Abstract
A brief period of global brain ischemia, such as that induced by cardiac arrest or cardiopulmonary bypass surgery, causes cell death in vulnerable hippocampal CA1 pyramidal neurons days after reperfusion. Although numerous factors have been suggested to account for this phenomenon, the mechanisms underlying it are poorly understood. We describe a cell death signal called the PIDDosome, a protein complex of p53-induced protein with a death domain (PIDD), receptor-interacting protein–associated ICH-1/CED-3 homologous protein with a death domain (RAIDD), and procaspase-2. We induced 5 min of transient global cerebral ischemia (tGCI) using bilateral common carotid artery occlusion with hypotension. Western blot analysis showed that expression of twice-cleaved fragment of PIDD (PIDD-CC) increased in the cytosolic fraction of the hippocampal CA1 subregion and preceded procaspase-2 activation after tGCI. Caspase-2 cleaved Bid in brain homogenates. Co-immunoprecipitation and immunofluorescent studies demonstrated that PIDD-CC, RAIDD, and procaspase-2 were co-localized and bound directly, which indicates the formation of the PIDD death domain complex. Furthermore, we tested inhibition of PIDD expression by using small interfering RNA (siRNA) treatment that was initiated 48 h before tGCI. Administration of siRNA against PIDD decreased not only expression of PIDD-CC, but also activation of procaspase-2 and Bid, resulting in a decrease in histological neuronal damage and DNA fragmentation in the hippocampal CA1 subregion after tGCI. These results imply that PIDD plays an important role in procaspase-2 activation and delayed CA1 neuronal death after tGCI. We propose that PIDD is a hypothetical molecular target for therapy against neuronal death after tGCI.
Keywords: apoptosis, caspase-2, p53, RAIDD, siRNA
It is well established that a brief period of global brain ischemia, such as that induced by cardiac arrest or cardiopulmonary bypass surgery, causes delayed cell death in vulnerable hippocampal CA1 pyramidal neurons days after reperfusion in animals and humans (1–3). Many studies have suggested various factors that contribute to this delayed vulnerability to neuronal death, such as glutamate neurotoxicity, calcium influx, expression of cell suicide genes, activation of apoptotic proteins, mitochondrial dysfunction, endoplasmic reticulum dysfunction, and oxygen free radicals. It has been demonstrated that caspase-3, caspase-9, and apoptosis-inducing factor, in particular, are involved in this neuronal death (4–6). However, the mechanisms are not well understood.
p53-induced protein with a death domain (PIDD) was originally identified as a gene that is dependent on p53 for its expression (7). PIDD induces cell-cycle arrest and apoptosis when overexpressed in p53-deficient human cell lines, suggesting that PIDD may act downstream of p53 in promoting cell death induced by p53. Recent reports have demonstrated that PIDD plays a critical role in the activation of caspase-2 (8–11). Full-length PIDD (PIDD-FL) is constitutively cleaved into three fragments by autoproteolysis: an N-terminal fragment and a C-terminal fragment (PIDD-C), which is further cleaved into PIDD-CC (8, 12). PIDD-CC, receptor-interacting protein-associated ICH-1/CED-3 homologous protein with a death domain (RAIDD), and procaspase-2 make a large protein complex, the PIDDosome, similar to the caspase-9 activating apoptosome complex (8). The PIDDosome structure is based on interaction of the death domains of PIDD and RAIDD and the caspase recruitment domains of RAIDD and procaspase-2 (13). The PIDDosome schematically dimerizes procaspase-2 molecules, resulting in caspase-2 activation (13). The PIDDosome likely regulates stress-induced apoptosis through a mitochondrial-dependent pathway (8). However, the role of PIDD in ischemia is unclear because most studies of PIDD have been conducted in the cancer field.
The purpose of this study was to detect the PIDDosome and determine its role in the activation of caspase-2 and subsequent neuronal death after transient global cerebral ischemia (tGCI). Five minutes of tGCI combined with hypotension were used to induce neuronal cell death in the hippocampal CA1 subregion in rats. We investigated expression of PIDD, activation of caspase-2, and interaction among PIDD, RAIDD, and procaspase-2, and expression of Bid as one of the downstream targets of caspase-2. Moreover, we administered PIDD siRNA to confirm the role of PIDD.
Results
Cytosolic Up-Regulation of PIDD and Subsequent Caspase-2 Activation in the Hippocampal CA1 Subregion After tGCI.
First, we tested the specificity of an anti-PIDD antibody. In Western blot analysis, PIDD-FL, PIDD-C, and PIDD-CC were detectable at ≈100, 53, and 37 kDa in the hippocampal lysate and positive control [supporting information (SI) Fig. S1A]. In the cytosolic fraction of the hippocampal CA1 subregion, PIDD-FL and PIDD-CC were detectable, but PIDD-C was not (Fig. S1A). Once an anti-PIDD antibody was neutralized with a blocking peptide, no PIDD bands were detected (Fig. S1B). These results suggest that bands detected by this anti-PIDD antibody were not nonspecific binding.
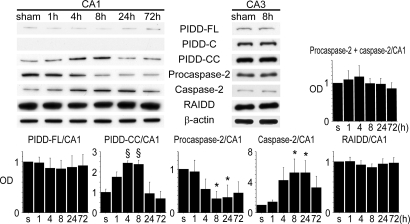
Next, we performed Western blotting with time course samples in the cytosolic fraction of the hippocampal CA1 subregion. This showed that PIDD-CC significantly increased after tGCI compared with the shams. It was detectable in the shams, was up-regulated 4 and 8 h after reperfusion, and was down-regulated by 72 h (Fig. 1). PIDD-FL was just detectable at all times and showed no significant difference at any time point (Fig. 1). PIDD-C was not detected (Fig. 1). Significant caspase-2 activation, detected by the antibody, which reacts with both procaspase-2 and caspase-2, was mainly observed 8 to 24 h after tGCI compared with the shams (Fig. 1). Procaspase-2 and caspase-2 were differentiated by their molecular weight. Procaspase-2 plus caspase-2 and RAIDD showed no significant difference at any time point (Fig. 1). Western blotting using CA3 samples showed that there was no up-regulation of PIDD-FL, PIDD-C, PIDD-CC, RAIDD, or caspase-2 after tGCI (Fig. 1). In summary, PIDD-CC increased in the hippocampal CA1 subregion after tGCI, followed by caspase-2 activation.
