Abstract
Endogenous 2-arachidonoylglycerol (2-AG) is antiinvasive in androgen-independent prostate carcinoma (PC-3) cells. Invasion of PC-3 cells is also inhibited by exogenously added noladin ether, a non-hydrolyzable analog of 2-AG. In contrast, exogenous 2-AG has the opposite effect. Cell invasion significantly increased with high concentrations of exogenous 2-AG. In PC-3 cells, arachidonic acid (AA) and 12-hydroxyeicosatetraenoic acid (12-HETE) concentrations increased along with exogenously added 2-AG, and 12-HETE concentrations increased with exogenously added AA. Invasion of PC-3 cells also increased with exogenously added AA and 12(S)-HETE but not 12(R)-HETE. The exogenous 2-AG-induced invasion of PC-3 cells was inhibited by 3-octylthio-1,1,1-trifluoropropan-2-one (OTFP, an inhibitor of 2-AG hydrolysis) and baicalein (a 12-LO inhibitor). Western blot and RT-PCR analyses indicated expression of 12-HETE producing lipoxygenases (LOs), platelet-type 12-LO (P-12-LO) and leukocyte-type 12-LO (L-12-LO), in PC-3 cells. These results suggest that exogenous 2-AG induced, rather inhibited, cell invasion because of its rapid hydrolysis to free AA, and further metabolism by 12-LO of AA to 12(S)-HETE, a promoter of PC cell invasion. The results also suggest that PC-3 cells and human prostate stromal (WPMY-1) cells released free AA, 2-AG, and 12-HETE. In the microenvironment of the PC cells, this may contribute to the cell invasion. The 2-AG hydrolysis and concentration of 2-AG in microenvironment are critical for PC cell's fate. Therefore, inhibitors of 2-AG hydrolysis could potentially serve as therapeutic agents for the treatment of prostate cancer.
Keywords: 2-arachidonoylglycerol, hydrolysis, cell invasion, 12-LO, prostate cancer
2-Arachidonoylglycerol (2-AG) is an endocannabinoid (endogenous ligand) for the cannabinoid (CB) receptors.1,2 The endocannabinoid signaling system is comprised of CB receptors, putative endogenous ligands and enzymes that synthesize and metabolize endocannabinoids. The endocannabinoid signaling system has recently been implicated in the regulation of various types of cancer.3-12 However, the role of 2-AG in prostate carcinoma cells are not well understood. 2-AG inhibits prolactin-induced proliferation in DU-145 cells by activation of the CB1 receptors.13 We previously demonstrated that 2-AG is endogenously produced in prostate carcinoma cells and acts as an endogenous antiinvasive factor in androgen-independent prostate carcinoma (PC-3 and DU-145) cells.14 Increasing the endogenous 2-AG concentration by inhibiting its hydrolysis or treating cells with a nonhydrolyzable analog of 2-AG, noladin ether (a CB1 receptor ligand), decreased cell invasion.14,15
Although the endogenous 2-AG and its stable analog inhibit invasion of prostate carcinoma cells, exogenously added 2-AG does not block cell invasion. 2-AG can be readily hydrolyzed to arachidonic acid (AA) and glycerol by 2 well characterized enzymes, monoacylglycerol lipase (MGL)16,17 and fatty acid amide hydrolase (FAAH).16 The 2-AG hydrolysis may possess 2 important effects; (i) it reduces 2-AG concentration as a ligand for binding to the CB receptors and (ii) it generates free AA as a substrate for enzymes in the AA metabolic cascade. Both these 2 pathways can promote invasion of prostate carcinoma cells. In this study, we investigated enzymatic pathways that contribute to detrimental effects of exogenous 2-AG on invasion of prostate carcinoma cells.
Material and methods
Materials
Androgen-independent prostate carcinoma (PC-3) cells, human prostate stromal cells (WPMY-1) and human fibroblast were obtained from the American Type Culture Collection (ATCC, Rockville, Maryland). AA, 2-AG, 12(R)-HETE, 12(S)-HETE, [2H8]AA, [2H8]2-AG, [2H8]12-HETE, and primary antibody against L-12-LO were obtained from Cayman Chemical (Ann Arbor, Michigan). Another primary antibody against L-12-LO was raised in rabbits in our laboratory.18,19 Human P-12-LO and porcine L-12-LO were obtained from Oxford Biochemical Research. (Oxford, Michigan). Goat anti-rabbit IgG-HRP was obtained from Zymed Laboratories. (South San Francisco, CA). Baicalein was obtained from Calbiochem (San Diego, CA). Thymidine [methyl-3H] (1 μCi/ml) was obtained from Applied Biosystems (Foster City, CA). Matrigel was obtained from BD Biosciences (Bedford, MA). 3-Octylthio-1,1,1-trifluoropropan-2-one (OTFP) was synthesized as previously described.20,21 [14C]2-AG was synthesized in Dr. J.R. Falck's laboratory. Western Lightning Chemiluminescence Reagent was obtained from Perkin Elmer (Boston, MA). Trizol Reagent was obtained from Invitrogen (Carlsbad, CA). Primers for P-12-LO and GAPDH were obtained from Integrated DNA Technologies (Coralville, IA). Adenosine-5′-triphosphate (γ-32P) was obtained from Perkin-Elmer Life Science (Wellesley, MA). C18 Bond Elut solid phase extraction (SPE) columns were obtained from Varian (Harbor City, CA). All other chemicals and solvents were of analytical or highest purity grades. Distilled, deionized water was used in all experiments.
Cells and cell culture
PC-3 cells, human fibroblasts and human prostate stromal (WPMY-1) cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, l-glutamine (2 mM), streptomycin (100 μg/ml) and penicillin (100 U/ml).14 Cells were grown in 75-cm2 polystyrene tissue culture flasks at 37°C in 5% CO2 in air to ∼70% confluency before use.
The media for human fibroblasts were changed to serum free RPMI and cultured overnight. Then, the conditioned media were used as a chemoattractant in invasion assay.
For conditioned media of PC-3 cells or WPMY-1 cells, the media were changed to serum free RPMI and the cells were incubated for 24 hr. Then, the conditioned media were analyzed for 2-AG, AA and 12-HETE, or used to treat PC-3 cells during invasion assay.
