1476. [DOI] [PubMed] [Google Scholar]
- 20.Osterman-Golkar S, Christakopoulos A, Zorcec V, Svensson K. Dosimetry of styrene 7,8-oxide in styrene- and styrene oxide-exposed mice and rats by quantification of haemoglobin adducts. Chem.-Biol. Interact. 1995;95:79–87. doi: 10.1016/0009-2797(94)03348-x. [DOI] [PubMed] [Google Scholar]
- 21.Sepai O, Anderson D, Street B, Bird I, Farmer PB, Bailey E. Monitoring of exposure to styrene oxide by GC-MS analysis of phenylhydroxyethyl esters in hemoglobin. Arch. Toxicol. 1993;67:28–33. doi: 10.1007/BF02072031. [DOI] [PubMed] [Google Scholar]
- 22.Ting D, Smith MT, Doane-Setzer P, Rappaport SM. Analysis of styrene oxide-globin adducts based upon reaction with Raney nickel. Carcinogenesis. 1990;11:755–760. doi: 10.1093/carcin/11.5.755. [DOI] [PubMed] [Google Scholar]
- 23.Rappaport SM, Ting D, Jin Z, Yeowell-O'Connell K, Waidyanatha S, McDonald T. Application of Raney nickel to measure adducts of styrene oxide with hemoglobin and albumin. Chem. Res. Toxicol. 1993;6:238–244. doi: 10.1021/tx00032a014. [DOI] [PubMed] [Google Scholar]
- 24.Rappaport SM, Yeowell-O'Connell K, Bodell W, Yager JW, Symanski E. An investigation of multiple biomarkers among workers exposed to styrene and styrene-7,8-oxide. Cancer Res. 1996;56:5410–5416. [PubMed] [Google Scholar]
- 25.Liu SF, Fang QM, Jin ZL, Rappaport MS. Investigation of protein-styrene oxide adducts as a molecular biomarker of humans exposed to styrene. J. Environ. Sci. (China) 2001;13:391–397. [PubMed] [Google Scholar]
- 26.Fustinoni S, Colosio C, Colombi A, Lastrucci L, Yeowell-O'Connell K, Rappaport SM. Albumin and hemoglobin adducts as biomarkers of exposure to styrene in fiberglass-reinforced-plastics workers. Int. Arch. Occup. Environ. Health. 1998;71:35–41. doi: 10.1007/s004200050247. [DOI] [PubMed] [Google Scholar]
- 27.Hinson JA, Pumford NR, Roberts DW. Mechanisms of acetaminophen toxicity: immunochemical detection of drug-protein adducts. Drug Metab. Rev. 1995;27:73–92. doi: 10.3109/03602539509029816. [DOI] [PubMed] [Google Scholar]
- 28.Martin JL, Meinwald J, Radford P, Liu Z, Graf ML, Pohl LR. Stereoselective metabolism of halothane enantiomers to trifluoroacetylated liver proteins. Drug Metab. Rev. 1995;27:179–189. doi: 10.3109/03602539509029822. [DOI] [PubMed] [Google Scholar]
- 29.Rombach EM, Hanzlik RP. Detection of adducts of bromobenzene 3,4-oxide with rat liver microsomal protein sulfhydryl groups using specific antibodies. Chem. Res. Toxicol. 1999;12:159–163. doi: 10.1021/tx980177v. [DOI] [PubMed] [Google Scholar]
- 30.Zheng J, Hammock BD. Development of polyclonal antibodies for detection of protein modification by 1,2-naphthoquinone. Chem. Res. Toxicol. 1996;9:904–909. doi: 10.1021/tx960014b. [DOI] [PubMed] [Google Scholar]
- 31.Halmes NC, McMillan DC, Oatis JE, Jr., Pumford NR. Immunochemical detection of protein adducts in mice treated with trichloroethylene. Chem. Res. Toxicol. 1996;9:451–456. doi: 10.1021/tx950171v. [DOI] [PubMed] [Google Scholar]
- 32.Hemminki K. Reactions of methylnitrosourea, epichlorohydrin, styrene oxide and acetoxyacetylaminofluorene with polyamino acids. Carcinogenesis. 1983;4:1–3. doi: 10.1093/carcin/4.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Yeowell-O'Connell K, Pauwels W, Severi M, Jin Z, Walker MR, Rappaport SM, Veulemans H. Comparison of styrene-7,8-oxide adducts formed via reaction with cysteine, N-terminal valine and carboxylic acid residues in human, mouse and rat hemoglobin. Chem.-Biol. Interact. 1997;106:67–85. doi: 10.1016/s0009-2797(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 34.Hemminki K. Binding of styrene oxide to amino acids, human serum proteins and hemoglobin. Arch. Toxicol. 1986;9:286–290. doi: 10.1007/978-3-642-71248-7_46. [DOI] [PubMed] [Google Scholar]
- 35.Basile A, Ferranti P, Mamone G, Manco I, Pocsfalvi G, Malorni A, Acampora A, Sannolo N. Structural analysis of styrene oxide/haemoglobin adducts by mass spectrometry: identification of suitable biomarkers for human exposure evaluation. Rapid Commun. Mass Spectrom. 2002;16:871–878. doi: 10.1002/rcm.655. [DOI] [PubMed] [Google Scholar]
- 36.Yagen B, Hernandez O, Bend JR, Cox RH. Synthesis and relative stereochemistry of the four mercapturic acids derived from styrene oxide and N-acetylcysteine. Chem.-Biol. Interact. 1981;34:57–67. doi: 10.1016/0009-2797(81)90090-9. [DOI] [PubMed] [Google Scholar]