Fig. 1.
Cytosolic up-regulation of PIDD-CC and subsequent cleavage of procaspase-2 in the hippocampal CA1 subregion after tGCI. Western blot analysis showed that PIDD-CC expression, which was detectable in the shams, significantly increased 4 and 8 h after tGCI (n = 4; §, P < 0.001). PIDD-FL expression was just detectable at all time points and no significant difference was seen at any time point. PIDD-C was not detected in the CA1 subregion. Caspase-2 expression significantly increased compared with the shams, peaking at 8 to 24 h and decreasing by 72 h after tGCI (n = 4; *, P < 0.05). In contrast, procaspase-2 expression significantly decreased 8 and 24 h after tGCI (n = 4; *, P < 0.05). Procaspase-2 plus caspase-2 stayed constant. RAIDD expression was evident, but no significant difference was seen at any time point. Western blotting using CA3 samples showed that there was no up-regulation of PIDD-FL, PIDD-C, PIDD-CC, RAIDD, or caspase-2 after tGCI. β-actin was used as an internal control. s, shams; OD, optical density.
Neuronal Expression of PIDD, RAIDD, and Procaspase-2/Caspase-2.
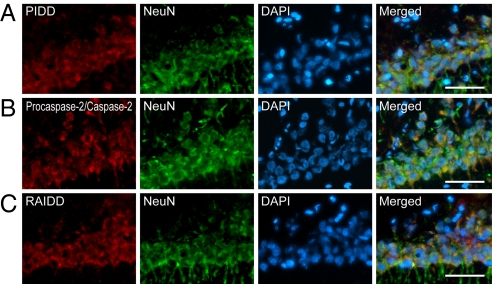
To investigate the subpopulations of PIDD, RAIDD, and procaspase-2/caspase-2 after tGCI, we performed double immunofluorescence of PIDD, RAIDD, or procaspase-2/caspase-2, and neuron-specific nuclear protein (NeuN). This demonstrated that PIDD, RAIDD, and procaspase-2/caspase-2 were expressed in hippocampal CA1 neurons (Fig. 2 A–C).
Fig. 2.
Neuronal expression of PIDD, procaspase-2/caspase-2, and RAIDD in the hippocampal CA1 subregion after tGCI. Fluorescent double staining of PIDD (A), procaspase-2/caspase-2 (B), or RAIDD (red) (C), and NeuN (green) in the hippocampal CA1 subregion. Nuclei were counterstained with DAPI (blue). NeuN showed neuronal distribution. Merged images demonstrate that PIDD-, RAIDD- and procaspase-2/caspase-2-positive cells co-localized with neurons. Scale bars, 50 μm.
Interaction of PIDD-CC, RAIDD, and Procaspase-2.
To investigate interaction among PIDD, RAIDD, and procaspase-2, we performed triple immunofluorescence, which demonstrated that PIDD was up-regulated in the cytosol of the hippocampal CA1 subregion 8 h after tGCI (Fig. S2). PIDD, RAIDD, and procaspase-2/caspase-2 were found together in many places (Fig. S2). No PIDD up-regulation was seen in the shams or the CA3 subregion (Fig. S2).
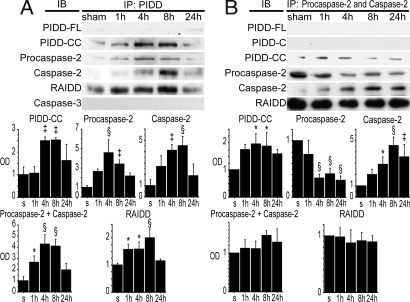
To reveal the functional relationship of PIDD-CC, RAIDD, and procaspase-2, we performed co-immunoprecipitation (co-IP) with a PIDD antibody in the cytosolic fraction after tGCI. We used an anti-PIDD antibody, which detects all fragments of PIDD, because there are no PIDD-CC-specific antibodies to our knowledge. RAIDD precipitated by PIDD significantly increased after tGCI compared with shams (Fig. 3A). Procaspase-2 precipitated by PIDD also significantly increased and peaked at 4 h (Fig. 3A). Caspase-2 precipitated by PIDD significantly increased and peaked 8 h after tGCI compared with the shams (Fig. 3A). Procaspase-2 plus caspase-2 significantly increased 1, 4, and 8 h after tGCI compared with shams, then decreased at 24 h (Fig. 3A), which may imply that caspase-2 remains on the PIDDosome for some time after it is formed. In contrast, caspase-3 was not precipitated by PIDD (Fig. 3A).
Fig. 3.