Determination of AA, 2-AG and 12-HETE by liquid chromatography-electrospray ionization-mass spectrometry
Cells were grown in T-75 flasks, rinsed with 5-ml HEPES buffer, pH 7.4, treated with OTFP (1 μM), baicalein (10 μM) or vehicle in HEPES buffer at 37°C for 15 min. In some experiments, cells were incubated with exogenous 2-AG following the 15-min pre-treatment with the inhibitors or vehicle as previously described.22 Cells were lysed, scraped and transferred into 15-ml tubes. An aliquot of 100 μl of cell lysates was saved for protein determination by Bio-Rad Protein Assay. [2H8]12-HETE (1 ng), [2H8]AA (60 ng), and [2H8]2-AG (30 ng) were added to the samples as internal standards. The samples were extracted by SPE as previously described,22 redissolved in 20 μl acetonitrile, and analyzed or kept at −80°C. Samples were analyzed by using liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) (Agilent 1100 LC-MSD, SL model).22 Briefly, the samples were separated on a reverse phase C18 column (Kromasil, 250 × 2 mm2, Phenomenex) using water/acetonitrile containing 0.005% acetic acid as a mobile phase at a flow rate of 0.2 ml/min. The gradient started at 15% acetonitrile, linearly increased to 80% acetonitrile in 40 min, then to 100% acetonitrile in 5 min, and was held at 100% acetonitrile for 10 min. Drying gas flow of the electrospray chamber was 12 l/min, drying gas temperature was 350°C, nebulizer pressure was 35 psig, vaporizer temperature was 350°C, capillary voltage was 3000 V, and fragmentor voltage was 120 V. The detection was made in the negative mode for 12-HETE and AA, and the positive mode for 2-AG. For quantitative measurement, the m/z 319, 327, 303, 311, 379 and 387 were used for 12-HETE, [2H8]12-HETE, AA, [2H8]AA, 2-AG, and [2H8]2-AG, respectively. The concentrations of 12-HETE, AA and 2-AG were calculated by comparing their ratios of peak areas (12-HETE to [2H8]12-HETE, AA to [2H8]AA, and 2-AG to [2H8]2-AG) to the standard curves. The results were normalized to the protein content.
Concentrations of AA, 2-AG and 12-HETE in conditioned media of PC-3 cells and WPMY-1 cells (cultured in serum free RPMI media for 24 hr) were also determined by LC-ESI-MS as earlier.
Determination of 2-AG metabolism by high performance liquid chromatography
To determine the conversion of 2-AG to AA in PC-3 cells, the cells were incubated with [14C]2-AG at 37°C for 30 min, lysed and extracted by SPE. Samples were separated on a C18 reverse phase column (4.6 × 250 mm2, Nucleosil, Phenomenex) using water:acetonitrile containing 0.1% acetic acid as a mobile phase at a flow rate of 1.0 ml/min. The mobile phase started at 50% acetonitrile and linearly increased to 100% acetonitrile in 35 min. The eluent was collected at 5 fractions/min and counted for radioactivity. The retention times of the radioactive peaks of 2-AG and AA in the samples were compared with the retention times of the 2-AG and AA standards.
For the determination of 12-HETE stereoisomers in PC-3 cells, the samples were separated by liquid chromatography using a chiral column (Chiracel OD, 4.6 × 250 mm2, Chiral Technologies, Exton, PA) with a mobile phase of hexane containing 0.1% isopropanol, 1% ethanol and 0.1% acetic acid at a flow rate of 1.0 ml/min. The UV absorption detection was made at the wavelength of 235 nm. The fractions corresponding to the retention times of 12(R)-HETE and 12(S)-HETE standards (19.32 and 21.45 min, respectively) were collected and then analyzed by LC-ESI-MS (Waters, Quattro micro API) using a similar protocol as described earlier.
Western blot analysis of L-12-LO
Cells were grown to 75% confluency and lysed in the presence of a “Complete Mini tablet” of protease inhibitors as described earlier. Protein samples (75 μg) were separated by SDS-PAGE (Ready Gels) and transferred to a 0.7-μm nitrocellulose membrane (BioRad, Hercules, CA). Human L-12-LO protein was used as a positive control. Primary antibodies against L-12-LO from 2 sources, Cayman Chemical. (1:500 dilution) and 1 produced in our laboratory (1:1000 dilution) were used. Then, goat anti-rabbit IgG-HRP (1:3000 dilution) was used to complex with the primary antibody. In some experiments, rabbit serum instead of the primary antibodies was used to determine nonspecficity of the antibodies. The detection was made by using Western lightning chemiluminescence reagent and captured by Fuji film X-ray (Tokyo, Japan).
Reverse-transcriptase polymerase chain reaction of P-12-LO
Since a primary antibody against human P-12-LO was not available for this study, the expression of P-12-LO mRNA in PC-3 cells was detected by RT-PCR as previously described with modifications.23 Briefly, PC-3 cells were washed twice with ice-cold PBS and RNA was extracted using Trizol followed by treatment with RNase-free DNA-Free (Ambion, Austin, TX). One microgram of total RNA was reverse-transcribed using oligo-dT primers with SuperScript III First-Strand Synthesis Kit (Invitrogen). Then, PCR was performed using PCR Master mix (Qiagen, Valencia, CA) with reported human P-12-LO primers (5′-GATGATCTACCTCCAAATATG and 3′-CTGGCCCCAGAAGATCTGATC)23 and amplified for 40 cycles. PCR products were separated on a 1.5% agarose gel supplemented with 20 μg of ethidium bromide, exposed to UV light and pictures for markers and GAPDH were captured. For P-12-LO detection, the bands was transferred to a zeta probe membrane (BioRad), and hybridized with a P-12-LO-specific probe (GTTTGAGGGCCATCTCCAGAGC) labeled with adenosine-5′-triphosphate (γ-32P) by T4 kinase (Invitrogen). Radioactivity was exposed overnight at −80°C using audioradiography film.
Cell invasion assay
Cell invasion assay was performed as previously described.14 Briefly, cells were incubated with thymidine [methyl-3H] (1 μCi/ml) in media containing 10% fetal bovine serum, rinsed with fresh complete media to remove unbound thymidine and detached. Cell suspensions of 50,000 cells containing the vehicle control or various concentrations of pharmacological agents [AA, 2-AG, 12(R)-HETE, 12(S)-HETE, OTFP and baicalein] were added to each upper compartment of Transwells containing Matrigel-coated 8.0-μm pore polyvinylpyrrolidone-free polycarbonate filters (Corning, Corning, NY). Fibroblast conditioned media (400 μl) was added in the bottom compartment of the well as a chemoattractant. Cells were incubated at 37°C in the incubator for 5 hr. The cells that passed into the lower compartment were detached and counted for radioactivity. Each treatment was performed on 6 wells. Two or three separate experiments were performed. The invasion was reported as the percentage of the invasion of the control cells.
For effects of conditioned media on cell invasion, the conditioned media from PC-3 cells or WPMY-1 cells were diluted with serum free media to 100, 50 and 25% of conditioned media and added in the upper compartments of the Transwells to treat PC-3 cells during the invasion assay. Cell invasion was measured as above.
Results
Metabolism of 2-arachidonoylglycerol in PC-3 cells
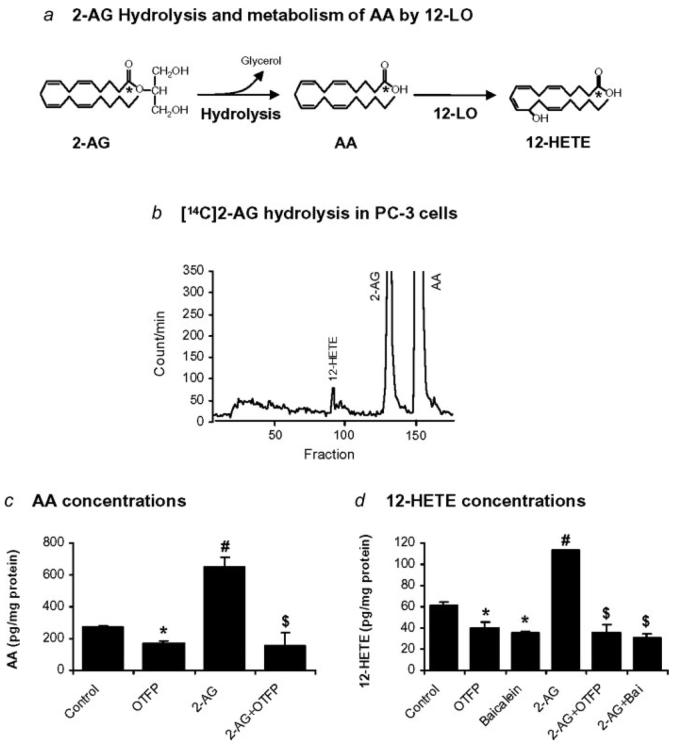
PC-3 cells were incubated with exogenous [1-14C]2-AG for 30 min and samples were extracted and analyzed by HPLC. Two major radiolabeled peaks comigrating with 2-AG and AA standards indicated that [1-14C]2-AG was hydrolyzed to [1-14C]AA (Fig. 1b). The chromatogram also contained small, more polar [1-14C]-radiolabeled peaks. One of these radioactive peaks comigrated with the 12-HETE standard (Fig. 1b).
Figure 1.
Metabolism of 2-AG in PC-3 cells. (a) Diagram depicting the hydrolysis of 2-AG to AA and the 12-LO metabolism of AA to 12-HETE. (b) Chromatogram of exogenous [14C]2-AG incubated with PC-3 cells for 30 min indicating the formation of AA and more polar products including a radiolabeled peak that comigrates with the 12-HETE standard. (c) Effects of OTFP (1 μM) and exogenous 2-AG (1 μM) on the concentrations of free AA in PC-3 cells. (d) Effects of OTFP (1 μM), baicalein (10 μM) and exogenous 2-AG (1 μM) on the concentrations of 12-HETE in PC-3 cells. Values are mean ± S.E.M. (n = 4–6). *, significantly lower than control, p < 0.05; #, significantly higher than control, p < 0.005; $, significantly lower than 2-AG treatment, p < 0.005.
Concentrations of free AA in PC-3 cells were analyzed by LC-ESI-MS. Free AA was present in PC-3 cells (control) and OTFP (1 μM) reduced the AA concentration (Fig. 1c). Exogenous 2-AG (1 μM) markedly increased AA concentration from the control cells. Again, OTFP reduced AA concentration (Fig. 1c). These results suggest that the exogenous 2-AG was hydrolyzed to AA and increased the concentration of free AA in PC-3 cells, and OTFP inhibited the hydrolysis of 2-AG and resulted in a decrease in concentration of free AA.
12-HETE, a major LO metabolite of AA in PC-3 cells, was also analyzed by LC-ESI-MS. PC-3 cells produced 12-HETE, and both OTFP (1 μM) and baicalein (a selective 12-LO inhibitor, 10 μM) significantly reduced the 12-HETE concentrations (Fig. 1d). Again, the exogenous 2-AG (1 μM) markedly increased the 12-HETE concentration from the control cells, and both OTFP (1 μM) and baicalein (10 μM) reduced the 12-HETE concentration (Fig. 1d). These results suggest that the exogenous 2-AG is hydrolyzed to AA and further metabolized by 12-LO to 12-HETE as illustrated in the schematic diagram (Fig. 1a). OTFP inhibited the hydrolysis of 2-AG to AA and reduced the 12-HETE concentration while baicalein inhibited the metabolism of AA by 12-LO and resulted in a decrease in 12-HETE concentration.