Interaction among PIDD, RAIDD, and procaspase-2/caspase-2 in the hippocampal CA1 subregion after tGCI. (A) Co-IP by PIDD using the cytosolic fraction of the hippocampal CA1 subregion. PIDD-CC precipitated by PIDD significantly increased 4 and 8 h after tGCI compared with the shams (n = 4, ‡, P < 0.005). Procaspase-2 precipitated by PIDD significantly increased after tGCI, peaked at 4 h, and started to decline at 24 h (n = 4; ‡, P < 0.005; §, P < 0.001). In contrast, caspase-2 precipitated by PIDD significantly increased after tGCI, peaked at 8 h, and declined at 24 h (n = 4, ‡, P < 0.005, §, P < 0.001). Procaspase-2 plus caspase-2 significantly increased 1, 4, and 8 h after tGCI compared with shams, then decreased at 24 h (n = 4; *, P < 0.05; §, P < 0.001). RAIDD precipitated by PIDD increased 1, 4, and 8 h after tGCI (n = 4; *, P < 0.05; §, P < 0.001). Caspase-3 immunoreactivity precipitated by PIDD was not detected. s, shams; IP, immunoprecipitation; IB, immunoblot; OD, optical density. (B) Co-IP by procaspase-2/caspase-2 using the cytosolic fraction of the hippocampal CA1 subregion. PIDD-CC precipitated by procaspase-2/caspase-2 significantly increased 4 and 8 h after tGCI compared with the shams (n = 4, *, P < 0.05). In contrast, PIDD-FL and PIDD-C were not precipitated by procaspase-2/caspase-2. Procaspase-2 precipitated by procaspase-2/caspase-2 was significantly down-regulated 4, 8, and 24 h after tGCI (n = 4, §, P < 0.001). Caspase-2 expression precipitated by procaspase-2/caspase-2 significantly increased 4, 8, and 24 h after tGCI (n = 4; *, P < 0.05, ‡, P < 0.005; §, P < 0.001). Procaspase-2 plus caspase-2 expression precipitated by procaspase-2/caspase-2 showed no significant difference at any time point. RAIDD precipitated by procaspase-2/caspase-2 showed no significant difference at any time point.
Next, we performed co-IP with a procaspase-2/caspase-2 antibody, which recognizes both procaspase-2 and caspase-2, in the cytosolic fraction after tGCI for further investigation of the interaction among PIDD-CC, RAIDD, and procaspase-2. PIDD-CC precipitated by procaspase-2/caspase-2 significantly increased and peaked 1 and 4 h after tGCI compared with the shams (Fig. 3B). In contrast, PIDD-FL or PIDD-C was not precipitated by procaspase-2/caspase-2 (Fig. 3B). Procaspase-2 plus caspase-2 precipitated by procaspase-2/caspase-2 showed no significant difference at any point (Fig. 3B). RAIDD precipitated by procaspase-2/caspase-2 was observed, but there was no significant difference at any time point (Fig. 3B). One possible reason why RAIDD stayed constant is that the total amount of procaspase-2 plus caspase-2 stayed constant, so RAIDD precipitated with procaspase-2/caspase-2 could also be constant.
In summary, these results cumulatively support the idea of direct binding and interaction among PIDD-CC, RAIDD, and procaspase-2 after tGCI, indicating formation of the PIDDosome and subsequent caspase-2 activation.
Bid Is Cleaved by Caspase-2 and Up-Regulated After tGCI.
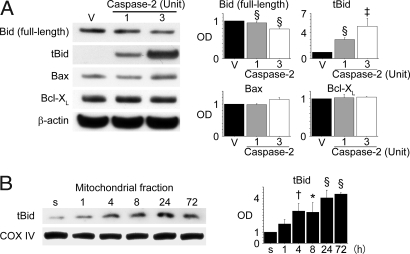
Bid is known to be one of the targets of caspase-2. To investigate the role of caspase-2, we added recombinant activated caspase-2 to the brain lysate. Western blot analysis revealed truncated Bid (tBid) up-regulation in a dose-dependent manner in samples incubated with caspase-2 compared with samples incubated with caspase-2 reaction buffer alone (Fig. 4A). Bax and Bcl-XL, two other Bcl-2 family proteins, were not significantly different between the two groups (Fig. 4A).
Fig. 4.
A downstream target of caspase-2. (A) In vitro processing of brain extract by recombinant activated caspase-2 using whole-cell extracts from fresh hippocampal CA1 subregions. The products were analyzed by SDS/PAGE. Bid cleavage was observed in a dose-dependent manner after incubation with caspase-2 (n = 4; ‡, P < 0.005; §, P < 0.001). Bax or Bcl-XL expression showed no significant difference. β-actin was used as an internal control. V, vehicle-treated sample (i.e., caspase-2 buffer); OD, optical density. (B) Mitochondrial up-regulation of tBid after tGCI. tBid expression significantly increased in the mitochondrial fraction of the hippocampal CA1 subregion 4, 8, 24, and 72 h after tGCI (n = 4; *, P < 0.05; †, P < 0.01; §, P < 0.001). Cytochrome oxidase subunit IV (COX IV) was used as an internal control. s, shams.
For further investigation, we examined tBid expression after tGCI. Western blot analysis showed that tBid expression significantly increased time-dependently in the mitochondrial fraction of the hippocampal CA1 subregion compared with the shams (Fig. 4B). In combination with the caspase-2 brain lysate data, these results indicate that Bid is cleaved, at least in part, by caspase-2 after tGCI in the hippocampal CA1 subregion.
Injection of PIDD-siRNA Silences PIDD Expression, and Activation of Caspase-2 is PIDD-Dependent.
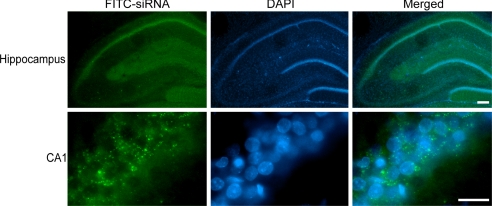
We injected FITC-conjugated non-targeting siRNA (FITC-siRNA) intracerebroventricularly. Immunofluorescence demonstrated the cytosolic distribution of FITC-siRNA predominantly in the dorsal hippocampus 48 h after injection (Fig. 5). These results indicate the successful transfection of siRNA. Because FITC-siRNA fluorescence 48 h after siRNA injection showed incorporation of siRNA in the CA1 subregion, we also used this time period to silence the PIDD protein using PIDD-siRNAs.
Fig. 5.
siRNA was distributed in the CA1 subregion. Fluorescent double staining of FITC-siRNA (green) and DAPI (blue) in the hippocampal CA1 subregion 48 h after injection of 1 μg of FITC-siRNA, which was distributed in the cytosol, predominantly in the dorsal hippocampus. [Scale bar, 200 μm (hippocampus), 20 μm (CA1).]
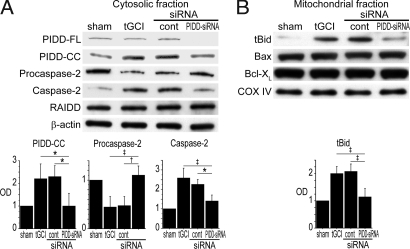
We then examined the effect of PIDD-siRNA on protein expression in the vulnerable hippocampal CA1 subregion after tGCI. We used 5 μg siSTABLE PIDD-siRNA to inhibit endogenous PIDD because a titration study indicated this dose effectively inhibited endogenous PIDD without a strong off-target effect (Fig. S3). Western blot analysis showed that PIDD expression was inhibited with PIDD-siRNA. Moreover, caspase-2 and tBid decreased in PIDD-siRNA-treated animals, indicating that activation of caspase-2 or Bid was PIDD-dependent, at least in part (Fig. 6). There was no significant difference in RAIDD (Fig. 6A) or mitochondrial Bax or Bcl-XL (Fig. 6B) among all groups. No significant difference was observed between non-treated animals and nontargeting control siRNA-treated animals, indicating that the off-target effect was not very strong in our siRNAs (Fig. 6).
Fig. 6.
Cleavage of procaspase-2 and Bid are PIDD-dependent. (A) Effect of siRNA against PIDD in the cytosol 8 h after tGCI. Western blot analysis showed that PIDD-FL and PIDD-CC expression significantly decreased in the 5-μg PIDD-siRNA-treated animals 8 h after tGCI (n = 4; *, P < 0.05). Cleavage of procaspase-2 was significantly inhibited in the PIDD-siRNA-treated animals (n = 4; *, P < 0.05; †, P < 0.01; ‡, P < 0.005). There was no significant difference in RAIDD expression in any groups. β-actin was used as an internal control. tGCI, nontreated; cont, control-siRNA-treated; OD, optical density. (B) Effect of siRNA against PIDD in mitochondria 8 h after tGCI. Western blot analysis demonstrated that mitochondrial tBid expression significantly decreased in the 5-μg PIDD-siRNA-treated animals 8 h after tGCI (n = 4; ‡, P < 0.005). Mitochondrial Bax and Bcl-XL showed no significant difference among all groups. Cytochrome oxidase subunit IV (COX IV) was used as an internal control.
Administration of PIDD siRNA Decreases Histological CA1 Neuronal Damage, TUNEL-Positive Cells, and DNA Fragmentation.
To examine the role of PIDD in delayed death of hippocampal CA1 neurons after tGCI, we performed histological analyses and a cell death assay using animals with the following treatments: shams, non-treated, control-siRNA-treated, and PIDD-siRNA-treated. In our model, the majority of CA1 pyramidal cells underwent degeneration 72 h after ischemia (14), which was the time point at which we collected samples for histological analysis and DNA fragmentation assay.
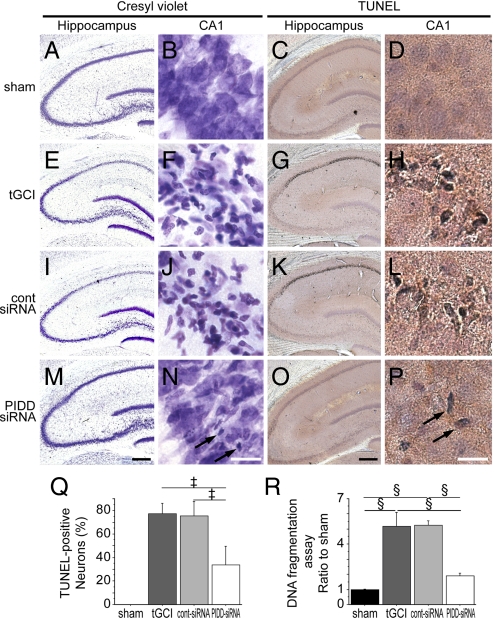
Histological analysis demonstrated that PIDD inhibition by siRNA decreased histological neuronal damage in the hippocampal CA1 subregion after tGCI. In the shams, CA1 neurons were intact 72 h after tGCI (Fig. 7 A and B). In contrast, more than 80% of CA1 neurons were degenerated in both the nontreated (Fig. 7 E and F) and control siRNA-treated animals (Fig. 7 I and J), which is compatible with our previous reports using the same model (14, 15). In the PIDD-siRNA-treated animals, neurons were fairly well preserved (Fig. 7 M and N); sparse damage was observed in the hippocampal CA1 subregion (Fig. 7N). Most of the morphologically damaged neurons stained with cresyl violet were also positive for TUNEL (Fig. 7 C, D, G, H, K, L, O, and P). Sparse TUNEL-positive cells were observed in the hippocampal CA1 subregion in the PIDD-siRNA-treated animals (Fig. 7P). Moreover, there were fewer TUNEL-positive cells (44% vs. nontreated animals and 45% vs. control siRNA-treated animals) in the PIDD-siRNA-treated animals (Fig. 7Q). No significant difference was observed between the nontreated and control siRNA-treated animals.
Fig. 7.