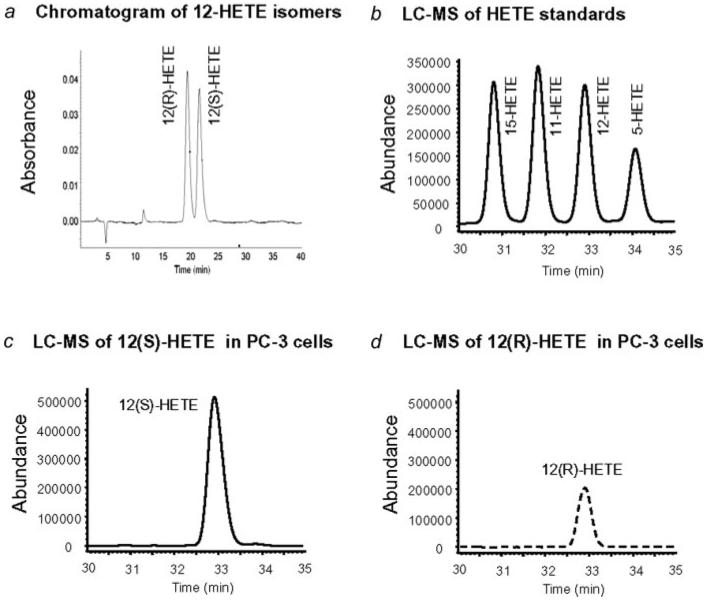
Identification of 12-HETE stereoisomers
To identify the stereoisomers of 12-HETE, PC-3 cells were treated with exogenous 2-AG (1 μM) for 30 min, lysed and extracted by SPE. The samples were separated on a chiral column and the fractions corresponding to the 12(S)-HETE and 12(R)-HETE standards (Fig. 2a) were collected. The fractions containing 12(S)-HETE and 12(R)-HETE were individually analyzed by LC-ESI-MS22 and compared with 12-HETE standard (Fig. 2b). The compositions of 12(S)-HETE and 12(R)-HETE were 62.5% ± 1.4% and 37.5% ± 1.4% (n = 4), respectively (Figs. 2c and 2d). Interestingly, baicalein (10 μM) reduced the 12-HETE concentrations in PC-3 cells (as shown in Fig. 1d) with a greater reduction in 12(S)-HETE than 12(R)-HETE (the compositions were 55.1 and 44.9%, respectively).
Figure 2.
Stereoisomers of 12-HETE. (a) Chromatogram of 12(R)-HETE and 12(S)-HETE standards separated on a chiral column and detected by UV detection. (b) Chromatogram of 15-, 11-, 12- and 5-HETE separated on a reverse phase C18 column and detected by ESI-MS. Note: The LC-ESI-MS technique used in this study cannot differentiate the 12-HETE stereoisomers. Chromatograms of (c) 12(S)-HETE and (d) 12(R)-HETE in PC-3 cells treated with exogenous 2-AG (1 μM). 12-HETE mixtures were first separated on an HPLC chiral column as in (a) and subsequently analyzed by LC-ESI-MS.
Expression of 12-LO in PC-3 cells
Western blot analysis of L-12-LO in PC-3 cell lysates exhibited an immunoreactive band corresponding to the L-12-LO protein standard at the molecular marker of 75 kDa (Fig. 3a). Both primary antibodies (Cayman Chemical and our laboratory) gave identical results. RT-PCR of P-12-LO mRNA indicated the gene expression of P-12-LO in PC-3 cells (Fig. 3b). Results from previous studies demonstrated an expression of P-12-LO and its role in cell invasion of PC-3 cells.24-26 The results from this study suggest that L-12-LO as well as P-12-LO are expressed and may contribute to production of 12(S)-HETE and cell invasion in PC-3 cells.
Figure 3.
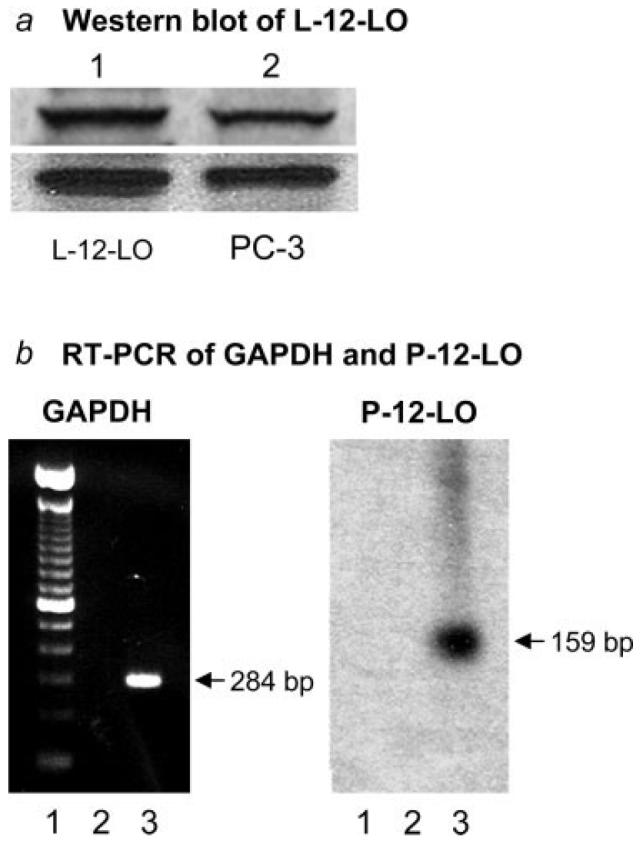
Expression of L-12-LO and P-12-LO in PC-3 cells. (a) Western blots depicting immunoreactive bands corresponding to L-12-LO (at 75 kDa of molecular weight markers). Lane 1 = L-12-LO protein standard and Lane 2 = PC-3 cell lysate. The upper blot was probed by a primary antibody (Cayman) that cross reacts with human L-12-LO and the lower blot was probed with the antibody raised in our laboratory against the NH2 terminus peptide sequence for the L-12-LO. (b) RT-PCR blots for P-12-LO mRNA in PC-3 cells. Left blot indicates the markers (Lane 1), blank negative control (Lane 2), and GAPDH (Lane 3) corresponding to marker of 284 bp. Right blot indicates the markers (Lane 1), negative control without reverse transcriptase (Lane 2), and the radioactive band of P-12-LO (Lane 3) corresponding to marker of 159 bp.