Cresyl violet staining (A, B, E, F, I, J, M, and N), TUNEL staining (C, D, G, H, K, L, O, and P), a cell-counting study of TUNEL-positive cells (Q), and a DNA fragmentation assay (R) 72 h after surgery. A, C, E, G, I, K, M, and O show low magnification of the entire hippocampus. B, D, F, H, J, L, N, and P show high magnification of the hippocampal CA1 subregion. In the shams, no hippocampal CA1 damage was observed (A and B). Seventy-two hours after tGCI, most CA1 neurons in the nontreated (E, F) and control-siRNA-treated (I and J) animals were damaged; they had shrunken, triangular-shaped, condensed nuclei (F and J). However, the normal features of the nuclei of many neurons were preserved in the PIDD-siRNA-treated animals (M and N). Sparse neuronal damage was observed in the hippocampal CA1 subregion in the PIDD-siRNA-treated animals (N, arrows). The majority of damaged neurons became TUNEL-positive (C, D, G, H, K, L, O, and P). Sparse TUNEL-positive cells were observed in the hippocampal CA1 subregion in the PIDD-siRNA-treated animals (P, arrows). The cell-counting study (Q) showed a significant decrease in TUNEL-positive cells in the hippocampal CA1 subregion 72 h after ischemia in the PIDD-siRNA-treated animals compared with the nontreated and control-siRNA-treated animals (n = 4, ‡, P < 0.005). The apoptotic DNA fragmentation assay (R) showed that fragmentation in the hippocampal CA1 subregion 72 h after tGCI increased significantly in the non-treated and control-siRNA-treated animals compared with the shams (n = 4, §, P < 0.001). DNA fragmentation in the hippocampal CA1 subregion 72 h after tGCI decreased significantly in the PIDD-siRNA-treated animals compared with the nontreated or control-siRNA-treated animals (n = 4, §, P < 0.001). tGCI, non-treated; cont-siRNA, control-siRNA-treated. [Scale bars: hippocampus, 400 μm; CA1, 20 μm.]
An apoptotic DNA fragmentation assay demonstrated that PIDD-siRNA administration decreased DNA fragmentation in the hippocampal CA1 subregion after tGCI. Fragmentation was significantly increased in this subregion 72 h after tGCI in the nontreated and control siRNA-treated animals (Fig. 7R). Less DNA fragmentation (37% vs. nontreated animals and 36% vs. control siRNA-treated animals) was observed in the PIDD-siRNA-treated animals (Fig. 7R).
To observe long-term cell viability, we also performed a histological analysis 7 days after tGCI. PIDD-siRNA-treated animals had less neuronal damage (Fig. S4). In conclusion, inhibition of PIDD using siRNA decreased histological neuronal damage and DNA fragmentation in the hippocampal CA1 subregion after tGCI.
Discussion
Until recently, the role of PIDD in the activation of caspase-2 and apoptosis had been studied primarily in the cancer field. The findings from our study indicate that PIDD also plays a role in ischemia by activating caspase-2, leading to neuronal cell death. We base these findings on the following results: PIDD-CC was up-regulated after tGCI not in the hippocampal CA3 region, but in hippocampal CA1 neurons, and preceded caspase-2 activation, followed by Bid cleavage (Figs. 1, 2, and 4 and Fig. S1). Furthermore, the direct binding of PIDD-CC, RAIDD, and procaspase-2 (which is referred to as the PIDDosome), was observed after tGCI (Fig. 3 and Fig. S2). The siRNA study indicates that activation of caspase-2 and Bid was PIDD-dependent, at least in part (Figs. 5 and 6 and Fig. S3). Moreover, inhibition of PIDD expression resulted in a decrease in neuronal death in the hippocampal CA1 subregion after tGCI (Fig. 7 and Fig. S4).
Previous studies demonstrated the roles of PIDD in cell death pathways (8, 12, 16, 17). PIDD could act as a switch for genotoxic stress through its fragments (17). PIDD-FL was constitutively cleaved into three fragments by autoproteolysis: an N-terminal fragment and a C-terminal fragment, PIDD-C, which was further cleaved into PIDD-CC (8, 12). PIDD-FL is thought to be a source of the other fragments. In agreement, endogenous PIDD-FL was hardly detectable or was absent (12). In our study, PIDD-FL showed only negligible expression, similar to the previous study.
PIDD-CC is thought to have a pro-apoptotic role. It is likely to regulate stress-induced apoptosis through a mitochondrial-dependent pathway (8, 12). We now report that PIDD may play a role in neuronal death because PIDD-CC is up-regulated in the vulnerable hippocampal CA1 subregion after tGCI, although PIDD is not up-regulated in the CA3 subregion. Furthermore, knockdown of PIDD reduced delayed neuronal death in this subregion after tGCI.
In contrast to PIDD-CC, PIDD-C is thought to have an anti-apoptotic role. In a recent study, PIDD-C formed a protein complex containing a nuclear factor-κB essential modulator and receptor-interacting protein 1, and PIDD-C was important for activating the transcription factor nuclear factor-κB pathway in response to genotoxic stress (16). We show that PIDD-C may not have a significant role in the mechanism of delayed neuronal death after tGCI, because it was not detected in the CA1 subregion at any time point after tGCI, although it was detected in the entire hippocampus and the positive control. In summary, our results using the different fragments of PIDD may suggest that PIDD acts as a “death switch” and that PIDD-CC plays a role after tGCI.
The next question addresses how PIDD induces delayed neuronal death. We believe one possibility is the formation of the PIDDosome and subsequent activation of caspase-2. In this study, we found direct binding among PIDD-CC, RAIDD, and procaspase-2 (which suggests formation of the PIDDosome complex) in the cytosol of vulnerable CA1 neurons after tGCI. Moreover, caspase-2 was activated in a time-dependent manner after formation of the PIDDosome. Furthermore, the siRNA study showed that activation of caspase-2 was PIDD-dependent. Our results are similar to those from other recent studies demonstrating that the PIDDosome activates caspase-2 and regulates stress-induced apoptosis (8, 18) and that knockdown of PIDD or RAIDD with siRNA results in downregulation of caspase-2 expression (8).