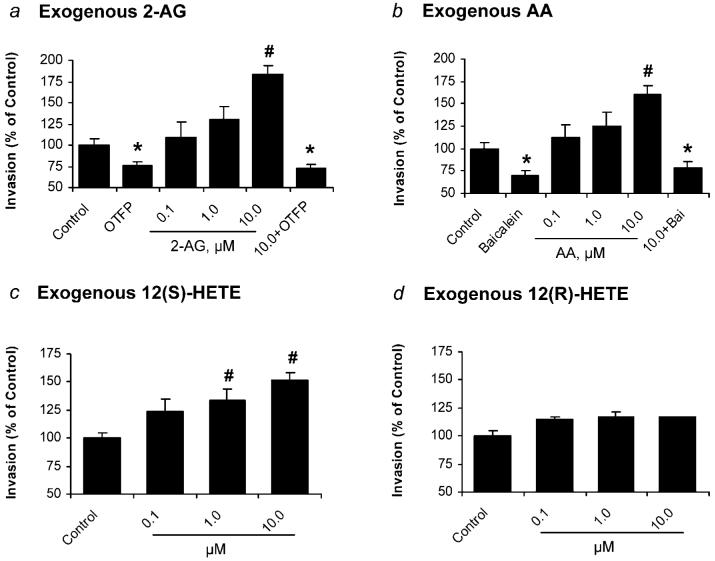
Effects of exogenous 2-AG, AA, and 12-HETE on cell invasion
Exogenously added 2-AG increased invasion of PC-3 cells, and OTFP (1 μM) blocked the invasion (Fig. 4a). Exogenously added AA increased invasion of PC-3 cells, and baicalein (10 μM) blocked the invasion (Fig. 4b). Exogenously added 12(S)-HETE also increased invasion of PC-3 cells (Fig. 4c). However, 12(R)-HETE did not significantly alter cell invasion (Fig. 4d). Taken together, these results suggest that the increase in cell invasion of PC-3 cells by the exogenous 2-AG, at least in part, because of the hydrolysis of 2-AG and metabolism of AA by 12-LO to produce 12(S)-HETE.
Figure 4.
Invasion of PC-3 cells treated with exogenous 2-AG, AA and 12-HETE. (a) Invasion of PC-3 cells treated with exogenous 2-AG at various concentrations (0.1–10.0 μM) and OTFP (1 μM). (b) Effect of exogenous AA at various concentrations (0.1–10.0 μM) and baicalein (10 μM) on invasion of PC-3 cells. (c) Effect of exogenous 12(S)-HETE at various concentrations (0.1–10.0 μM) on invasion of PC-3 cells. (d) Effect of exogenous 12(R)-HETE at various concentrations (0.1–10.0 μM) on invasion of PC-3 cells. Invasion was normalized to the control cells. Values are mean ± SEM (n = 12–18). *, significantly lower than control with p < 0.01; #, significantly higher than control with p < 0.005.
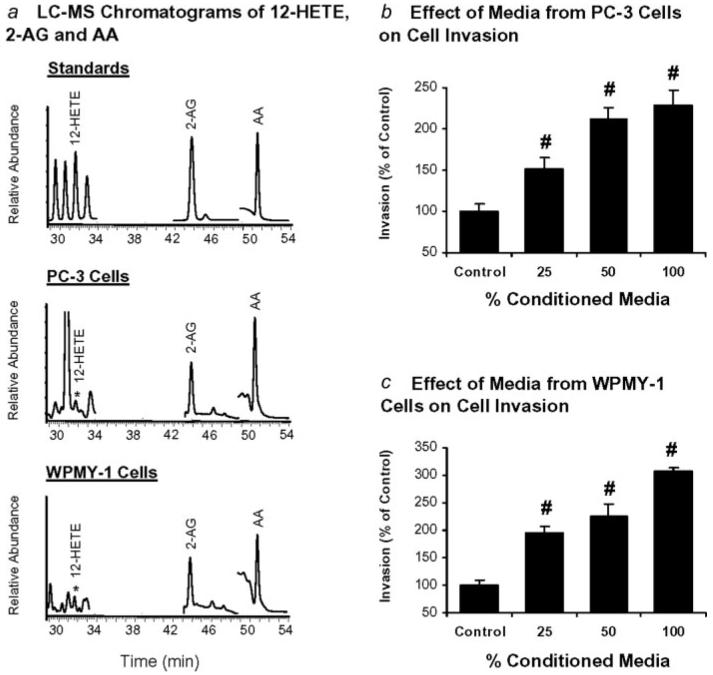
Release of 2-AG, AA and 12-HETE from PC-3 cells and WPMY-1 cells
To demonstrate the presence of exogenous 2-AG, AA and 12-HETE that may influence the invasion of prostate carcinoma cells, these lipids in the conditioned media of PC-3 cells and WPMY-1 cells were determined by LC-ESI-MS. Figure 5a shows the LC-MS chromatograms of 12-HETE, 2-AG, and AA standards (upper panel), in conditioned media of PC-3 cells (middle panel), and in conditioned media of WPMY-1 cells (lower panel), respectively. These results indicate that PC-3 cells and WPMY-1 cells release free 2-AG, AA and 12-HETE into the media.
Figure 5.
Release of 2-AG, AA, and 12-HETE by PC-3 cells and WPMY-1 cells and effects of conditioned media on invasion of PC-3 cells. (a) LC-MS chromatograms of 12-HETE, 2-AG and AA standards (upper panel), in conditioned media of PC-3 cells (middle panel), and in conditioned media of WPMY-1 cells (lower panel), respectively. (b) Effects of PC-3 cell conditioned media at 25, 50 and 100% on invasion of PC-3 cells. (c) Effects of WPMY-1 cell conditioned media at 25, 50 and 100% on invasion of PC-3 cells. Invasion was normalized to the control cells. Values are mean ± SEM (n = 6–12). #, significantly higher than control with p < 0.005.
PC-3 cells were treated with conditioned media from PC-3 cells or WPMY-1 cells at different dilutions, and cell invasion was determined as above. The effects of conditioned media from PC-3 cells and WPMY-1 cells on invasion of PC-3 cells are shown in Figures 5b and 5c. Invasion of PC-3 cells increased with the percentage of conditioned media from either PC-3 cells or WPMY-1 cells. These results suggest that 2-AG, AA and 12-HETE, among other factors released by PC-3 cell or WPMY-1 cells, may contribute to an increase of invasion of PC-3 cells.
Discussion
This study demonstrated that endogenous 2-AG is anti-invasive in prostate carcinoma (PC-3 cells) while exogenous 2-AG causes the opposite effect, an increase of cell invasion. The rapid hydrolysis of the exogenous 2-AG to AA and subsequent 12-LO metabolism is responsible for the increase invasion of prostate carcinoma PC-3 cells.
The basal AA concentration was reduced with OTFP. This is consistent with our previous findings that the inhibition of endogenous 2-AG hydrolysis by OTFP increases 2-AG concentrations and decreased invasion in PC-3 cells.15 Exogenously added 2-AG markedly increased free AA concentration. Again, the increase in AA concentration was reduced by OTFP. Both OTFP and baicalein reduced the 12-HETE concentrations in PC-3 cells by reducing the availability of free AA from 2-AG hydrolysis and by inhibiting the 12-LO metabolism of AA to 12-HETE, respectively. These results indicate that 2-AG is one of the sources of free AA and its 12-LO metabolite, 12-HETE.
Platelet-type, leukocyte-type and epidermal-type are the characterized forms of 12-LO that metabolize AA to 12(S)-HETE.27,28 12(R)-LO has been cloned from human keratinocytes,29 and produces 12(R)-HETE. P-12-LO has been investigated in androgen-independent PC-3 cells. P-12-LO promotes prostate cancer metastasis and angiogenesis.23-26,30-32 L-12-LO has been investigated in the vascular systems.23,33,34 The expression and function of L-12-LO have been characterized in human breast cancer tissues and cells35 and rat W256 cells36 but not in prostate cancer. Western blot analysis demonstrated that L-12-LO is expressed in PC-3 cells. These results suggest that L-12-LO may have a role in prostate carcinoma cells, which needs further investigation.
P-12-LO and L-12-LO are enantiomeric selective enzymes that produce only 12(S)-HETE.37,38 About two-thirds of the 12-HETE detected in PC-3 cells is 12(S)-HETE, suggesting that enzyme(s) other than P-12-LO and L-12-LO metabolize AA to 12(R)-HETE in PC-3 cells. 12(R)-HETE can be produced by cytochrome P450s39 or 12(R)-LO.29 Cytochrome P450 inhibitors such as miconazole (30 μM) and 17-octadecynoic acid (10 μM) did not reduce the 12(R)-HETE or 12(S)-HETE concentrations in PC-3 cells (data not shown), suggesting that 12(R)-HETE may be derived from 12(R)-LO. At present, the function of 12(R)-HETE in prostate carcinoma cells is unknown.
Exogenously added 2-AG increased cell invasion in PC-3 cells and OTFP inhibited the increase of cell invasion. Exogenously added AA also increased cell invasion which was inhibited by baicalein. Similarly, exogenously added 12(S)-HETE increased cell invasion consistent with previous studies demonstrating enhanced prostate carcinoma cell invasion with 12-(S)-HETE.37 These studies indicate metabolism of 2-AG to AA and 12-HETE is required for increased invasion.
The role of 12(S)-HETE in promoting prostate cancer growth and metastasis is established.25,26 However, prostate carcinoma cells also produce 12(R)-HETE. Since 12(R)-HETE is functionally inactive, AA metabolism to 12(R)-HETE may be desirable to reduce prostate cancer invasion.
Studies of the metabolism and effects of exogenous 2-AG is more than an experimental curiosity. These results suggest that exogenous 2-AG in the microenvironment of the prostate carcinoma cells may play a role in prostate tumor metastasis. In the microenvironment of the prostate carcinoma cells, other cell types can synthesize and release 2-AG and may be metabolized to AA and 12-HETE and influence carcinoma cell growth and migration. Although PC-3 cells and WPMY-1 cells might release several factors that can induce invasion of PC-3 cells, they also released 2-AG and its enzymatic metabolites (AA and 12-HETE) into the microenvironment and may promote the invasion of prostate carcinoma cells. These results suggest the release of 2-AG and its metabolites by surrounding cells such as other carcinoma cells and stromal cells may enhance the metastatic potential of prostate carcinoma cells. We have previously shown that endothelial cells release 2-AG in response to agonist.40 Platelets are another source of this lipid.41-44 The local concentrations of these lipids at close proximity or in microenvironment of prostate carcinoma cells can be high. Thus, 2-AG from these other cell types will behave as exogenous 2-AG to promote prostate carcinoma cell invasion.
In conclusion, an increase of invasion in PC-3 cells by exogenous 2-AG is mediated, at least in part, through the hydrolysis of 2-AG to AA and further AA metabolism by 12-LO. Since the binding sites of the CB receptors are located in the plasma membrane,45 the exogenous 2-AG will be susceptible to hydrolysis before it can bind to the receptors. These studies emphasize the critical role of enzymes that metabolize endogenous and exogenous 2-AG. These enzymes represent molecular switches that determine if invasion is turned off or invasion is turned on. Whereas endogenous 2-AG inhibits cell invasion, the metabolism of 2-AG to AA and 12-HETE results in the opposite effect, an increase in invasion. Understanding the regulation of 2-AG metabolism and the enzymes responsible for its metabolism is important to our understanding of prostate cancer. Molecular and pharmacological approaches to reducing 2-AG metabolism represent a therapeutic approach of great potential for controlling prostate cancer invasion.
Acknowledgements
The authors thank Dr. Lijie Cui, Mr. Dan Brody, and Miss Morgan Depas for their technical assistance. M.P.E. was supported by Predoctoral Fellowship Award from Emory T. Clark Foundation and C.E.W. was supported by NIH Post Doctoral Training Grant T32 DK07355-22. Support was also provided by NIESH Center for Environmental Health Sciences Grant P30 ES05707 (B.D.H), and NIH (GM-31278, J.R.F).
Grant sponsor: National Institute of Health; Grant numbers: DA 09155, T32 DK07355-22, GM-31278, RR-17824; Grant sponsors: Wisconsin Breast Cancer Showhouse for a Cure, Ridin' for Research Funds, Cancer Center of the Medical College of Wisconsin; NIEHS; Grant number: R37 ES02710; Grant sponsor: NIESH; Grant number: P42 ES04699; Grant sponsor: NIESH Center for Environmental Health Sciences; Grant number: P30 ES05707.