Although we have shown in this study that PIDD may play a role in activating caspase-2 and in subsequent delayed neuronal death after tGCI, the role of caspase-2 in apoptosis remains controversial; procaspase-2/caspase-2 is unique because it has characteristics of both initiator and effector caspases. It has an N-terminal pro-domain and is very similar in sequence to initiator caspases, but its cleavage specificity is closer to the effector caspases (19, 20). Previous studies have demonstrated the role of caspase-2 in apoptosis. Under certain conditions, procaspase-2/caspase-2 seems to be downstream of caspase-9/caspase-3 (21–23). However, a number of studies have shown that caspase-2 acts upstream of mitochondria by inducing Bid cleavage, Bax translocation to mitochondria, and subsequent cytochrome c release in stress-induced apoptosis (22, 24–26). Although Bid is a substrate of caspase-2, Golgin-160 and caspase-8 are also potential substrates of caspase-2. Golgin-160 was cleaved by caspase-2 under certain apoptotic conditions, such as staurosporine- and UV-induced cell death (27). Procaspase-8 was cleaved by caspase-2 in human cancer cell lines under tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptotic conditions (28). Moreover, recent studies demonstrated that caspase-2 can directly interact with mitochondria and that this interaction occurs independently of its proteolytic activity (29, 30). Finally, caspase-2 may play roles in apoptosis through direct interaction with mitochondria or by cleaving Bid, Golgin-160, or caspase-8.
Although the role of caspase-2 in apoptosis remains controversial, caspase-2 has been reported to have roles in neuronal cell death; it mediates death by trophic factor deprivation in PC12 cells and sympathetic neurons (31, 32). It is also involved in neuronal cell death induced by β-amyloid (33), seizure (34), or HIV infection (35). Procaspase-2 mRNA and its level of protein expression increased after focal ischemia (36, 37) and tGCI (38). Moreover, inhibition of caspase-2 by z-VDVAD-FMK was neuroprotective (37). In our study, activation of caspase-2 occurred after tGCI, as previously reported (38). Incubation of whole cell lysate from CA1 subregions with caspase-2 resulted in Bid cleavage, even though no change in Bax or Bcl-XL was observed. tBid expression was up-regulated after tGCI. Moreover, knockdown of PIDD expression by siRNA resulted in downregulation of caspase-2 and tBid and protection against subsequent delayed neuronal death in the hippocampal CA1 subregion after tGCI. Although our results do not show evidence that caspase-2 cleaves Bid directly or indirectly, these results suggest an important role for caspase-2 in Bid cleavage and subsequent selective CA1 neuronal death after tGCI.
In contrast to reports on the role of caspase-2 in neuronal cell death, several studies using caspase-2-null mice have suggested that caspase-2 may play less of an apoptotic role in neurons. These mice were reported to have very few apoptotic defects, and studies showed no defects in cultured sympathetic neurons that underwent nerve growth factor deprivation-induced death (23, 39). These discrepancies may be caused by up-regulation of the caspase-9 pathway in compensation for the lack of caspase-2 in these mice (40).
In our study, we used an siRNA technique to specifically inhibit the PIDD-caspase-2 pathway. Baptiste-Okoh et al. (41) also used the siRNA method to inhibit this pathway. The advantages of this method are as follows: first, siRNA is more specific than a drug such as a caspase-2 inhibitor. Second, we avoided continuous overexpression of the caspase-9 pathway, which was observed in caspase-2-null mice. Our siRNA study strongly supports the role of caspase-2 in apoptosis and neuronal cell death.
We have demonstrated a role for PIDD in the activation of caspase-2 and subsequent neuronal cell death using the siRNA technique. Transfection of siRNAs into animal cells results in potent, long-lasting, posttranscriptional silencing of specific genes (42). To evaluate siRNA effectiveness, selectivity should be a concern. We have closely followed the detailed recommendation of an editorial in Nature Cell Biology regarding controls for siRNA specificity (43).
In our siRNA study, siSTABLE nontargeting control siRNA was used as a scrambled control, and it showed no significant nonspecific knockdown of target proteins. This control siRNA was also used in other reports (44, 45). For basic control, Western blotting showed that siRNA administration decreased expression of PIDD, the target protein. We did not detect mRNA because we had a functional control. For quantitative control, the highest dose of siRNA (5 μg) had maximum knockdown of target protein in the titration study. Because more concentrated siRNA precipitated before administration, further concentrated siRNA could not be injected, although it may have had a greater knockdown effect. A large amount of siRNA was needed because we applied the siRNA technique to an in vivo rat model. For functional control, all siRNA we used contained the modification against 3′-untranslated regions (46). 3′-Untranslated regions are associated with RNAi off-targets (47). For the multiplicity controls, we used predesigned PIDD-siRNA and siSTABLE PIDD-siRNA. Although siRNAs tend to reduce the expression of PIDD, siSTABLE PIDD-siRNA had a stronger knockdown of PIDD.
In conclusion, our results imply that PIDD plays a role in activating caspase-2 and subsequent delayed CA1 neuronal death after tGCI. Based on our studies, we propose that PIDD is a hypothetical molecular target for therapy against neuronal death after tGCI.
Materials and Methods
Details beyond the descriptions here and in the Results are given in SI Materials and Methods.
Global Cerebral Ischemia.
Five minutes of tGCI was induced by bilateral common carotid artery occlusion combined with hypotension in male Sprague-Dawley rats (14). All animals were treated in accordance with Stanford University guidelines and the animal protocols were approved by Stanford University's Administrative Panel on Laboratory Animal Care (see SI Materials and Methods).
siRNA Administration.
All siRNAs were administered intracerebroventricularly with 10 mM of jetSI (403–05; Polyplus Transfection) as a transfection agent. To evaluate transfection efficiency, we used FITC-siRNA (sc-36869; Santa Cruz Biotechnology). Animals were killed 48 h after administration of FITC-siRNA and observed under a fluorescent microscope. To inhibit PIDD expression, we used predesigned ON-TARGETplus PIDD-siRNA (L-0852440–01; Dharmacon) and siSTABLE PIDD-siRNA (Dharmacon). The siSTABLE option chemically modifies the enhancing stability of siRNAs. ON-TARGETplus and siSTABLE option contain the modification against 3′-untranslated regions (46). siSTABLE control-siRNA (D-001700–10−05; Dharmacon) was used as an off-site siRNA control. For titration, control-siRNA, predesigned PIDD siRNA, and siSTABLE PIDD-siRNA were injected (0.2, 1, and 5 μg). Animals were killed 48 h after injection and Western blotting was performed. For inhibition of PIDD after tGCI, 5 μg of control siRNA and siSTABLE PIDD-siRNA were injected 48 h before tGCI based on titration (see SI Materials and Methods).