Abbreviations
- AA
arachidonic acid
- FAAH
fatty acid amide hydrolase
- HETE
hydroxyeicosatetraenoic acid
- LO
lipoxygenase
- MGL
monoacylglycerol lipase
- OTFP
3-octylthio-1,1,1-trifluoropropan-2-one
- 2-AG
2-arachidonoylglycerol
References
- 1.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 2.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 3.Di Marzo V, Bisogno T, De Petrocellis L. Endocannabinoids: new targets for drug development. Curr Pharm Des. 2000;6:1361–80. doi: 10.2174/1381612003399365. [DOI] [PubMed] [Google Scholar]
- 4.Ligresti A, Bisogno T, Matias I, De Petrocellis L, Cascio MG, Cosenza V, D'Argenio G, Scaglione G, Bifulco M, Sorrentini I, Di Marzo V. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology. 2003;125:677–87. doi: 10.1016/s0016-5085(03)00881-3. [DOI] [PubMed] [Google Scholar]
- 5.De Petrocellis L, Melck D, Bisogno T, Di Marzo V. Endocannabinoids and fatty acid amides in cancer, inflammation and related disorders. Chem Phys Lipids. 2000;108:191–209. doi: 10.1016/s0009-3084(00)00196-1. [DOI] [PubMed] [Google Scholar]
- 6.Parolaro D, Massi P, Rubino T, Monti E. Endocannabinoids in the immune system and cancer. Prostaglandins Leukot Essent Fatty Acids. 2002;66:319–32. doi: 10.1054/plef.2001.0355. [DOI] [PubMed] [Google Scholar]
- 7.Bifulco M, Laezza C, Valenti M, Ligresti A, Portella G, Di Marzo V. A new strategy to block tumor growth by inhibiting endocannabinoid inactivation. FASEB J. 2004;18:1606–8. doi: 10.1096/fj.04-1754fje. [DOI] [PubMed] [Google Scholar]
- 8.Grimaldi C, Pisanti S, Laezza C, Malfitano AM, Santoro A, Vitale M, Caruso MG, Notarnicola M, Iacuzzo I, Portella G, Di Marzo V, Bifulco M. Anandamide inhibits adhesion and migration of breast cancer cells. Exp Cell Res. 2006;312:363–73. doi: 10.1016/j.yexcr.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Bari M, Battista N, Fezza F, Gasperi V, Maccarrone M. New insights into endocannabinoid degradation and its therapeutic potential. Mini Rev Med Chem. 2006;6:257–68. doi: 10.2174/138955706776073466. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsson SO, Wallin T, Fowler CJ. Inhibition of rat C6 glioma cell proliferation by endogenous and synthetic cannabinoids. Relative involvement of cannabinoid and vanilloid receptors. J Pharmacol Exp Ther. 2001;299:951–9. [PubMed] [Google Scholar]
- 11.Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Orlando P, Wagner JA, Kunos G. Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. Eur J Biochem. 1999;264:258–67. doi: 10.1046/j.1432-1327.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- 12.Bisogno T, Sepe N, Melck D, Maurelli S, De Petrocellis L, Di Marzo V. Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochem J. 1997;322(Part 2):671–7. doi: 10.1042/bj3220671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melck D, De Petrocellis L, Orlando P, Bisogno T, Laezza C, Bifulco M, Di Marzo V. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology. 2000;141:118–26. doi: 10.1210/endo.141.1.7239. [DOI] [PubMed] [Google Scholar]
- 14.Nithipatikom K, Endsley MP, Isbell MA, Falck JR, Iwamoto Y, Hill-ard CJ, Campbell WB. 2-Arachidonoylglycerol: a novel inhibitor of androgen-independent prostate cancer cell invasion. Cancer Res. 2004;64:8826–30. doi: 10.1158/0008-5472.CAN-04-3136. [DOI] [PubMed] [Google Scholar]
- 15.Nithipatikom K, Endsley MP, Isbell MA, Wheelock CE, Hammock BD, Campbell WB. A new class of inhibitors of 2-arachidonoylglycerol hydrolysis and invasion of prostate cancer cells. Biochem Biophys Res Commun. 2005;332:1028–33. doi: 10.1016/j.bbrc.2005.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goparaju SK, Ueda N, Taniguchi K, Yamamoto S. Enzymes of porcine brain hydrolyzing 2-arachidonoylglycerol, an endogenous ligand of cannabinoid receptors. Biochem Pharmacol. 1999;57:417–23. doi: 10.1016/s0006-2952(98)00314-1. [DOI] [PubMed] [Google Scholar]
- 17.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–24. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nithipatikom K, McCoy MJ, Hawi SR, Nakamoto K, Adar F, Campbell WB. Characterization and application of Raman labels for confocal Raman microspectroscopic detection of cellular proteins in single cells. Anal Biochem. 2003;322:198–207. doi: 10.1016/j.ab.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Patel HH, Fryer RM, Gross ER, Bundey RA, Hsu AK, Isbell M, Eusebi LO, Jensen RV, Gullans SR, Insel PA, Nithipatikom K, Gross GJ. 12-lipoxygenase in opioid-induced delayed cardioprotection: gene array, mass spectrometric, and pharmacological analyses. Circ Res. 2003;92:676–82. doi: 10.1161/01.RES.0000065167.52922.F6. [DOI] [PubMed] [Google Scholar]
- 20.Wheelock CE, Colvin ME, Uemura I, Olmstead MM, Sanborn JR, Nakagawa Y, Jones AD, Hammock BD. Use of ab initio calculations to predict the biological potency of carboxylesterase inhibitors. J Med Chem. 2002;45:5576–93. doi: 10.1021/jm020072w. [DOI] [PubMed] [Google Scholar]
- 21.Wheelock CE, Severson TF, Hammock BD. Synthesis of new carboxylesterase inhibitors and evaluation of potency and water solubility. Chem Res Toxicol. 2001;14:1563–72. doi: 10.1021/tx015508+. [DOI] [PubMed] [Google Scholar]
- 22.Nithipatikom K, Isbell MA, See WA, Campbell WB. Elevated 12- and 20-hydroxyeicosatetraenoic acid in urine of patients with prostatic diseases. Cancer Lett. 2006;233:219–25. doi: 10.1016/j.canlet.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Gu JL, Natarajan R, Ben-Ezra J, Valente G, Scott S, Yoshimoto T, Yamamoto S, Rossi JJ, Nadler JL. Evidence that a leukocyte type of 12-lipoxygenase is expressed and regulated by angiotensin II in human adrenal glomerulosa cells. Endocrinology. 1994;134:70–7. doi: 10.1210/endo.134.1.8275971. [DOI] [PubMed] [Google Scholar]