Western Blot Analysis.
Fresh hippocampal CA1 and CA3 tissue was removed after 1, 4, 8, 24, and 72 h of reperfusion. Protein extraction of cytosolic and mitochondrial fractions was performed using a multiple centrifugation method. Jurkat cell lysate (sc-2204; Santa Cruz Biotechnology) was used as a positive control. PIDD blocking peptide was used to confirm the specificity of the antibody (see SI Materials and Methods).
Co-IP.
The Co-IP procedure applied in this study is detailed in the SI Materials and Methods.
Assay of Bid Cleavage.
Forty micrograms of whole-cell extract from the hippocampal CA1 subregion were reacted with 1 or 3 units of recombinant activated caspase-2 (CC127; Millipore) or without caspase-2 for 1 h at 37°C. The products were immunoblotted (see SI Materials and Methods).
Immunofluorescent Staining.
The immunofluorescent staining performed in this study is detailed in SI Materials and Methods.
Histological Analysis of Hippocampal Injury and In Situ Labeling of DNA Fragmentation.
Histological analysis of hippocampal injury and in situ labeling of DNA fragmentation is detailed in SI Materials and Methods.
Cell Death Assay.
The cell death assay performed in this study is detailed in SI Materials and Methods.
Cell-Counting Procedure.
Details of the cell-counting procedure used in the present study are found in SI Materials and Methods.
Statistical Analysis.
To evaluate the results of the Western blotting, co-IP, cell-counting study, and cell death assay, comparisons among the data obtained from each group (n = 4) were performed with a one-way ANOVA, followed by a Scheffé post-hoc analysis (SigmaStat software; Jandel). The data are expressed as mean ± SD and significance was accepted with P < 0.05.
Supplementary Material
Acknowledgments.
We thank Liza Reola, Bernard Calagui, and Trisha Crandall for technical assistance; Cheryl Christensen for editorial assistance; and Elizabeth Hoyte for figure preparation. This work was supported by National Institutes of Health Grants P50 NS014543, R01 NS025372, R01 NS036147, and R01 NS038653.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806222105/DCSupplemental.
References
- 1.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 2.Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37:1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- 3.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 4.Cao G, et al. Caspase-activated DNase/DNA fragmentation factor 40 mediates apoptotic DNA fragmentation in transient cerebral ischemia and in neuronal cultures. J Neurosci. 2001;21:4678–4690. doi: 10.1523/JNEUROSCI.21-13-04678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, et al. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci. 1998;18:4914–4928. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao G, et al. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin Y, Ma W, Benchimol S. Pidd, a new death-domain-containing protein, is induced by p53 and promotes apoptosis. Nat Genet. 2000;26:124–127. doi: 10.1038/79102. left. [DOI] [PubMed] [Google Scholar]
- 8.Tinel A, Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science. 2004;304:843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- 9.Berube C, et al. Apoptosis caused by p53-induced protein with death domain (PIDD) depends on the death adapter protein RAIDD. Proc Natl Acad Sci USA. 2005;102:14314–14319. doi: 10.1073/pnas.0506475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren J, et al. The Birc6 (Bruce) gene regulates p53 and the mitochondrial pathway of apoptosis and is essential for mouse embryonic development. Proc Natl Acad Sci USA. 2005;102:565–570. doi: 10.1073/pnas.0408744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seth R, Yang C, Kaushal V, Shah SV, Kaushal GP. p53-dependent caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in renal tubular epithelial cell injury. J Biol Chem. 2005;280:31230–31239. doi: 10.1074/jbc.M503305200. [DOI] [PubMed] [Google Scholar]
- 12.Tinel A, et al. Autoproteolysis of PIDD marks the bifurcation between pro-death caspase-2 and pro-survival NF-κB pathway. EMBO J. 2007;26:197–208. doi: 10.1038/sj.emboj.7601473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park HH, et al. Death domain assembly mechanism revealed by crystal structure of the oligomeric PIDDosome core complex. Cell. 2007;128:533–546. doi: 10.1016/j.cell.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugawara T, Lewén A, Noshita N, Gasche Y, Chan PH. Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA1 subregion in rats. J Neurotrauma. 2002;19:85–98. doi: 10.1089/089771502753460268. [DOI] [PubMed] [Google Scholar]
- 15.Chan PH, et al. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J Neurosci. 1998;18:8292–8299. doi: 10.1523/JNEUROSCI.18-20-08292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssens S, Tinel A, Lippens S, Tschopp J. PIDD mediates NF-κB activation in response to DNA damage. Cell. 2005;123:1079–1092. doi: 10.1016/j.cell.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z-H, Mabb A, Miyamoto S. PIDD: a switch hitter. Cell. 2005;123:980–982. doi: 10.1016/j.cell.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Bradley G, et al. The expression of p53-induced protein with death domain (Pidd) and apoptosis in oral squamous cell carcinoma. Br J Cancer. 2007;96:1425–1432. doi: 10.1038/sj.bjc.6603745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey NL, Butt AJ, Kumar S. Functional activation of Nedd2/ICH-1 (caspase-2) is an early process in apoptosis. J Biol Chem. 1997;272:13134–13139. doi: 10.1074/jbc.272.20.13134. [DOI] [PubMed] [Google Scholar]
- 20.Li H, et al. Activation of caspase-2 in apoptosis. J Biol Chem. 1997;272:21010–21017. doi: 10.1074/jbc.272.34.21010. [DOI] [PubMed] [Google Scholar]
- 21.Slee EA, et al. Ordering the cytochrome c-initiated caspase cascade: Hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paroni G, Henderson C, Schneider C, Brancolini C. Caspase-2-induced apoptosis is dependent on caspase-9, but its processing during UV- or tumor necrosis factor-dependent cell death requires caspase-3. J Biol Chem. 2001;276:21907–21915. doi: 10.1074/jbc.M011565200. [DOI] [PubMed] [Google Scholar]
- 23.O'Reilly LA, et al. Caspase-2 is not required for thymocyte or neuronal apoptosis even though cleavage of caspase-2 is dependent on both Apaf-1 and caspase-9. Cell Death Differ. 2002;9:832–841. doi: 10.1038/sj.cdd.4401033. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Vaux DL. A Cinderella caspase takes center stage. Science. 2002;297:1290–1291. doi: 10.1126/science.1076118. [DOI] [PubMed] [Google Scholar]
- 25.Lassus P, Opitz-Araya X, Lazebnik Y. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science. 2002;297:1352–1354. doi: 10.1126/science.1074721. and erratum (2004) 306:1683. [DOI] [PubMed] [Google Scholar]
- 26.Robertson JD, Enoksson M, Suomela M, Zhivotovsky B, Orrenius S. Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J Biol Chem. 2002;277:29803–29809. doi: 10.1074/jbc.M204185200. [DOI] [PubMed] [Google Scholar]
- 27.Mancini M, et al. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J Cell Biol. 2000;149:603–612. doi: 10.1083/jcb.149.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin S, et al. Caspase-2 primes cancer cells for TRAIL-mediated apoptosis by processing procaspase-8. EMBO J. 2005;24:3532–3542. doi: 10.1038/sj.emboj.7600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enoksson M, et al. Caspase-2 permeabilizes the outer mitochondrial membrane and disrupts the binding of cytochrome c to anionic phospholipids. J Biol Chem. 2004;279:49575–49578. doi: 10.1074/jbc.C400374200. [DOI] [PubMed] [Google Scholar]
- 30.Robertson JD, et al. Processed caspase-2 can induce mitochondria-mediated apoptosis independently of its enzymatic activity. EMBO Rep. 2004;5:643–648. doi: 10.1038/sj.embor.7400153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troy CM, Stefanis L, Greene LA, Shelanski ML. Nedd2 is required for apoptosis after trophic factor withdrawal, but not superoxide dismutase (SOD1) downregulation, in sympathetic neurons and PC12 cells. J Neurosci. 1997;17:1911–1918. doi: 10.1523/JNEUROSCI.17-06-01911.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefanis L, Troy CM, Qi H, Shelanski ML, Greene LA. Caspase-2 (Nedd-2) processing and death of trophic factor-deprived PC12 cells and sympathetic neurons occur independently of caspase-3 (CPP32)-like activity. J Neurosci. 1998;18:9204–9215. doi: 10.1523/JNEUROSCI.18-22-09204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Troy CM, et al. Caspase-2 mediates neuronal cell death induced by β-amyloid. J Neurosci. 2000;20:1386–1392. doi: 10.1523/JNEUROSCI.20-04-01386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henshall DC, Skradski SL, Bonislawski DP, Lan J-Q, Simon RP. Caspase-2 activation is redundant during seizure-induced neuronal death. J Neurochem. 2001;77:886–895. doi: 10.1046/j.1471-4159.2001.00291.x. [DOI] [PubMed] [Google Scholar]
- 35.Kolson DL, Sabnekar P, Baybis M, Crino PB. Gene expression in TUNEL-positive neurons in human immunodeficiency virus-infected brain. J Neurovirol. 2004;10(Suppl 1):102–107. doi: 10.1080/753312760. [DOI] [PubMed] [Google Scholar]
- 36.Asahi M, et al. Expression of interleukin-1β converting enzyme gene family and bcl-2 gene family in the rat brain following permanent occlusion of the middle cerebral artery. J Cereb Blood Flow Metab. 1997;17:11–18. doi: 10.1097/00004647-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Kitagawa H, et al. Reduction of ischemic brain injury by topical application of glial cell line-derived neurotrophic factor after permanent middle cerebral artery occlusion in rats. Stroke. 1998;29:1417–1422. doi: 10.1161/01.str.29.7.1417. [DOI] [PubMed] [Google Scholar]
- 38.Jin K, et al. Two caspase-2 transcripts are expressed in rat hippocampus after global cerebral ischemia. J Neurochem. 2002;81:25–35. doi: 10.1046/j.1471-4159.2002.00781.x. [DOI] [PubMed] [Google Scholar]
- 39.Bergeron L, et al. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev. 1998;12:1304–1314. doi: 10.1101/gad.12.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Troy CM, et al. Death in the balance: Alternative participation of the caspase-2 and -9 pathways in neuronal death induced by nerve growth factor deprivation. J Neurosci. 2001;21:5007–5016. doi: 10.1523/JNEUROSCI.21-14-05007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baptiste-Okoh N, Barsotti AM, Prives C. A role for caspase 2 and PIDD in the process of p53-mediated apoptosis. Proc Natl Acad Sci USA. 2008;105:1937–1942. doi: 10.1073/pnas.0711800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elbashir SM, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. left. [DOI] [PubMed] [Google Scholar]
- 43.Editorial. Whither RNAi? Nat Cell Biol. 2003;5:489–490. doi: 10.1038/ncb0603-490. [DOI] [PubMed] [Google Scholar]
- 44.Jackson LN, et al. PI3K/Akt activation is critical for early hepatic regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1401–G1410. doi: 10.1152/ajpgi.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molnar GA, et al. Glucocorticoid-related signaling effects in vascular smooth muscle cells. Hypertension. 2008;51:1372–1378. doi: 10.1161/HYPERTENSIONAHA.107.105718. [DOI] [PubMed] [Google Scholar]
- 46.Jackson AL, et al. Position-specific chemical modification of siRNAs reduces “off-target” transcript silencing. RNA. 2006;12:1197–1205. doi: 10.1261/rna.30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Birmingham A, et al. 3′ UTR seed matches, but not overall identity, are associated RNAi off-targets. Nat Methods. 2006;3:199–204. doi: 10.1038/nmeth854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.