- 24.Timar J, Raso E, Dome B, Li L, Grignon D, Nie D, Honn KV, Hagmann W. Expression, subcellular localization and putative function of platelet-type 12-lipoxygenase in human prostate cancer cell lines of different metastatic potential. Int J Cancer. 2000;87:37–43. doi: 10.1002/1097-0215(20000701)87:1<37::aid-ijc6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.Kandouz M, Nie D, Pidgeon GP, Krishnamoorthy S, Maddipati KR, Honn KV. Platelet-type 12-lipoxygenase activates NF-κB in prostate cancer cells. Prostaglandins Other Lipid Mediat. 2003;71:189–204. doi: 10.1016/s1098-8823(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 26.Pidgeon GP, Tang K, Cai YL, Piasentin E, Honn KV. Overexpression of platelet-type 12-lipoxygenase promotes tumor cell survival by enhancing α(v)β(3) and α(v)β(5) integrin expression. Cancer Res. 2003;63:4258–67. [PubMed] [Google Scholar]
- 27.Funk CD, Furci L, FitzGerald GA. Molecular cloning, primary structure, and expression of the human platelet/erythroleukemia cell 12-lipoxygenase. Proc Natl Acad Sci USA. 1990;87:5638–42. doi: 10.1073/pnas.87.15.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimoto T, Suzuki H, Yamamoto S, Takai T, Yokoyama C, Tanabe T. Cloning and sequence analysis of the cDNA for arachidonate 12-lipoxygenase of porcine leukocytes. Proc Natl Acad Sci USA. 1990;87:2142–6. doi: 10.1073/pnas.87.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boeglin WE, Kim RB, Brash AR. A 12R-lipoxygenase in human skin: mechanistic evidence, molecular cloning, and expression. Proc Natl Acad Sci USA. 1998;95:6744–9. doi: 10.1073/pnas.95.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pidgeon GP, Kandouz M, Meram A, Honn KV. Mechanisms controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase inhibition in prostate cancer cells. Cancer Res. 2002;62:2721–7. [PubMed] [Google Scholar]
- 31.Nie D, Nemeth J, Qiao Y, Zacharek A, Li L, Hanna K, Tang K, Hillman GG, Cher ML, Grignon DJ, Honn KV. Increased metastatic potential in human prostate carcinoma cells by overexpression of arachidonate 12-lipoxygenase. Clin Exp Metastasis. 2003;20:657–63. doi: 10.1023/a:1027302408187. [DOI] [PubMed] [Google Scholar]
- 32.Gao X, Grignon DJ, Chbihi T, Zacharek A, Chen YQ, Sakr W, Porter AT, Crissman JD, Pontes JE, Powell IJ, Honn KV. Elevated 12-lipoxygenase mRNA expression correlates with advanced stage and poor differentiation of human prostate cancer. Urology. 1995;46:227–37. doi: 10.1016/s0090-4295(99)80198-8. [DOI] [PubMed] [Google Scholar]
- 33.Kim JA, Gu JL, Natarajan R, Berliner JA, Nadler JL. A leukocyte type of 12-lipoxygenase is expressed in human vascular and mononuclear cells. Evidence for upregulation by angiotensin II. Arterioscler Thromb Vasc Biol. 1995;15:942–8. doi: 10.1161/01.atv.15.7.942. [DOI] [PubMed] [Google Scholar]
- 34.Natarajan R, Gu JL, Rossi J, Gonzales N, Lanting L, Xu L, Nadler J. Elevated glucose and angiotensin II increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proc Natl Acad Sci USA. 1993;90:4947–51. doi: 10.1073/pnas.90.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Natarajan R, Esworthy R, Bai W, Gu JL, Wilczynski S, Nadler J. Increased 12-lipoxygenase expression in breast cancer tissues and cells. Regulation by epidermal growth factor. J Clin Endocrinol Metab. 1997;82:1790–8. doi: 10.1210/jcem.82.6.3990. [DOI] [PubMed] [Google Scholar]
- 36.Pidgeon GP, Tang K, Rice RL, Zacharek A, Li L, Taylor JD, Honn KV. Overexpression of leukocyte-type 12-lipoxygenase promotes W256 tumor cell survival by enhancing αvβ5 expression. Int J Cancer. 2003;105:459–71. doi: 10.1002/ijc.11134. [DOI] [PubMed] [Google Scholar]
- 37.Dailey LA, Imming P. 12-Lipoxygenase: classification, possible therapeutic benefits from inhibition, and inhibitors. Curr Med Chem. 1999;6:389–98. [PubMed] [Google Scholar]
- 38.Reddy RG, Yoshimoto T, Yamamoto S, Funk CD, Marnett LJ. Expression of porcine leukocyte 12-lipoxygenase in a baculovirus/insect cell system and its characterization. Arch Biochem Biophys. 1994;312:219–26. doi: 10.1006/abbi.1994.1302. [DOI] [PubMed] [Google Scholar]
- 39.Capdevila J, Yadagiri P, Manna S, Falck JR. Absolute configuration of the hydroxyeicosatetraenoic acids (HETEs) formed during catalytic oxygenation of arachidonic acid by microsomal cytochrome P-450. Biochem Biophys Res Commun. 1986;141:1007–11. doi: 10.1016/s0006-291x(86)80144-9. [DOI] [PubMed] [Google Scholar]
- 40.Gauthier KM, Baewer DV, Hittner S, Hillard CJ, Nithipatikom K, Reddy DS, Falck JR, Campbell WB. Endothelium-derived 2-arachidonylglycerol: an intermediate in vasodilatory eicosanoid release in bovine coronary arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1344–H1351. doi: 10.1152/ajpheart.00537.2004. [DOI] [PubMed] [Google Scholar]
- 41.Pryor SR. Is platelet release of 2-arachidonoyl-glycerol a mediator of cognitive deficits? An endocannabinoid theory of schizophrenia and arousal. Med Hypotheses. 2000;55:494–501. doi: 10.1054/mehy.2000.1100. [DOI] [PubMed] [Google Scholar]
- 42.Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–44. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- 43.Chau LY, Tai HH. Diglyceride/monoglyceride lipases pathway is not essential for arachidonate release in thrombin-activated human platelets. Biochem Biophys Res Commun. 1983;113:241–7. doi: 10.1016/0006-291x(83)90457-6. [DOI] [PubMed] [Google Scholar]
- 44.Chau LY, Tai HH. Release of arachidonate from diglyceride in human platelets requires the sequential action of a diglyceride lipase and a monoglyceride lipase. Biochem Biophys Res Commun. 1981;100:1688–95. doi: 10.1016/0006-291x(81)90713-0. [DOI] [PubMed] [Google Scholar]
- 45.Klein TW, Lane B, Newton CA, Friedman H. The cannabinoid system and cytokine network. Proc Soc Exp Biol Med. 2000;225:1–8. doi: 10.1177/153537020022500101. [DOI] [PubMed] [Google Scholar]